Abstract
Aims/hypothesis
Quantitative sensory testing (QST) allows the identification of individuals with rapid progression of diabetic sensorimotor polyneuropathy (DSPN) based on certain sensory phenotypes. Hence, the aim of this study was to investigate the relationship of these phenotypes with the structural integrity of the sciatic nerve among individuals with type 2 diabetes.
Methods
Seventy-six individuals with type 2 diabetes took part in this cross-sectional study and underwent QST of the right foot and high-resolution magnetic resonance neurography including diffusion tensor imaging of the right distal sciatic nerve to determine the sciatic nerve fractional anisotropy (FA) and cross-sectional area (CSA), both of which serve as markers of structural integrity of peripheral nerves. Participants were then assigned to four sensory phenotypes (participants with type 2 diabetes and healthy sensory profile [HSP], thermal hyperalgesia [TH], mechanical hyperalgesia [MH], sensory loss [SL]) by a standardised sorting algorithm based on QST.
Results
Objective neurological deficits showed a gradual increase across HSP, TH, MH and SL groups, being higher in MH compared with HSP and in SL compared with HSP and TH. The number of participants categorised as HSP, TH, MH and SL was 16, 24, 17 and 19, respectively. There was a gradual decrease of the sciatic nerve’s FA (HSP 0.444, TH 0.437, MH 0.395, SL 0.382; p=0.005) and increase of CSA (HSP 21.7, TH 21.5, MH 25.9, SL 25.8 mm2; p=0.011) across the four phenotypes. Further, MH and SL were associated with a lower sciatic FA (MH unstandardised regression coefficient [B]=−0.048 [95% CI −0.091, −0.006], p=0.027; SL B=−0.062 [95% CI −0.103, −0.020], p=0.004) and CSA (MH β=4.3 [95% CI 0.5, 8.0], p=0.028; SL B=4.0 [95% CI 0.4, 7.7], p=0.032) in a multivariable regression analysis. The sciatic FA correlated negatively with the sciatic CSA (r=−0.35, p=0.002) and markers of microvascular damage (high-sensitivity troponin T, urine albumin/creatinine ratio).
Conclusions/interpretation
The most severe sensory phenotypes of DSPN (MH and SL) showed diminishing sciatic nerve structural integrity indexed by lower FA, likely representing progressive axonal loss, as well as increasing CSA of the sciatic nerve, which cannot be detected in individuals with TH. Individuals with type 2 diabetes may experience a predefined cascade of nerve fibre damage in the course of the disease, from healthy to TH, to MH and finally SL, while structural changes in the proximal nerve seem to precede the sensory loss of peripheral nerves and indicate potential targets for the prevention of end-stage DSPN.
Trial registration
ClinicalTrials.gov NCT03022721
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Diabetic sensorimotor polyneuropathy (DSPN) embodies the most common type of neuropathy in individuals with diabetes, affecting up to half of those with type 2 diabetes [1]. Painful subtypes of DSPN are a major reason for the impairment of quality of life caused by the disease, with a substantial economic burden due to the resultant healthcare costs [2, 3]. However, to date, the pathophysiology and the natural course of the disease are incompletely understood and therapies are limited to symptomatic strategies [4, 5]. Novel diagnostic approaches now aim to identify distinct sensory phenotypes in individuals with diabetes, with the goal of improving patient care by identifying those at risk and enabling individual prognoses on the progression of DSPN, thereby potentially contributing to the elucidation of the natural course and pathophysiological of the disease [6,7,8]. Quantitative sensory testing (QST), a neurophysiological and psychological test battery of 13 sensory variables introduced by the German Research Network on Neuropathic Pain (DFNS) to assess large and small-fibre functions [9], has recently been applied to stratify individuals with polyneuropathy into four sensory clusters: healthy; sensory loss (SL); thermal hyperalgesia (TH); and mechanical hyperalgesia (MH) [7]. SL is characterised by a loss of small- and large-fibre function with coexisting paradoxical heat sensations (PHS). In TH the function of large and small fibres is relatively preserved, with the key feature being cold and heat hyperalgesia. Individuals in the MH cluster suffer from diminished cold and heat sensory functions, while pressure and pinprick hyperalgesia, as well as dynamic mechanical allodynia (DMA), are present. While these sensory clusters were suggested to be related to distinct pathophysiological mechanisms [7], it remains unclear whether they define entirely different phenotypes or whether they reflect an order of severity and chronological sequence of nerve fibre damage in individuals with polyneuropathies such as DSPN. It could, however, be hypothesised, that from a functional standpoint, the MH and SL clusters exhibiting loss of thermal sensation and loss of both thermal and mechanical sensation, respectively, may represent the most severe phenotypes in DSPN.
High-resolution magnetic resonance neurography (MRN) at 3 Tesla (3T) represents an additional sophisticated method that has provided valuable insights into pathophysiological mechanisms of DSPN in individuals with type 2 diabetes through the application of multimodal sequence techniques [10,11,12]. Diffusion tensor imaging (DTI), a technique that measures the restricted diffusion of water molecules along the axons of peripheral nerves, allows analysis of the structural integrity of peripheral nerves in vivo [13]. The fractional anisotropy (FA) embodies the main readout parameter showing correlations with electrophysiological variables of axonal and myelin sheath integrity and thereby reflects the structural integrity of peripheral nerves [14,15,16].
Consequently, DTI-MRN and sensory profiling by QST were combined to investigate whether the structural integrity of the sciatic nerve differs between the four clusters of sensory phenotypes and to test the hypothesis of whether nerve damage may follow a predefined cascade in individuals with type 2 diabetes.
Methods
Study design and participants
This cross-sectional study was approved by the local ethics committee of Heidelberg University Hospital (Heidelberg Study on Diabetes and Complications [HEIST-DiC]; ClinicalTrials.gov registration no. NCT03022721; local ethics no. S-383/2016) and was conducted according to the principles of the declaration of Helsinki. All participants gave written informed consent. Sex was investigator assessed or self-reported. The study took place between January 2016 and November 2019. Out of a total of 262 participants with type 2 diabetes in the HEIST-DiC study, 76 were included in the current study, all 76 having undergone MRN of the right leg (22 women, 54 men) and had a full set of MRN, QST, electrophysiological, clinical and laboratory data. A cohort of individuals without type 2 diabetes, which did not differ from the cohort of individuals with type 2 diabetes regarding age, sex and BMI and having undergone identical phenotyping were examined as a control group (n=13). The screening and recruitment of the participants, as well as QST, electrophysiological and serological examinations of all participants, were performed by the department of Endocrinology, Diabetology and Clinical Chemistry (Internal Medicine 1) [15]. Sex and gender were not considered during the process of screening and recruitment of participants.
To preclude factors other than type 2 diabetes as being responsible for peripheral nerve damage we excluded individuals suffering from other conditions predisposing to peripheral neuropathy (alcoholism, hypovitaminosis, malignant or infectious diseases, monoclonal gammopathy, chronical neurological diseases such as multiple sclerosis, or previous exposure to neurotoxic agents), as described previously [15]. Further, we excluded individuals with a history of lumbar surgery or disk protrusion, those with contraindications for MRN, pregnant women and individuals under 18 years of age or with an eGFR <60 ml/min per 1.73 m2[15]. Subsequently, 239 individuals with type 2 diabetes were eligible to participate in this study. Of these, 76 underwent the full study protocol consisting of DTI-MRN, QST of the right foot and electrophysiological as well as serological exams and were subsequently included in cross-sectional analysis. To justify the appropriateness of the size of the cohort, we performed a post hoc power analysis based on a previously published study investigating associations of DTI-MRN parameters with clinical, serological and electrophysiological variables [15], which yielded a power of 0.83. Subsequently for an α level of 0.05, an effect size of 0.4 and a power of 0.8, 76 participants were required to conduct perform one-way ANOVA for four groups.
Clinical and electrophysiological examination
Blood was drawn and urine was taken under fasting conditions in the morning, and samples were immediately processed in the Central Laboratory of University Hospital of Heidelberg under standardised conditions. HbA1c, lipid profile, urinary albumin/creatinine ratio (ACR), eGFR, high-sensitivity troponin T (hsTNT) and high-sensitivity C-reactive protein (hsCRP) were assessed. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [17] and ACR was determined in mg/g.
To evaluate the presence of neuropathic symptoms, the neuropathy symptom score (NSS) was estimated, while clinical neurological deficits of the lower extremities were evaluated using the neuropathy disability score (NDS). Nerve conduction studies on each participant’s right leg included measurements of nerve conduction velocity (NCV) and sensory nerve action potential (SNAP) of the sural nerve. NCVs as well as compound motor action potentials (CMAP) of the common peroneal and tibial nerves were performed maintaining a skin temperature of at least 32°C. Non-detectable excitation of the sural nerve bilaterally was considered indicative of advanced sural nerve damage. The corresponding missing NCV and SNAP values were imputed with the lowest values measured in the HEIST-DiC cohort (30.9 m/s and 2.54 μV, respectively). As additional sensitivity analysis for group comparisons, sural NCV and SNAP as well as common peroneal and tibial NCVs and CMAPs were converted into categorical variables (normal/abnormal) using the 2.5 percentile values in the HEIST-DiC cohort (38.6, 37.0, 38.0 m/s and 2.54, 1.14, 2.72 μV for sural, common peroneal and tibial nerves, respectively) as cut-offs.
QST and diagnosis of neuropathy
Participants underwent QST according to the protocol of the DFNS as described in detail elsewhere [11, 18]. The 13 domains of the DFNS QST protocol (cold detection threshold, warm detection threshold, perception of alternating warm and cold stimuli including PHS, cold and heat pain thresholds, mechanical and vibration detection thresholds, pinprick and blunt pressure pain thresholds, stimulus/response functions for pinprick sensitivity, DMA and pain summation to repetitive pinprick stimuli, known as the wind-up ratio) were measured on the right foot. Participants were subsequently grouped in to the four outlined sensory phenotypes according to the deterministic version of the sorting algorithm established by Vollert et al [8]. Using this algorithm, based on the greatest concordance of the 13 items of the QST profile, each participant with type 2 diabetes was assigned to a single one of the four sensory phenotypes (Fig. 1): (1) healthy sensory profile (HSP), representing individuals with type 2 diabetes whose sensory profile resembled those of healthy individuals; (2) TH; (3) MH; and (4) SL. [8]
Graphic presentation of the 13-domain QST profiles of the four subgroups of participants with type 2 diabetes. (a) Presentation of the 11 z-transformed QST domains. The z-transformation normalises differences due to age, sex and tested anatomical region. Positive and negative z scores denote gain (hyperalgesia) or loss (hypoalgesia or hypoaesthesia) of function, respectively. (b) Presentation of numeric pain ratings for dynamic mechanical allodynia (0–100) on a log10 scale and of frequency of paradoxical heat sensation (×/3 denotes number of paradoxical heat sensations [0–3] for 3× repeated testing). Grey circles, HSP, (n=16); red circles, TH (n=24); yellow circles, MH (n=17); blue circles, SL (n=19). MPT, mechanical pain threshold; NRS, numerical rating scale; PPT pressure pain threshold; WUR, wind-up ratio
According to current guidelines, the diagnosis of DSPN through the sole performance of QST is not recommended, so we applied the Toronto consensus criteria to diagnose ‘confirmed neuropathy’[19] in order to investigate whether sensory profiles are associated with established diagnostic criteria of DSPN. Diagnosis of DSPN was given where there were abnormal results of the nerve conduction study of the sural nerve and additional abnormal nerve conduction studies of the common peroneal or tibial nerve together with an NSS and/or NDS of ≥3.
MRI imaging protocol and data analysis
Magnetic resonance neurography imaging protocol
High-resolution MRN of the right leg was performed in a 3.0 Tesla MR scanner (Magnetom TIM-TRIO; Siemens, Erlangen, Germany) using a 15-channel transmit–receive extremity coil with the participant positioned in the supine position. An axial high-resolution T2-weighted (T2w) turbo spin echo two-dimensional sequence with spectral fat saturation of the right mid-thigh (A) and a DTI with an axial fat-suppressed, diffusion-weighted two-dimensional echo-planar sequence (B) were applied with the following parameters:
-
(A)
repetition time (TR) 5970 ms, echo time (TE) 55 ms, field of view (FOV) 160×160 mm2, matrix size 512×512, slice thickness 4 mm, interslice gap 0.35 mm, voxel size 0.3×0.3×4.0 mm3, three averages, 24 images.
-
(B)
TR 5100 ms; TE 92.8 ms; b=0 and 1000 s/mm2; directions 20; FOV 160×160 mm2; matrix size 128×128; slice thickness 4 mm; voxel size 1.3×1.3×4 mm3; no interslice gap, three averages, 24 slices, 1512 images.
Image post-processing
All images were pseudonymised and post-processed by two neuroradiologists with 3 and 7 years of experience in MRN imaging, respectively. The analysis of DTI was performed using Nordic BRAINEX (NordicNeuroLab, Norway, version 2.2, 2019), an automated software applied for the calculation and reconstruction of fibre tracts in diffusion-weighted imaging. Hereby, the sciatic nerve’s FA was calculated [15, 20]: first, automatic co-registration of the axial T2w sequence with the DTI sequence was performed (B); then the anatomical region of the sciatic nerve was manually delineated and the sciatic nerve’s mean FA was automatically calculated by Nordic BRAINEX. Settings for DTI analysis were chosen as follows and as performed previously: value of >0.1 for the nerve’s FA, a tract turning angle of 41.4°, a minimum fibre length of 20 mm and one seed per voxel as cut-off values for automated fibre tracking [15, 20]. The FA is a dimensionless parameter, with values ranging between 0 and 1 being representative of the structural integrity of peripheral nerves [15, 21, 22] and associated with electrophysiological markers of axonal and myelin sheath integrity [12, 15].
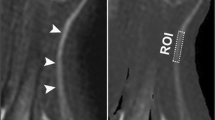
The manual anatomical segmentation of the tibial compartment of the sciatic nerve on the axial fat-saturated turbo spin echo two-dimensional sequence of the right mid-thigh was performed using ImageJ (US National Institutes of Health, Bethesda, Maryland, USA, version 1.53k) and analysed the mean cross-sectional area (CSA).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) and SPSS 28 (IBM, Armonk, NY, USA). Dichotomous variables are presented as absolute numbers and percentages. The normality of the distribution of continuous variables was tested using the Kolmogorov–Smirnov test. Normally and non-normally distributed quantitative variables are presented as means (SD) or medians (25, 75 IQR). Comparisons across the four phenotype groups (HSP, SL, TH, MH) regarding qualitative characteristics were made with the χ2 test; quantitative variables were compared using one-way ANOVA or the Kruskal–Wallis non-parametric test, depending on the normality of their distribution. Where there were significant differences across the four groups, between-group post hoc comparisons with Bonferroni correction were carried out. To examine the correlations between pairs of continuous variables, the Pearson r was calculated, while correlations between categorical and continuous variables were examined via binary logistic regression. To ascertain the independence of observed associations we used partial correlation or multivariable linear regression analysis. Independent variables exhibiting a markedly skewed distribution (e.g. duration of diabetes, hsTNT, ACR) were log-transformed prior to correlation analysis. All statistical tests were two-sided and a p value of <0.05 was considered statistically significant.
Results
Upon application of the sorting algorithm within the type 2 diabetes participant cohort, a total of 16, 24, 17 and 19 participants were categorised as HSP, TH, MH and SL, respectively. In Fig. 1 and the electronic supplementary material (ESM Results and ESM Table 1) we provide QST profiles and group comparisons of each subgroup.
Group comparisons across the four clusters showed no differences in age, sex composition, number of active smokers, BMI, waist circumference, duration of type 2 diabetes, type of glucose-lowering medication, total serum cholesterol, eGFR and hsCRP (Table 1).
There was a gradual increase in ACR (median [IQR]: HSP 0.07 [0.05, 0.26] mg/mmol; TH 0.11 [0.06, 0.14] mg/mmol; MH 0.12 [0.05, 0.47] mg/mmol; SL 0.39 [0.11, 1.66] mg/mmol; p=0.034) across the groups, yet, without significant differences in post hoc tests. Likewise, hsTNT also showed a pattern of gradual increase (median [IQR]: HSP 8.5 [5.8, 9.3] ng/ml; TH 9.0 [8.0, 11.0] ng/ml; MH 9.0 [9.0, 12.0] ng/ml; SL 13.5 [6.0, 16.0] ng/ml; p=0.050) and was higher in the SL compared with the healthy cluster (p=0.042) (Table 1).
There were no differences across the groups with respect to overall subjective neuropathic symptoms indexed by NSS (Table 1, Fig. 2a). Nevertheless, among NSS-related symptoms, a greater proportion of those with SL reported the presence of pain in lower extremities (Table 1). Objective neurological deficits showed a gradual increase across HSP, TH, MH and SL, being higher in MH compared with HSP and in SL compared with HSP and TH (Table 1, Fig. 2b).
Comparative presentation of clinical neurological and electrophysiological features across the four groups of participants with type 2 diabetes. (a) No difference in the severity of neuropathic symptoms across the four phenotypes. (b) The magnitude of objective clinical deficits indexed by the NDS shows a gradual increase, culminating in those with SL. (c) There are no noted differences in sural NCV. (d) Pronounced differences in sural SNAP across the groups, with the greatest magnitude of abnormalities observed in SL. See also Table 1. *p<0.05, **p<0.05, ***p<0.001 in pairwise comparisons after Bonferroni correction
There were no significant differences in sural NCV across the four groups (p=0.058), although the prevalence of an abnormally low NCV showed a gradual increase (31.3% vs 33.3% vs 58.8% vs 78.9%, respectively, p=0.008), being significantly higher in SL compared with HSP and TH (Table 1, Fig. 2c). In contrast, differences regarding sural SNAP were considerably more pronounced across the groups either as continuous or categorical variables (pANOVA=0.001 for both), culminating in lowest and highest values, respectively, in the SL subgroup (Table 1, Fig. 2d). The prevalence of confirmed DSPN according to the Toronto consensus was higher in the SL subgroup compared with all the others, while those in the MH subgroup also showed a higher DSPN frequency compared with those in the HSP subgroup.
Analysis of the FA of the sciatic nerve (HSP 0.444, TH 0.437, MH 0.395, SL 0.382; p=0.005) revealed a gradual decrease across the clusters (HSP>TH>MH>SL) with a significantly lower FA in SL compared with HSP (p=0.024) and TH (p=0.029) (Table 1, Fig. 3). In parallel a gradual increase of sciatic nerve CSA at the level of the distal thigh was found (HSP 21.7, TH 21.5, MH 25.9, SL 25.8 mm2; p=0.011), reaching a maximum in those with MH and SL, yet, without significant differences between subgroups in post hoc testing (Table 1, Fig. 3).
MRN-derived sciatic nerve variables among the different sensory subtypes in participants with type 2 diabetes. (a) Disruption of nerve integrity indexed by FA increases across HSP, TH, MH and SL (p=0.005), being significantly lower in SL compared with HSP (p=0.024) and TH (p=0.029). (b) An inverse pattern is observed regarding sciatic nerve CSA (p=0.012), without significant pairwise differences after Bonferroni correction. *p<0.05 in pairwise comparisons after Bonferroni correction
Comparison with healthy control individuals without type 2 diabetes
Participants with type 2 diabetes were compared with 13 healthy control participants who had similar age, sex and BMI (Table 2). Compared with the sum of the type 2 diabetes groups, there were expected differences in waist circumference, HbA1c and cholesterol levels and the sum of tested peripheral clinical and neurophysiological variables. Accordingly, z scores for thermal detection thresholds (cold detection threshold [CDT], warm detection threshold [WDT], thermal sensory limen [TSL]), thermal pain (cold pain threshold [CPT], heat pain threshold [HPT]) and mechanical detection thresholds (mechanical detection threshold [MDT], vibration detection threshold [VDT]), and mechanical pain sensitivity (MPS) were lower among participants with type 2 diabetes, while PHS and DMA were more prevalent among those with type 2 diabetes (ESM Table 1). Additionally, participants with type 2 diabetes exhibited lower FA (0.415 vs 0.459, p=0.001) and higher CSA values (23.6 vs 19.9 mm2, p=0.032) compared with healthy control participants (Table 2).
Group comparison of healthy control individuals with the HSP subgroup yielded higher hsTNT, HbA1c and NSS among the HSP subgroup. QST profiles were also similar between the healthy control and HSP groups, except for slight differences on zTSL (z score of TSL; 0.45 vs −0.22, p=0.012) and zHPT (z score of HPT; 0.88 vs −0.31, p=0.036) (ESM Table 1).
Impact of different sensory phenotypes on FA
Within the cohort of participants with type 2 diabetes, multivariable regression analysis was carried out with sural NCV, sural SNAP and the sciatic nerve’s FA and CSA as the dependent variables and the three sensory phenotypes as independent variables (Table 3). MH and SL were both associated with lower sural SNAP (MH B=−2.2 [95% CI −4.1, −0.3], p=0.022; SL B=−3.8 [95% CI −5.6, −1.96], p<0.001), lower sciatic FA (MH B=−0.048 [95% CI −0.091, −0.006], p=0.027; SL B=−0.062 [95% CI −0.103, −0.020], p=0.004) and a higher sciatic CSA (MH B=4.3 [95% CI 0.5, 8.0], p=0.028; SL B=4.0 [95% CI 0.4, 7.7], p=0.032). Further, SL was associated with a lower sural NCV (B=−7.0 [95% CI −13.0, −1.1], p=0.021).
After including sural NCV in the multivariable regression model, the association of SL with lower FA remained robust (B=−0.05 [95% CI −0.093, −0.007], p=0.023), although that of MH with FA was marginally non-significant (B=−0.042 [95% CI −0.085, 0.001], p=0.055) (Table 4). The inclusion of sural SNAP in the multivariable model instead resulted in the abolishment of all associations between sensory phenotypes and FA (Table 4), hence implying that the different phenotypes are distinguished by progressive axonal loss, concurrently reflected by both diminishing SNAP and FA.
Sciatic FA negatively correlates with CSA and markers of microvascular damage
Correlation analysis over the sum of participants with type 2 diabetes revealed that the sciatic FA correlates negatively with the sciatic CSA (r=−0.346, p=0.002; Table 5), a finding which persisted after adjustment for sex (r=−0.269, p=0.02). Furthermore, sciatic FA correlated negatively with log10(ACR) (r=−0.316, p=0.006) and the correlation was not substantially affected after adjustment for HbA1c and log(type 2 diabetes duration) (r=−0.328, p=0.009). FA also showed a negative correlation with log10(hsTNT) (r=−0.382, p=0.002) and the correlation remained robust after adjusting for age and eGFR (r=−0.317, p=0.014).
Discussion
This study is the first to investigate associations of sensory phenotyping through QST supplemented by DTI-MRN at 3T. The main findings were as follows: (1) in individuals with type 2 diabetes the sciatic nerve’s FA gradually decreases across the sensory phenotypes (HSP>TH>MH>SL) in conjunction with increasing clinical deficits and increased DSPN prevalence before the onset of subjective symptoms; (2) MH and SL are both associated with lower sural SNAP, lower sciatic FA and a higher sciatic CSA, all of which may serve as an additional marker of structural deterioration, while SL was also associated with a lower sural NCV; (3) over all participants the sciatic nerve’s FA negatively correlates with sciatic CSA and markers of microvascular damage, namely ACR and hsTNT. Furthermore, electrophysiological analyses suggest that diminishing FA across the different sensory phenotypes is mediated by progressive axonal loss and constitutes the structural and functional equivalents of sensory nerve damage in DSPN.
Although comparison with a cohort of control participants with similar age, sex and BMI revealed higher sciatic nerve FA and lower CSA compared with the sum of participants with type 2 diabetes, indicating greater structural integrity, there was no difference in sciatic FA and CSA compared with HSP (Table 2). This further indicates that the peripheral sensory profile is decisively determined by the degree of structural nerve damage, rather than by type 2 diabetes diagnosis or other potential confounders accompanying the type 2 diabetes status.
The finding that the FA of the sciatic nerve as a measure of peripheral nerve integrity [14, 15] was lower in SL compared with HSP and TH in combination with MH and SL being associated with lower sciatic FA values in multiple regression analysis suggests that individuals with type 2 diabetes may experience a predefined continuous cascade of nerve fibre damage in the course of the disease from HSP to TH, to MH and finally SL. Previous studies showed that predominant small-fibre damage is an early finding in individuals with diabetes [23, 24], and may be observed in those who primarily suffer from TH, which is mediated by an impairment of thin myelinated Aδ fibres and small unmyelinated C fibres. Hence, the fact that FA was not lower in the TH group compared with HSP group may potentially be explained through a low cumulative amount of nerve fibre damage with retained nerve structural integrity in this early disease stage. This is underlined by the result that TH was also not associated with lower sural NCV and SNAP, in line with the assumption that the cumulative nerve fibre damage is relatively sparse and small-fibre-dominant and can therefore not be detected by nerve conduction studies [25, 26]. Nonetheless, in MH, which is defined by loss of small-fibre function and mechanical allodynia/hyperalgesia, and in SL, which is characterised through small- and large-fibre loss [7, 18], FA was lower than in the HSP group, suggesting that a progression of small-fibre damage and involvement of large-fibres occur in the transition from MH to SL resulting in a lower structural nerve integrity of the sciatic nerve. Furthermore, sural SNAP was found to considerably differ across the four phenotypes, with a gradual monotonous decrease across HSP, TH, MH and SL, while differences regarding NCV were far more subtle. Likewise, the significant associations of MH and SL with a lower FA were largely retained after adjustment for sural NCV. However, this was not the case for sural SNAP, the inclusion of which in the multivariable model attenuated the aforementioned findings. SNAP reductions constitute an earlier feature of axonal loss in DSPN, temporally preceding the decrease in NCV [27]. Taken together, this evidence further adds to the notion that the patterns of diminishing FA across the different sensory phenotypes are mediated by progressive axonal loss and constitute the structural and functional equivalents of sensory nerve damage in DSPN. This is supported by a previous study which found that the amount of sciatic nerve lesions diagnosed through high-resolution MRN is associated with the severity of DSPN indicating that peripheral nerve damage may accumulate in the course of the disease and determine the onset and severity of DSPN [11]. In addition, a gradual increase of the NDS, with significantly higher scores in participants with MH and SL compared with HSP, was found and further indicates a higher degree neurological deficit in individuals with MH and SL. In further support of this observation, the prevalence of confirmed DSPN was highest in those with SL and higher in those with MH than in the HSP subgroup (Table 1).
Our hypothesis finds further support in the finding that a higher sciatic CSA is associated with sensory profiles of increasing neuropathic severity such as MH and SL and that the sciatic CSA correlates inversely with sciatic FA in a correlation analysis over all participants with type 2 diabetes. Several studies on peripheral nerve pathologies of various aetiologies previously demonstrated that an increase of the CSA was associated with more severe nerve damage [28,29,30,31,32]. A greater peripheral (most prominently, sciatic or tibial) nerve CSA assessed by ultrasound or MRN has been also ascertained in DSPN compared with control individuals with HSP, albeit with considerable overlap of CSA values and without convincing evidence of an association between CSA and the severity of DSPN [33,34,35]. Moreover, in individuals with carpal tunnel syndrome, an inverse correlation of the nerve’s FA and CSA was found, with the FA being associated with a higher severity of neurological deficits [36]. However, our study is the first to show such correlation in individuals with polyneuropathy, underlining that an increased CSA is associated with a compromised nerve function in individuals with polyneuropathies such as DSPN. Subsequently it may be hypothesised that the CSA of peripheral nerves gradually increases in the course of type 2 diabetes while the structural integrity gradually decreases.
We ascertained a negative correlation between the sciatic FA and markers of microvascular damage, namely hsTNT and ACR. Furthermore, perturbations of those markers were found to gradually increase in severity across HSP, TH and MH and peak in those with SL, thus exhibiting a pattern similar to FA and SNAP. This may indicate that microangiopathic mechanisms may play a primary role in nerve damage and sensory dysfunction in type 2 diabetes, as suggested by previous studies using MRN [10, 12, 37, 38]. Even though no association between HbA1c and FA or differences among sensory phenotypes were found in the current study, the positive correlation between HbA1c and ACR highlights the implication of hyperglycaemia in the pathogenesis of microangiopathy.
Our results add to the findings of previous studies and emphasises the advantages of disease stratification through sensory phenotypes, potentially allowing a more individualised severity-adapted management of individuals with type 2 diabetes and DSPN [7, 39, 40].
Strengths of our study include the implementation of comprehensive measures of nerve structural and functional assessment (MRN, nerve conduction studies, QST) for a detailed phenotyping of participants in our cohort. Further, comparative analysis was conducted among participant subgroups exhibiting different sensory phenotypes but they did not differ regarding several variables pertinent to nerve damage in type 2 diabetes such as age, sex, the number of active smokers per group, BMI, waist circumference, duration of type 2 diabetes, HbA1c, eGFR and hsCRP. This allowed for unconfounded observations regarding our study hypothesis, despite the relatively small sample size.
Some limitations have to be acknowledged. First, due to the cross-sectional design of the study we cannot draw definite conclusions concerning a distinct cascade of nerve damage and sensory profiles through which individuals with type 2 diabetes pass until they experience a complete loss of sensory nerve fibre function. However, the increase in the severity of objective findings from TH over MH towards SL suggests a natural course of DSPN involving TH and MH as transitional phenotypes. Additionally, not all potential confounding factors in this study can be precluded due to the sample size. However, compared with other studies, participants were well phenotyped according to established factors that exert an impact on the natural course of DSPN. Furthermore, we lack data from subgroups with younger age or lower BMI, both of which would have contributed to the generalisability of our results. Nonetheless, the compared groups did not differ regarding age and BMI, while no correlations were found between those factors and FA or CSA and hence it is unlikely that this limitation constitutes a significant source of bias for the current study. Another limitation of the study is the lack of implementation of specialised scales or questionnaires to assess the presence and intensity of pain in lower extremities, other than reported pain as part of the NSS score. QST is limited by dependence on an individual’s attentiveness and compliance, as well as variable reproducibility. Nonetheless, QST was carried out by experienced and trained personnel and was strictly adherent to the protocol of DFNS, in order to minimise reproducibility issues. Further, QST represents a highly sophisticated method. Nevertheless, it is also a notably labour-intensive approach demanding personnel with specialised training. As a result, the application of QST is reserved for settings with specific clinical indications in specialised centres as well as clinical research. Another important aspect is that the sample size of the study cohort did not allow us to conduct analyses separated for sex or gender. Subsequently, our results do not provide insights into gender- or sex-specific differences regarding associations of sensory phenotypes with the structural integrity of peripheral nerves.
In summary, our study represents the first to find associations of the structural integrity of peripheral nerves with sensory profiles based on QST as suggested by Baron et al [7] indicating that individuals with type 2 diabetes may pass through a predefined cascade of nerve damage from HSP, to symptoms of predominating TH to predominating MH and ultimately experience complete loss of sensory sensation. These patterns of sensory clusters may be accompanied by a gradual decrease of structural integrity of the sciatic nerve as measured by the FA, mediated by progressive axonal loss, along with a simultaneous increase of the nerve’s CSA. However, longitudinal data are required to confirm whether nerve damage follows a certain cascade predominantly mediated by nerve fibre loss.
Abbreviations
- ACR:
-
Albumin/creatinine ratio
- CMAP:
-
Compound motor action potential
- CDT:
-
Cold detection threshold
- CPT:
-
Cold pain threshold
- CSA:
-
Cross-sectional area
- DFNS:
-
German Research Network on Neuropathic Pain
- DMA:
-
Dynamic mechanical allodynia
- DSPN:
-
Diabetic sensorimotor polyneuropathy
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- HEIST-DiC:
-
Heidelberg Study on Diabetes and Complications
- HPT:
-
Heat pain threshold
- hsCRP:
-
High-sensitivity C-reactive protein
- HSP:
-
Healthy sensory profile
- hsTNT:
-
High-sensitivity troponin T
- MDT:
-
Mechanical detection threshold
- MH:
-
Mechanical hyperalgesia
- MPS:
-
Mechanical pain sensitivity
- MRN:
-
Magnetic resonance neurography
- NCV:
-
Nerve conduction velocity
- NDS:
-
Neuropathy disability score
- NSS:
-
Neuropathy symptom score
- PHS:
-
Paradoxical heat sensations
- QST:
-
Quantitative sensory testing
- SL:
-
Sensory loss
- SNAP:
-
Sensory nerve action potential
- T2w:
-
T2-weighted
- TH:
-
Thermal hyperalgesia
- TSL:
-
Thermal sensory limen
- VDT:
-
Vibration detection threshold
- WDT:
-
Warm detection threshold
- WUR:
-
Wind-up ratio
References
Pop-Busui R, Ang L, Boulton AJM et al (2022) Diagnosis and treatment of painful diabetic peripheral neuropathy. ADA Clinical Compendia 2022(1):1–32. https://doi.org/10.2337/db-2022-01
Galer BS, Gianas A, Jensen MP (2000) Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 47(2):123–128. https://doi.org/10.1016/s0168-8227(99)00112-6
Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA (2003) The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 26(6):1790–1795. https://doi.org/10.2337/diacare.26.6.1790
Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM (2015) Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes 6(3):432–444. https://doi.org/10.4239/wjd.v6.i3.432
Yang H, Sloan G, Ye Y et al (2019) New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol (Lausanne) 10:929. https://doi.org/10.3389/fendo.2019.00929
Themistocleous AC, Crombez G, Baskozos G, Bennett DL (2018) Using stratified medicine to understand, diagnose, and treat neuropathic pain. Pain 159(Suppl 1):S31–S42. https://doi.org/10.1097/j.pain.0000000000001301
Baron R, Maier C, Attal N et al (2017) Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 158(2):261–272. https://doi.org/10.1097/j.pain.0000000000000753
Vollert J, Maier C, Attal N et al (2017) Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. Pain 158(8):1446–1455. https://doi.org/10.1097/j.pain.0000000000000935
Rolke R, Baron R, Maier C et al (2006) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 123(3):231–243. https://doi.org/10.1016/j.pain.2006.01.041
Mooshage CM, Schimpfle L, Kender Z et al (2023) Diametrical effects of glucose levels on microvascular permeability of peripheral nerves in patients with type 2 diabetes with and without diabetic neuropathy. Diabetes 72(2):290–298. https://doi.org/10.2337/db22-0548
Groener JB, Jende JME, Kurz FT et al (2020) Understanding diabetic neuropathy-from subclinical nerve lesions to severe nerve fiber deficits: a cross-sectional study in patients with type 2 diabetes and healthy control subjects. Diabetes 69(3):436–447. https://doi.org/10.2337/db19-0197
Jende JME, Groener JB, Kender Z et al (2020) Troponin T parallels structural nerve damage in type 2 diabetes: a cross-sectional study using magnetic resonance neurography. Diabetes 69(4):713–723. https://doi.org/10.2337/db19-1094
Jeon T, Fung MM, Koch KM, Tan ET, Sneag DB (2018) Peripheral nerve diffusion tensor imaging: overview, pitfalls, and future directions. J Magn Reson Imaging 47(5):1171–1189. https://doi.org/10.1002/jmri.25876
Heckel A, Weiler M, Xia A et al (2015) Peripheral nerve diffusion tensor imaging: assessment of axon and myelin sheath integrity. PLoS One 10(6):e0130833. https://doi.org/10.1371/journal.pone.0130833
Jende JME, Mooshage C, Kender Z et al (2021) Magnetic resonance neurography reveals smoking-associated decrease in sciatic nerve structural integrity in type 2 diabetes. Front Neurosci 15:811085. https://doi.org/10.3389/fnins.2021.811085
Kender Z, Jende JME, Kurz FT et al (2023) Sciatic nerve fractional anisotropy and neurofilament light chain protein are related to sensorimotor deficit of the upper and lower limbs in patients with type 2 diabetes. Front Endocrinol (Lausanne) 14:1046690. https://doi.org/10.3389/fendo.2023.1046690
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Kopf S, Groener JB, Kender Z et al (2018) Deep phenotyping neuropathy: an underestimated complication in patients with pre-diabetes and type 2 diabetes associated with albuminuria. Diabetes Res Clin Pract 146:191–201. https://doi.org/10.1016/j.diabres.2018.10.020
Tesfaye S, Boulton AJ, Dyck PJ et al (2010) Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33(10):2285–2293. https://doi.org/10.2337/dc10-1303
Jende JME, Kender Z, Morgenstern J et al (2021) Fractional anisotropy and Troponin T parallel structural nerve damage at the upper extremities in a group of patients with prediabetes and type 2 diabetes - a study using 3T magnetic resonance neurography. Front Neurosci 15:741494. https://doi.org/10.3389/fnins.2021.741494
Lehmann HC, Zhang J, Mori S, Sheikh KA (2010) Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol 223(1):238–244. https://doi.org/10.1016/j.expneurol.2009.10.012
Vaeggemose M, Haakma W, Pham M et al (2020) Diffusion tensor imaging MR neurography detects polyneuropathy in type 2 diabetes. J Diabetes Complications 34(2):107439. https://doi.org/10.1016/j.jdiacomp.2019.107439
Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M (2003) The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 60(1):108–111. https://doi.org/10.1212/wnl.60.1.108
Dyck PJ, Dyck PJ, Larson TS, O’Brien PC, Velosa JA (2000) Patterns of quantitative sensation testing of hypoesthesia and hyperalgesia are predictive of diabetic polyneuropathy: a study of three cohorts. Nerve growth factor study group. Diabetes Care 23(4):510–517. https://doi.org/10.2337/diacare.23.4.510
Hovaguimian A, Gibbons CH (2011) Diagnosis and treatment of pain in small-fiber neuropathy. Curr Pain Headache Rep 15(3):193–200. https://doi.org/10.1007/s11916-011-0181-7
Fabry V, Gerdelat A, Acket B et al (2020) Which method for diagnosing small fiber neuropathy? Front Neurol 11:342. https://doi.org/10.3389/fneur.2020.00342
Perkins B, Bril V (2014) Electrophysiologic testing in diabetic neuropathy. Handb Clin Neurol 126:235–248. https://doi.org/10.1016/B978-0-444-53480-4.00018-7
Kollmer J, Hegenbart U, Kimmich C et al (2020) Magnetization transfer ratio quantifies polyneuropathy in hereditary transthyretin amyloidosis. Ann Clin Transl Neurol 7(5):799–807. https://doi.org/10.1002/acn3.51049
Rother C, Bumb JM, Weiler M et al (2022) Characterization and quantification of alcohol-related polyneuropathy by magnetic resonance neurography. Eur J Neurol 29(2):573–582. https://doi.org/10.1111/ene.15127
Pitarokoili K, Kronlage M, Baumer P et al (2018) High-resolution nerve ultrasound and magnetic resonance neurography as complementary neuroimaging tools for chronic inflammatory demyelinating polyneuropathy. Ther Adv Neurol Disord 11:1756286418759974. https://doi.org/10.1177/1756286418759974
Kronlage M, Baumer P, Pitarokoili K et al (2017) Large coverage MR neurography in CIDP: diagnostic accuracy and electrophysiological correlation. J Neurol 264(7):1434–1443. https://doi.org/10.1007/s00415-017-8543-7
Pulikkottil BJ, Schub M, Kadow TR, Wang W, Fowler JR (2016) Correlating median nerve cross-sectional area with nerve conduction studies. J Hand Surg Am 41(10):958–962. https://doi.org/10.1016/j.jhsa.2016.08.018
Senarai T, Pratipanawatr T, Yurasakpong L et al (2022) Cross-sectional area of the tibial nerve in diabetic peripheral neuropathy patients: a systematic review and meta-analysis of ultrasonography studies. Medicina (Kaunas) 58(12):1696. https://doi.org/10.3390/medicina58121696
Evans MC, Wade C, Hohenschurz-Schmidt D et al (2021) Magnetic resonance imaging as a biomarker in diabetic and HIV-associated peripheral neuropathy: a systematic review-based narrative. Front Neurosci 15:727311. https://doi.org/10.3389/fnins.2021.727311
Kelle B, Evran M, Balli T, Yavuz F (2016) Diabetic peripheral neuropathy: correlation between nerve cross-sectional area on ultrasound and clinical features. J Back Musculoskelet Rehabil 29(4):717–722. https://doi.org/10.3233/BMR-160676
Vo NQ, Hoang NT, Nguyen DD et al (2022) Quantitative parameters of diffusion tensor imaging in the evaluation of carpal tunnel syndrome. Quant Imaging Med Surg 12(6):3379–3390. https://doi.org/10.21037/qims-21-910
Jende JME, Mooshage C, Kender Z et al (2022) Sciatic nerve microvascular permeability in type 2 diabetes decreased in patients with neuropathy. Ann Clin Transl Neurol 9(6):830–840. https://doi.org/10.1002/acn3.51563
Jende JME, Mooshage C, Kender Z et al (2022) Troponin T is negatively associated with 3 Tesla magnetic resonance peripheral nerve perfusion in type 2 diabetes. Front Endocrinol (Lausanne) 13:839774. https://doi.org/10.3389/fendo.2022.839774
Rosenberger DC, Blechschmidt V, Timmerman H, Wolff A, Treede RD (2020) Challenges of neuropathic pain: focus on diabetic neuropathy. J Neural Transm (Vienna) 127(4):589–624. https://doi.org/10.1007/s00702-020-02145-7
Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D (2008) Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain 138(2):343–353. https://doi.org/10.1016/j.pain.2008.01.006
Tsilingiris D, Schimpfle L, Mooshage C et al (2023) Changes in sural nerve structural integrity are associated with distinct peripheral sensory phenotypes in individuals with type 2 diabetes. Diabetologie und Stoffwechsel 18(S1):35–46. https://doi.org/10.1055/s-0043-1767921
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
The authors thank D. Willich (Department of Neuroradiology, Heidelberg University Hospital) for her ongoing support and excellent technical performance of all MRN examinations. The results of our study were partly presented in the Diabetes Kongress of the German Diabetes Association (17–20 May 2023, Berlin, Germany) and included as an abstract in the corresponding issue of Diabetologie und Stoffwechsel [41].
Data availability
Data will be provided by the corresponding author upon reasonable request.
Funding
The German Research Council (DFG, SFB 1158) provided financial support for personnel expenditure, MR imaging costs and costs for the technical equipment required for electrophysiological and serological analysis. The DFG had no influence on the study design, collection and analysis of data or on the writing of the article. JS received grants from the DFG (SFB 1116; SFB 1118), DZD and the Ministry of Science, Research and the Arts of the State of Baden-Württemberg, and travel grants and personal fees from Boehringer Ingelheim Pharma. SHer received grants from the DFG (TRR 205/2; TRR 333/1; SFB 1231/1; SFB 1118), Ministry of Science, Research and the Arts (DZHK Standortprojekt; DZHK VHB Säule B project; VIP+ project MIRANDA), the Helmholtz Association (Helmholtz International Research School for Diabetes), Edith-Haberland-Wagner (EHW) Foundation and Else Kröner-Fresenius-Stiftung (EKFS). PN received grants from the DZD (SFB 1118 and 1158) and from Novo Nordisk. SHei received a grant from the Dietmar Hopp Foundation and the DFG (SFB 1118). MB received grants and personal fees from Codman, Guerbet, Bayer and Novartis, personal fees from Roche, Teva, Springer, Boehringer, Grifols and Braun, and grants from the European Union, Siemens, the Dietmar Hopp Foundation, Stryker and the DFG (SFB 1118 and 1158). FK was supported by the German Research Foundation (KU 3555/1-1) and the Hoffmann-Klose Foundation of Heidelberg University Hospital, and received a research grant from Heidelberg University Hospital. JJ further acknowledges support from the International Foundation for Research in paraplegia (IRP) and the EKFS. ZK received grants from the German Center of Diabetes Research (DZD) and from German Diabetes Association (DDG).
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
CM, DT, LSc, LSe, OE, TA, AH, SHer, JS, PN, SHei, MB, FK, SK, JJ and ZK contributed to the study design, data collection, analysis and interpretation, and drafting and critical revision of the article. All authors approved the final version to be published. CM, DT and ZK are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christoph M. Mooshage and Dimitrios Tsilingiris contributed equally and therefore share first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mooshage, C.M., Tsilingiris, D., Schimpfle, L. et al. A diminished sciatic nerve structural integrity is associated with distinct peripheral sensory phenotypes in individuals with type 2 diabetes. Diabetologia 67, 275–289 (2024). https://doi.org/10.1007/s00125-023-06050-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-06050-y