Abstract
Aims/hypothesis
Type 2 diabetes is associated with increased risk of cognitive decline although the pathogenic basis for this remains obscure. Deciphering diabetes-linked molecular mechanisms in cells of the cerebral cortex could uncover novel therapeutic targets.
Methods
Single-cell transcriptomic sequencing (scRNA-seq) was conducted on the cerebral cortex in a mouse model of type 2 diabetes (db/db mice) and in non-diabetic control mice in order to identify gene expression changes in distinct cell subpopulations and alterations in cell type composition. Immunohistochemistry and metabolic assessment were used to validate the findings from scRNA-seq and to investigate whether these cell-specific dysfunctions impact the neurovascular unit (NVU). Furthermore, the behavioural and cognitive alterations related to these dysfunctions in db/db mice were assessed via Morris water maze and novel object discrimination tests. Finally, results were validated in post-mortem sections and protein isolates from individuals with type 2 diabetes.
Results
Compared with non-diabetic control mice, the db/db mice demonstrated disrupted brain function as revealed by losses in episodic and spatial memory and this occurred concomitantly with dysfunctional NVU, neuronal circuitry and cerebral atrophy. scRNA-seq of db/db mouse cerebral cortex revealed cell population changes in neurons, glia and microglia linked to functional regulatory disruption including neuronal maturation and altered metabolism. These changes were validated through immunohistochemistry and protein expression analysis not just in the db/db mouse cerebral cortex but also in post-mortem sections and protein isolates from individuals with type 2 diabetes (74.3 ± 5.5 years) compared with non-diabetic control individuals (87.0 ± 8.5 years). Furthermore, metabolic and synaptic gene disruptions were evident in cortical NVU cell populations and associated with a decrease in vascular density.
Conclusions/interpretation
Taken together, our data reveal disruption in the cellular and molecular architecture of the cerebral cortex induced by diabetes, which can explain, at least in part, the basis for progressive cognitive decline in individuals with type 2 diabetes.
Data availability
The single-cell sequencing data that supports this study are available at GEO accession GSE217665 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE217665).
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Type 2 diabetes constitutes a huge burden on healthcare systems worldwide due to multi-morbidities such as blindness, renal failure and cardiovascular-related death [1]. There is mounting evidence that type 2 diabetes is also associated with cognitive decline [2,3,4]. Individuals with type 2 diabetes have an approximate twofold increased risk of developing Alzheimer’s disease compared with non-diabetic, age-matched individuals [3].
Clinical evidence and pre-clinical data suggest that common pathways may be involved in type 2 diabetes and cognitive decline, including glial dysfunction, neurodegeneration and inflammation [5, 6], arising from dysfunctional insulin signalling, chronic hyperglycaemia and oxidative stress. These pathways may contribute to neurovascular unit (NVU) dysfunction in the brain and retina during type 2 diabetes [4]. However, the underlying pathways that drive neurodegeneration and cognitive decline in type 2 diabetes are not fully understood.
The NVU plays an important homeostatic role in the central nervous system and is formed by interdependency of vascular cells, glia, neurons and professional immune cells. Besides ensuring integrity of the blood–brain barrier, the NVU is important for immune privilege and regulating blood flow to meet the oxygenation and nutrient demands of the highly metabolically demanding neuropile. A recent study revealed human-specific transcriptomic signatures in the cerebrovasculature in Huntington’s disease [7] and another study revealed several Alzheimer’s disease risk genes mapping to endothelial and microglial cell populations [8]. Furthermore, neurodegenerative disease-linked shifts in brain cell populations have been reported [9]. Neuronal and glial population changes have been observed in Alzheimer’s disease [10] and may co-occur with reduced cortical thickness in individuals [11].
Analysis from a single-cell RNA sequencing (scRNA-seq) dataset revealed profound changes to the transcriptome of vascular, glial and neuronal cells from the retina of db/db mice, showing a useful linkage to the pathogenesis of diabetic retinopathy [12]. The scRNA-seq approach has also been used to characterise hippocampal changes in db/db mice, indicating the role of microglial populations in diabetes-induced cognitive decline [13]. The impact of type 2 diabetes on the brain NVU, particularly in the cortex, have not been fully explored. Moreover, whether the cognitive decline in type 2 diabetes is associated with disruption in brain cell populations remains unknown.
The aim of the present study was to provide novel insights into type 2 diabetes-associated cognitive decline and the underlying associated pathology in the cerebral cortex by combining state-of-the-art physiological and molecular measurements in db/db mice as a model and validation in human samples.
Methods
Animals
db/db (C57BLKS/heterozygous db/+ mice [BKS.Cg-+Leprdb/+Leprdb/OlaHds]) (RRID: IMSR_ENV:HSD-173) were purchased from Harlan Laboratories (Netherlands) or Janvier (France). Since heterozygous (db/+) mice do not show a specific phenotype [14], wild-type (WT) and db/+ mice are commonly considered a single control group [15, 16]. For For immunohistochemistry (IHC) and behavioural studies, WT mice (BKS.Cg-/OlaHds) belonging to the same colony were used as the control group. For transcriptional studies and cortical metabolic assessment, db/+ mice were used as the control group. Mice were housed in a standard pathogen-free experimental facility, exposed to a 12 h light–dark cycle and had free access to food and water. Five or six male/female mice per group aged 14–16 weeks (representing an established but not yet advanced stage of diabetes) were used. Metabolic determinations (Table 1) were carried out as described in electronic supplementary material (ESM) Methods.
Actimetry and new object discrimination task
Episodic memory for ‘what,’ ‘where’ and ‘when’ paradigms was analysed, following procedures shown in ESM Methods. ‘What’ was the difference in time exploring familiar and recent objects, ‘where’ was the difference in time exploring displaced and non-displaced objects and ‘when’ was the difference between time exploring familiar non-displaced and recent non-displaced objects. All measurements were performed in triplicate and data were expressed as percentage of control values. Discrimination index was calculated as the percentage of time exploring recent objects/total exploration time.
Morris water maze
Spatial learning and memory abilities were analysed in the Morris water maze (MWM) (see ESM Methods). Time required to locate the platform in the acquisition phase, number of entrances in quadrant 2 and swimming velocity during the retention phase were analysed using Smart software version 3.0.04 (Panlab S.L.U., Barcelona, Spain).
Tissue dissociation
For scRNA-seq, one male homozygous (db/db) mouse and one male heterozygous (db/+) mouse were killed at 16 weeks of age. Whole cortex was dissected and dissociated using the papain dissociation system (Worthington, Lakewood, NJ, USA). Cortex samples were digested in papain (20 U/ml) for 45 min at 37°C. After treating with ovomucoid, cells were re-suspended in PBS with 0.04% wt/vol. BSA. Cell counts and viability was checked using Trypan Blue (Sigma-Aldrich, Missouri, USA).
scRNA-seq library preparation, sequencing and associated analysis
scRNA-seq libraries were generated using the 10x Genomics Chromium Single Cell 3′ Reagent Kit v3 and sequenced on Illumina NovaSeq6000 S2 200 flow cell at the Genomics Core Technology Unit (GCTU), Queen’s University Belfast. Analysis was completed as discussed in ESM Methods using Seurat R package v.4.2.0 (https://satijalab.org/seurat/; developed by P. Hoffman, S. Lab and collaborators, New York City, NY, USA).
Identification of cell types, marker genes and differential expression analysis
Following procedures detailed in ESM Methods, cortical cell types were identified by expression of established markers (Table 2). Differential gene expression, single-cell regulatory network, weighted gene correlation network (WGCN) [17] and pseudotime trajectory [18] analyses were performed.
Tissue preparation and staining
Immediately after mice were killed via overdose of pentobarbital, db/db and control mouse brains were harvested and weighed. Left hemispheres were fixed in 4% wt/vol. paraformaldehyde for 1 week and 30 µm coronal brain sections were obtained in a Microm HM450 microtome (ThermoFisher, Spain). Sections were stored in 50% wt/vol. glycerol at −20°C until used. For staining procedures, consecutive sections were selected at 1.5, 0.5, −0.5, −1.5, −2.5 and −3.5 mm from Bregma. Staining was carried out for neuronal nuclear protein (NeuN) [19], BrdU and doublecortin (DCX) [20] as previously described.
Cresyl violet staining
Sections were processed as described [21] (see ESM Methods). Images were acquired using an optical Olympus Bx60 microscope with an attached Olympus DP71 camera (Olympus, Tokyo, Japan) and mmi CellTools software version 4.3 (Molecular Machines & Industries, Eching, Germany). Hemisection size and cortical thickness were measured using Adobe Photoshop Elements 2.0 version 6.2 (Adobe, USA) and Image J version 1.49 (National Institutes of Health, MD, USA) software.
Iba1 and GFAP staining
Sections were pre-treated with Tween-20 (BP337; Fisher Scientific, UK) (0.05%) (wt/vol.) in citrate buffer (10 mmol/l) for 2 h at 65°C and blocked in 3% (wt/vol.) BSA (A7906; Sigma-Aldrich, Madrid, Spain) and 0.5% (wt/vol.) Triton X100 (BP151; Fisher Scientific) for 1 h. Sections were incubated with anti-Iba1 (ionised calcium binding adaptor molecule 1) (ESM Table 1) and anti-GFAP (glial fibrillary acidic protein) (ESM Table 1) antibodies overnight at 4°C in 3% (wt/vol.) BSA and 0.5% (wt/vol.) Triton X100 followed by incubation with secondary antibodies (diluted in PBS) (ESM Table 1) for 1 h at room temperature.
Vascular staining
Antigen retrieval was performed using citrate buffer at 95°C for 30 min in a water bath. Slides were permeabilised in 2% (wt/vol.) Triton-X for 30 min. Slides were blocked in 5% donkey serum in 0.2% (wt/vol.) Triton-X for 1 h at room temperature. Primary antibodies (ESM Table 1) were added and slides were incubated overnight at 4°C in 0.5% donkey serum and 0.2% Triton-X followed by incubation with secondary antibodies (ESM Table 1) for 2 h at room temperature, in PBS.
Image analysis
Images were taken via Olympus DP71 camera or Leica SP8 confocal. Counting of cell populations was performed by selecting 15–20 regions of interest (ROIs) (7960.215 µm2/ROI) from each cortical section (333–417 ROIs/group) and counting using Image J Software version 1.49 (National Institutes of Health, MD, USA).
Metabolic assessment of cortical brain punches
The method published by Qi et al (2021) [22] was adapted in order to assess glycolysis in acute coronal slices from the cortex of five male db/db and five male db/+ mice, as described in ESM Methods. The ‘glycolysis stress test’ protocol from Agilent (USA) was optimised for use with cortex slices (ESM Table 2). Protocol length and injection timings were as follows: baseline, 4 cycles; glucose injection, 5 cycles; oligomycin A injection, 5 cycles; and 2-deoxy-d-glucose (2-DG), 10 cycles. Each cycle consisted of 3 min mix, 1 min wait, 2 min measure. Analysis of basal glycolysis, glycolytic capacity and glycolytic reserve was performed in Wave Software version 2.6.3.5 (Agilent).
Human tissue
For IHC staining, post-mortem brain samples of the frontal cortex of eight individuals were provided by the Netherlands Brain Bank, Amsterdam. The brain samples were from donors with or without diabetes mellitus. Classification of the donors was based on clinical diagnosis and confirmed by autopsy. The staining procedures were performed as described in ESM Methods. Antibody information is included in ESM Table 3.
For western blotting, post-mortem brain samples were obtained as frozen pieces of cortical tissue from the neurological tissue biobank of Hospital Clínic – IDIBAPS in Barcelona, Spain. Control donors did not have signs of neurological alterations. Samples from individuals with type 2 diabetes clinically diagnosed with dementia were excluded from the study. The most relevant associated comorbidities are presented in ESM Table 4. Proteins were extracted and western blots were run as described in ESM Methods. Antibody information is included in ESM Table 3.
Analysis and statistics
Randomisation and blinding at the experimental phase was not possible due to the obese nature of db/db mice. Quantification and analysis of mouse data was done by two independent, blinded researchers. Student’s t test for independent samples, or Mann–Whitney test was used to compare control and db/db mouse groups. Two-way ANOVA (group × session) was used to analyse the acquisition phase in the MWM. IBM SPSS Statistics software version 29.0.0.0 (IBM Corporation, NY, USA) was used for all statistical analysis and results are expressed as mean ± SEM.
Study approval
The Animal Care and Use Committee of the University of Cadiz, in accordance with the Guidelines for Care and Use of experimental animals (European Commission Directive 2010/63 /UE and Spanish Royal Decree 53/2013), approved all experimental procedures. The Animal Welfare Ethical Review Body at Queen’s University Belfast approved this study, and all procedures were conducted under the regulation of the UK Home Office Animals Scientific Research Act 1986. The collection of the human brain material for immunostaining was done in accordance with Dutch laws and the rules outlined by the local ethics committee. For western blotting, samples were obtained by the biobank after corresponding ethical approvals and following the regulations of local ethics committees in Universitat de Barcelona, and in accordance with the Helsinki Declaration and the Convention of the Council of Europe on Human Rights and Biomedicine.
Results
db / db mice exhibit altered memory function and reduced cortical thickness
The db/db mouse displays key characteristics of type 2 diabetes including obesity, hyperglycaemia and hyperinsulinaemia (Table 1) [21, 23]. A schematic of the study is shown in Fig. 1a.
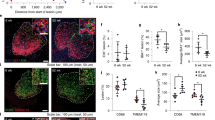
Cognitive impairment and brain atrophy in db/db mice. (a) Schematic detailing study design and time points. (b) The new object discrimination test showed that episodic memory was not affected in db/db mice when ‘what’ (p=0.441) or ‘where’ (p=0.484) paradigms were analysed. However, the ‘when’ paradigm was significantly impaired in db/db mice (*p=0.016). Data are representative of five mice. (c) Discrimination index was not affected in db/db mice (p=0.530). Data are representative of five or six mice. (d) Learning impairment was observed in db/db mice in the acquisition phase of the MWM. Times required to locate the platform were significantly longer in db/db mice than control mice in sessions 5–8 (session 1 p=0.729, session 2 p=0.637, session 3 p=0.294, session 4 p=0.168, session 5 ***p<0.001 vs control, session 6 ***p<0.001 vs control, session 7 **p=0.003 vs control, session 8 *p=0.025 vs control). Data are representative of five or six mice. White squares, mean value for db/db mice; grey squares, mean value for control mice. (e) The number of entries into quadrant 2 (where the platform was located along the acquisition phase) was significantly lower in db/db mice (*p<0.05). (f) Brain weight was reduced in db/db mice when compared with control mice (**p<0.001 vs control). (g) Hemisection size was significantly reduced in db/db mice (*p=0.039 vs control). (h) Cortical thickness was significantly reduced in db/db mice (*p=0.013 vs control). (i) Representative images showing reduced cortical thickness in db/db mice. Scale bar, 250 µm. Schematic in (a) was created with BioRender.com
Episodic memory was compromised in db/db mice (Fig. 1b). While no differences were detected for ‘what’ or ‘where’ paradigms, a significant impairment was observed for ‘when’ paradigm. A similar discrimination index was observed when comparing the groups of mice (Fig. 1c). Assessment of spatial memory using the MWM revealed no significant group × session effect (F(1,7)=1.009, p=0.427), although individual session assessment revealed that db/db mice had great difficulty in learning the location of the hidden platform. This was reflected from session 5 onwards (Fig. 1d). The number of entries into the quadrant where the platform used to be located was significantly lower in db/db mice (Fig. 1e). Swimming speed was reduced in db/db mice (ESM Table 5) and while this may impact the results of the MWM, we have also shown that walking speed (and therefore motor activity) is not compromised in these mice. At the time of death, brain weight was significantly lower in db/db mice (Fig. 1f) [24]. Cresyl violet staining revealed that hemisection sizes were also significantly smaller in db/db mice (Fig. 1g). db/db mice presented significantly thinner cortices when compared with control mice (Fig. 1h,i).
Single-cell transcriptome of db/db mouse cortex reveals massive changes in identity and composition of various key cell types
Single-cell transcriptome analysis of cortex from db/db mice revealed significant changes in neuronal and glial populations (Fig. 2a,b). There was a marked increase in the microglia population of the db/db mouse cerebral cortex accompanied by a loss of astrocytes and mature neurons (Fig. 2c). An increased fraction of immature neurons was observed, alongside an increase in oligodendrocyte progenitors and concomitant decrease in mature oligodendrocytes (Fig. 2c). Pseudotime trajectory on glia populations (astrocytes and oligodendrocytes together) (ESM Fig. 1) showed cellular heterogeneity and an altered lineage trajectory in db/db vs control mice. These results suggest altered neuronal development, glial development and maturation trajectory in the db/db mouse cortex.
Single-cell sequencing of diabetic mouse brain reveals changes in glial and neuronal populations. (a) Schematic of scRNA-seq data derivation. db/db and db/+ (control) mouse cortex was dissociated in papain when mice were 16 weeks old. The resulting single-cell solution was sequenced via 10× genomics and analysis was performed in R. (b) Clustering of the cortex showed similar cell populations in diabetic and control mouse brain. (c) Proportion of each cell type in db/db and db/+ mouse brain cortex, indicating the difference in astrocytes, microglia and neuronal populations. (d–g) Significant differential expression of genes (all p<0.001) in astrocytes (d), microglia (e), mature neurons (f) and immature neurons (g). Expression levels for individual genes are presented as the normalised counts. Schematic in (a) was created with BioRender.com. ABC, arachnoid barrier cells; ASC, astrocytes; CPC, choroid plexus cells; Ctrl, control; DC, dendritic cells; EC, endothelial cells; EPC, ependymocytes; ImmN, immature neurons; MAC, macrophages; MG, microglia; mNEUR, mature neurons; OEG, olfactory ensheathing glia; OLG, oligodendrocytes; OPC, oligodendrocyte progenitor cells; PC, pericytes; VLMC, vascular leptomeningeal cells
Molecular analysis of cortical cell populations reveals critical changes in diabetic mouse brains
Focused molecular analysis on neuronal and glial specific transcripts (Fig. 2d–g) demonstrated that genes encoding ribosomal protein S21 and S29 (Rps21 and Rps29, respectively) were downregulated in immature neurons as well as astrocytes, with upregulation of ribosomal protein SA (encoded by Rpsa) in microglia. Transcripts linked to inflammation and microglial activation were altered in db/db mouse microglia: expression of genes encoding apolipoprotein E (Apoe) [25] and prostaglandin D2 synthase (Ptgds) [26] was upregulated. Furthermore, downregulated genes in mature neurons were major mediators and regulators of synaptic neuronal functions such as the genes encoding glutamate ionotropic receptor AMPA type subunit 1 (Gria1) [27] and myelin basic protein (Mbp) [28]. Mature neurons showed downregulation of genes encoding maternally expressed gene 3 (Meg3) and β-actin (Actb). Astrocytes displayed downregulation of actin polymerisation regulating genes tetraspanin 7 (Tspan7) [29] and metallothionein III (Mt3) [30]. Immature neurons showed downregulation of the gene encoding early growth response-1 (Egr1), an important transcription factor in memory formation [31]. This occurred alongside upregulation of HbA-a2 (encoding a globin family protein) and Gad2, which are known to be linked to schizophrenia and bipolar disorder [32]. Analysis by the algorithm pySCENIC identified enhanced expression of the transcription factor genes (Hes5, Lhx2, Sox21, Sox2) and neurogenesis regulatory factors in db/db mouse astrocytes (Fig. 3a–e), suggesting a potentially ambiguous lineage trajectory between db/db and control mice. Top microglial regulons were slightly discrete between control and db/db mice (Fig. 3f–j). The mature neuronal population (db/db) showed top regulons that are known transcription factors (Elk1 and Egr4) regulating early immediate genes (ESM Fig. 2a–e), while immature neuronal cells (db/db) had increased expression of marker genes for inhibitory neuronal characteristics (Sp9 and Meis1) (ESM Fig. 2f–j). These outcomes suggest a shift in the gene regulatory networks and driver transcription factors underlying distinct cell fates in db/db mouse cortex. The corresponding scale independence and mean connectivity are shown in ESM Fig. 3.
Regulon analysis. (a–d) Top regulons (a, b) and top unique motifs (c, d) in db/+ (a, c) and db/db (b, d) mouse astrocytes. (e) Differential expression patterns of top regulon genes in db/+ and db/db mouse astrocyte cell populations. (f–j) Top regulons (f, g) and unique motifs (h, i) in db/+ (f, h) and db/db (g, i) mouse microglia. (j) Differential expression patterns of top regulon genes in db/+ and db/db mouse microglia cell populations. Expression levels for individual genes are presented as the normalised counts. Neuronal populations are shown in ESM Fig. 2
Ex vivo investigation validates neuronal and glial population alterations in db/db mice
Immunohistochemistry (Fig. 4a) was used to quantify the numbers of immature and mature neurons (Fig. 4b–g). NeuN+ cell/DAPI ratio was significantly reduced in db/db mice (Fig. 4b). A slight increase in the density of DCX+ and proliferating cells was observed in db/db mice (Fig. 4c,d). Since the cortex is not a classical neurogenic niche, proliferation and neurogenesis were also assessed in the subventricular zone (SVZ). Both DCX burden and BrdU+ cell density in the SVZ were significantly increased in db/db mice when compared with control mice (Fig. 4e–g).
Neuron compromise and microglia burden are increased in db/db mice. (a) Schematic describing the staining process. Indirect staining with primary antibody and secondary fluorescently conjugated antibody was used to identify cell subtypes (immature neurons, mature neurons, astrocytes and microglia). (b) NeuN/DAPI ratio was significantly lower in the cortex from db/db mice, when compared with control mice (***p<0.001 vs control). Representative images of cortical sections from control and db/db mice, with NeuN (red) and DAPI (blue) staining, are shown. Scale bar, 50 µm. (c, d) No differences were observed when DCX (p=0.407) (c) or BrdU (p=0.267) (d) densities were compared in the cortex from db/db and control mice. (e, f) DCX burden (e) was significantly increased in the SVZ from db/db when compared with control mice (*p=0.012 vs control). Similarly, BrdU density (f) was significantly higher in the SVZ from db/db mice (**p=0.004 vs control). (g) Representative images of the SVZ from control and db/db mice (green, BrdU+; red, DCX+). Scale bar, 50 μm. (h) Microglia burden was increased in db/db mice when compared with control mice (*p=0.046). Representative images of microglia staining in the cortex from control and db/db mice (green, Iba-1) are shown. Scale bar, 25 μm. (i) Astrocyte burden was not affected in db/db mice when compared with control mice (p=0.142). Representative images of astrocyte staining in the cortex from control and db/db mice (red, GFAP) are shown. Scale bar, 25 μm. Schematic in (a) was created with BioRender.com. ASC, astrocytes; ImmN, immature neurons; MG, microglia; mNEUR, mature neurons
Microglia burden was increased in the cortex from db/db mice when compared with control mice (Fig. 4h), while no differences in astrocyte burden were observed (Fig. 4i).
Correlated gene modules reveal the altered cellular behaviour of metabolism, synapse and inflammatory pathways in db/db mouse cortex
WGCN analysis was performed (Fig. 5a–d). Gene ontology (GO) analysis of correlated unique gene modules in cells revealed altered mRNA metabolism in immature neurons and changes in glycogen and lipid metabolism in astrocytes of diabetic mouse brains (Fig. 5e). Furthermore, pathways implicated in inflammation and phagocytosis in microglia were uniquely altered in diabetic cortex, whereas mature neurons had altered pathways relating to synaptic processes (Fig. 5e).
WGCN analysis and GO terms for correlated modules. (a, b) Cluster dendrogram for gene modules showing modules of high interconnection in control (a) and db/db (b) mice. Height in cluster dendrograms indicates the inter-cluster correlation distance and modules are marked by different colours in the horizontal bar (grey represents unassigned genes). (c, d) The relationship between gene sets and cell types is shown for control (c) and db/db (d). Each column is a cell type and each row represents module eigengenes. Each unit is coloured in the correlation coefficient (R) on a scale of −1 to +1 (black, negative; red, positive; white, null). (e) Unique GO terms for correlated gene module in db/db mouse cell types. Cell images were created with BioRender.com. ABC, arachnoid barrier cells; ASC, astrocytes; CPC, choroid plexus cells; DC, dendritic cells; EC, endothelial cells; EPC, ependymocytes; ImmN, immature neurons; MAC, macrophages; MG, microglia; mNeur, mature neurons; OLG, oligodendrocytes; OPC, oligodendrocyte progenitor cells; PC, pericytes; VLMC, vascular leptomeningeal cells; VSMC, vascular smooth muscle cells
Vascular abnormalities observed in db/db mouse cortex
Previous reports have suggested that brain vasculature is affected in type 2 diabetes models [24]. We observed reduced laminin+ vessels in db/db mice (Fig. 6a, d). ‘Hotspots’ of leakage were observed in the db/db mouse cortex but not in the control mice cortex (Fig. 6e). Expression of endothelial marker genes including Lgals1 (encoding galectin 1) and Lamc3 (encoding laminin γ subunit 3) was reduced in db/db mice (ESM Fig. 4). The top differentially expressed genes (DEGs) in db/db mouse endothelial cells included Malat (encoding metastasis-associated lung adenocarcinoma transcript 1), which may be involved in angiogenesis [33], and Cts3, which is dysregulated in cerebral amyloid angiopathy (Fig. 6h) [34]. There was an increase in acellular capillaries in the db/db mouse cortex (Fig. 6b,f) alongside a loss of pericyte coverage (Fig. 6c,g). The top DEGs in pericytes included Cald1 (encoding downregulation of caldesmon 1), which is involved in smooth muscle contraction [35]. Dysregulation of metabolic genes was observed in db/db mouse pericytes, with a loss of mitochondrial complex-related genes such as mt-Co1 and mt-Nd4 (encoding mitochondrial NADH dehydrogenase 4) (Fig. 6i).
Vascular alterations in db/db mouse cortex. (a) Laminin+ vessel area was significantly reduced at various levels of Bregma in the cortex of db/db mice (*p<0.05, **p<0.01). (b) A significant increase in acellular capillaries was observed in db/db mouse cortex (*p<0.05) at Bregma level +0.5 mm. (c) A significant loss of pericyte coverage (PDGFR-β) was observed in db/db mouse cortex at Bregma +0.5 mm (**p<0.01). (d–g) Representative images: laminin staining (purple) (d); ‘hot-spot’ (arrow) area of vessel leakage in db/db mouse cortex (blue, laminin; red, IgG; green, albumin) (e); lectin (green) and Col-IV (red) in control and db/db mouse cortex for assessment of acellular capillaries (arrows) (f); and lectin (green) and PDGFR-β (red) in the control and db/db mouse cortex for measurement of pericyte coverage (g). Scale bar, 50 µm. (h) DEGs in endothelial cells. (i) DEGs in pericytes. Expression levels for individual genes (all p<0.001) are presented as the normalised counts
Cortical metabolism is significantly altered in db/db mice, with dysregulated metabolic pathway genes observed in specific cortical populations
Our results indicated that metabolic dysfunction in the type 2 diabetes brain may be a consequence of NVU impairment. To validate these findings in vivo, metabolic profiling of the cortex was performed using acute brain slices (Fig. 7a). Glycolytic function was increased in db/db mouse cortex compared with controls (Fig. 7b). Non-glycolytic acidification (Fig. 7c), basal glycolysis (Fig. 7d) and glycolytic capacity (Fig. 7e) were all significantly increased in the db/db mouse cortex; however, no significant difference was observed in glycolytic reserve (Fig. 7f).
Metabolic profiling of db/db mouse cortex. (a) Schematic describing the process of obtaining acute coronal sections for metabolic profiling by Seahorse X FE 96. Male db/db or control mice aged 16 weeks (n=5 mice per group) were used to generate acute coronal slices (250 µm) using the Leica VT100s Vibratome. The Seahorse analyser was used to assess glycolytic function, via an adapted glycolytic stress test protocol. (b) Traces from n=5 db/db and n=5 control mice (representative of seven or eight punches per mouse) following the adapted glycolysis stress test protocol. (c) Non-glycolytic acidification was significantly increased in db/db mice. (d) Basal glycolysis was significantly increased in db/db mice. (e) Glycolytic capacity was significantly increased in db/db mice. (f) No significant changes were observed between db/db and control mice when comparing glycolytic reserve. n=5 mice per group; seven or eight cortical punches per mouse. *p<0.05, **p<0.01. Unpaired Student’s t test. Data are presented as mean values ± SEM. (g) Metabolism-related DEGs (all p<0.001) in db/+ (control) and db/db mouse cortex. Heatmap depicts normalised expression counts scaled between +1 and −1. Schematic in (a) was created with BioRender.com. ECAR, extracellular acidification rate
A number of metabolism-related genes were differentially expressed in the db/db mouse cortex (Fig. 7g). For example, astrocytes and mature neurons showed increased expression of electron transport chain subunit genes and NADH dehydrogenase subunits. Together, these data indicate that the metabolic processes of cortical NVU cell populations are altered in db/db mice.
Cortical cell populations are also changed in humans with type 2 diabetes
To validate our db/db mouse model findings, we studied samples from people with type 2 diabetes. Neuronal and glial cell populations were assessed in a small population of cortex samples from non-diabetic individuals and from individuals with type 2 diabetes. Staining of NeuN in frontal cortex sections indicated a qualitative reduction in neuronal cells in the frontal cortex of individuals with type 2 diabetes (Fig. 8a), although semi-quantitative analysis of NeuN western blots from parietal, temporal and frontal cortex samples showed no significant difference (Fig. 8c). A similar observation was seen after analysis of postsynaptic density protein 95 (PSD95) (ESM Fig. 5a). Noticeably, a reduction in GFAP protein was observed in samples from type 2 diabetes temporal cortex only (Fig. 8b,d). GFAP staining of human cortex showed an increased localisation with blood vessels (stained with wheat germ agglutinin) (Fig. 8b). Iba-1 was not significantly changed in any cortical region in individuals with type 2 diabetes when compared with healthy control individuals. A reduction in zonula occludens-1 (ZO-1) protein levels was observed in individuals with type 2 diabetes in the parietal and temporal cortex (ESM Fig. 5b). Occludin was unexpectedly increased in the parietal cortex of individuals with type 2 diabetes (ESM Fig. 5b). A statistically significant loss of platelet-derived growth factor receptor (PDGFR) α/β was observed in the parietal cortex of individuals with type 2 diabetes (ESM Fig. 5c).
Cortex samples from individuals with type 2 diabetes reveal alterations in neuronal and glial cell populations. (a) Representative images show reduced NeuN staining (yellow) in frontal cortex from a donor with type 2 diabetes compared with cortex from a control donor. Laminin staining is shown in purple, alongside DAPI in blue, n=4 or 5 individuals per group. (b) Representative images showing GFAP staining of the frontal cortex (purple) alongside wheat germ agglutinin (yellow), n=4 or 5 patients per group. (c) Western blotting of human cortex samples (parietal, temporal and frontal) shows the expression of NeuN in each location, n=3 individuals per group. (d) Western blotting of human cortex samples (parietal, temporal and frontal) shows the expression of GFAP and Iba-1 in each location. GFAP expression was reduced in the temporal cortex (*p<0.05 vs control samples), n=3 individuals per group. Western blot quantification was analysed by normalising the protein levels to the Ponceau stain. C, control; T2D, type 2 diabetes; WGA, wheat germ agglutinin
Discussion
Recent evidence has shown a relationship between type 2 diabetes and cognitive decline, dementia and Alzheimer’s disease [2-4]. Hyperglycaemia, insulin resistance, hypertension and dyslipidaemia in type 2 diabetes have a profound effect on the vasculature, leading to complications such as stroke, cardiovascular disease and diabetic retinopathy. Type 2 diabetes also has a damaging effect on neurons and glia, often relating to cell–cell communication defects across the retinal NVU [12]. The current study has detailed how db/db mice manifest cognitive deficiencies in unison with reduced brain weight and cortex size. Here, for the first time, we have catalogued a map of transcriptional profiles of the cerebral cortex from db/db mice, with a focus on the cell populations of the NVU.
Our scRNA-seq analysis identified a decrease in mature neurons, alongside an increase in microglia and immature neurons in the db/db mouse cortex. These novel findings, including disruption of several key neuronal regulatory genes, were complemented by the observed brain atrophy and cognitive decline in the db/db mouse. Meg3 and Mbp both showed reduced expression in db/db mice, indicating functional disruption. Downregulation of Mbp in mature neurons may indicate that golli-Mbp [28] (a known protein found outside the myelin sheath), which is involved in neuronal development [36] and Ca2+ homeostasis, may be affected in type 2 diabetes. We also observed some evidence of NeuN reduction in individuals with type 2 diabetes. Together, these data suggest that type 2 diabetes may affect neuronal maturation, thereby having a profound impact on cognition. This finding is reinforced by similar findings in Alzheimer’s disease [37] and other neurodegenerative diseases associated with progressive cognitive decline [38].
Previous reports have suggested that glial GFAP+ area is increased in the db/db mouse cortex [39]. Our findings in db/db mice and humans with type 2 diabetes remain ambiguous. Nevertheless, we observed a distinct transcriptional profile of cortical astrocytes in db/db mice. A notable example is Mt3 downregulation in db/db mouse astrocytes. This change in expression has been related to reduced uptake of Aβ leading to extracellular accumulation in Alzheimer’s disease [40], highlighting a link between Alzheimer’s disease pathology and diabetes-induced cognitive decline. Mt1 and Mt3 are also involved in protection against oxidative stress [30] and their downregulation in db/db mouse astrocytes may contribute to oxidative damage and enhanced inflammation in the type 2 diabetes cortex.
Cortical astrocyte metabolism was affected in db/db mice, including upregulation of genes involved in the formation of mitochondrial complexes (e.g. mt-Co2, mt-Nd1, mt-Nd2) and dysregulated lipid metabolism. A previous study reported that alterations in astrocyte lipid metabolism may be linked to degeneration of motor neurons [41]. Astrocytes play an important role in NVU function, interacting with and supporting neurons and blood vessels [42] and these changes in metabolic function in cortical astrocytes may contribute to NVU dysfunction in db/db mice.
Microglia are important modulators of homeostasis in the cerebral cortex. We found an increased number of microglia in the cortex of db/db mice, occurring in unison with overexpression of inflammatory pathways and genes involved in myelin damage, indicating neuronal injury in the diabetic brain [43]. ApoE is an established genetic risk factor for Alzheimer’s disease with roles in Aβ clearance, glucose metabolism and proinflammatory responses [44]. However, high levels of leptin can influence ApoE expression [45], therefore this finding should be explored in other diabetic models before a conclusion is made. In diabetic retinopathy, neurodegeneration is known to be an important early event (prior to vascular complications [46]), and is thought to be driven by chronic inflammation. A recent study showed that depleting microglia in a high-fat-diet-induced model of diabetes was able to improve cerebrovasculature deficits [47]. Further understanding of the neuroinflammatory pathways mediated by microglia in the type 2 diabetes cortex will be beneficial in understanding the role of neuroinflammation in the progress of diabetes-related cognitive decline.
Diabetes is known to have a profound effect on vascular function. Our IHC data indicated reduced laminin+ vascular density in the db/db mouse cortex, mirrored by increased acellular capillaries and loss of pericytes. This is in line with findings in the db/db retina, where vascular alterations can be seen from 10 weeks [48]. We also observed evidence of vascular leakage in specific ‘hot spots’ in the db/db mouse cortex, although this requires further characterisation. Top DEGs in endothelial cells suggested alterations to angiogenesis and vessel disease, while smooth muscle contraction and metabolism were dysregulated in pericytes. We saw a statistically significant reduction of ZO-1 protein expression in the parietal and temporal cortex of individuals with type 2 diabetes. We also observed region-specific increases in occludin expression, although the cellular source of this upregulation remains to be clarified. Loss of vascular density and pericyte coverage have been reported in diabetic retinopathy [49] and also in the cortex in Alzheimer’s disease [8], strengthening the assertion that progressive NVU dysfunction in the brain during type 2 diabetes is linked to neuronal depletion and cognitive decline. Together, these findings of altered neuronal maturation, astrocyte metabolism and microglial inflammation in the db/db mouse cortex highlight the impact of complex neuroglial interactions in type 2 diabetes-induced cognitive decline.
We report that db/db mouse cortex showed a significant increase in glycolysis and alterations to a number of genes involved in oxidative phosphorylation (e.g. mt-Nd2 and mt-Nd5 were reduced in immature neurons). This result suggests that at least some cells in the type 2 diabetes cortex may switch to anaerobic glycolysis as the main supply of energy (Warburg effect). Interestingly, this Warburg effect metabolism shift has been shown to drive neuronal degeneration in sporadic Alzheimer’s disease [50]. Metabolism was also dysregulated in mature neurons and astrocytes. Mitochondrial dysfunction in diabetes has been widely described [51]. We propose that altered metabolic function of key component cells of the brain NVU may play a major role in the loss of vascular integrity and homeostasis leading to increased risk of neurodegeneration and cognitive decline.
Our study reveals distinct changes to the cells of the cerebral cortex in type 2 diabetes. One limitation of our approach is the use of IHC quantification to validate changes to cortical NVU populations, as IHC is primarily a qualitative technique. Future analysis should focus on characterisation and quantitative validation of cortical cellular heterogeneity and subcellular vulnerability in type 2 diabetes. Our dataset facilitates opportunities for molecular analysis and their validation in cell–cell communication in diabetes-related cortical dysfunction. Future studies in such diabetes models will provide further insights into the therapeutic potential of targeting these gene alterations in the NVU for treatment of diabetic comorbidities of memory and cognition.
Change history
20 August 2023
Open Access license text has been added.
Abbreviations
- DCX:
-
Doublecortin
- DEG:
-
Differentially expressed gene
- GFAP:
-
Glial fibrillary acidic protein
- GO:
-
Gene ontology
- IHC:
-
Immunohistochemistry
- MWM:
-
Morris water maze
- NeuN:
-
Neuronal nuclear protein
- NVU:
-
Neurovascular unit
- ROI:
-
Region of interest
- scRNA-seq:
-
Single-cell RNA sequencing
- SVZ:
-
Subventricular zone
- WGCN:
-
Weighted gene correlation network
- WT:
-
Wild-type
References
Karuranga S, da Rocha Fernandes J, Huang Y, Malanda B (2017) IDF diabetes atlas eighth edition 2017. International Diabetes Federation, Brussels
Koekkoek PS, Janssen J, Kooistra M et al (2016) Case-finding for cognitive impairment among people with type 2 diabetes in primary care using the Test Your Memory and Self-Administered Gerocognitive Examination questionnaires: the Cog-ID study. Diabet Med 33(6):812–819. https://doi.org/10.1111/dme.12874
Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5(1):64–74. https://doi.org/10.1016/S1474-4422(05)70284-2
Little K, Llorián-Salvador M, Scullion S et al (2022) Common pathways in dementia and diabetic retinopathy: understanding the mechanisms of diabetes-related cognitive decline. Trends Endocrinol Metab 33(1):50–71. https://doi.org/10.1016/j.tem.2021.10.008
Ma T, Li Z-Y, Yu Y et al (2022) Gray and white matter abnormality in patients with T2DM-related cognitive dysfunction: a systemic review and meta-analysis. Nutr Diabetes 12(1):39. https://doi.org/10.1038/s41387-022-00214-2
Dutta BJ, Singh S, Seksaria S, Das Gupta G, Singh A (2022) Inside the diabetic brain: insulin resistance and molecular mechanism associated with cognitive impairment and its possible therapeutic strategies. Pharmacol Res 182:106358. https://doi.org/10.1016/j.phrs.2022.106358
Garcia FJ, Sun N, Lee H et al (2022) Single-cell dissection of the human brain vasculature. Nature 603(7903):893–899. https://doi.org/10.1038/s41586-022-04521-7
Yang AC, Vest RT, Kern F et al (2022) A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603(7903):885–892. https://doi.org/10.1038/s41586-021-04369-3
Velmeshev D, Schirmer L, Jung D et al (2019) Single-cell genomics identifies cell type-specific molecular changes in autism. Science 364(6441):685–689. https://doi.org/10.1126/science.aav8130
Mathys H, Davila-Velderrain J, Peng Z et al (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570(7761):332–337. https://doi.org/10.1038/s41586-019-1195-2
Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC (2005) Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex 15(7):995–1001. https://doi.org/10.1093/cercor/bhh200
Niu T, Fang J, Shi X et al (2021) Pathogenesis study based on high-throughput single-cell sequencing analysis reveals novel transcriptional landscape and heterogeneity of retinal cells in type 2 diabetic mice. Diabetes 70(5):1185–1197. https://doi.org/10.2337/db20-0839
Ma S, Bi W, Liu X et al (2022) Single-cell sequencing analysis of the db/db mouse hippocampus reveals cell-type-specific insights into the pathobiology of diabetes-associated cognitive dysfunction. Front Endocrinol 13:891039. https://doi.org/10.3389/fendo.2022.891039
Jiménez-Palomares M, Ramos-Rodríguez JJ, López-Acosta JF et al (2012) Increased Aβ production prompts the onset of glucose intolerance and insulin resistance. Am J Physiol Endocrinol Metab 302(11):1373–1380. https://doi.org/10.1152/ajpendo.00500.2011
Infante-Garcia C, Ramos-Rodriguez JJ, Galindo-Gonzalez L, Garcia-Alloza M (2016) Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology 65:15–25. https://doi.org/10.1016/j.psyneuen.2015.12.001
Zheng H, Zheng Y, Zhao L et al (1863) (2017) Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. Biochim Biophys Acta Mol Basis Dis 1:266–273. https://doi.org/10.1016/j.bbadis.2016.11.003
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9(1):559. https://doi.org/10.1186/1471-2105-9-559
Qiu X, Mao Q, Tang Y et al (2017) Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 14(10):979–982. https://doi.org/10.1038/nmeth.4402
Carranza-Naval MJ, del Marco A, Hierro-Bujalance C et al (2021) Liraglutide reduces vascular damage, neuronal loss, and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Front Aging Neurosci 13(December):1–17. https://doi.org/10.3389/fnagi.2021.741923
Hierro-Bujalance C, del Marco A, José Ramos-Rodríguez J et al (2020) Cell proliferation and neurogenesis alterations in Alzheimer’s disease and diabetes mellitus mixed murine models. J Neurochem 154(6):673–692. https://doi.org/10.1111/jnc.14987
Ramos-Rodriguez JJ, Ortiz O, Jimenez-Palomares M et al (2013) Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 38(11):2462–2475. https://doi.org/10.1016/j.psyneuen.2013.05.010
Qi G, Mi Y, Yin F (2021) Characterizing brain metabolic function ex vivo with acute mouse slice punches. STAR Protoc 2(2):100559. https://doi.org/10.1016/j.xpro.2021.100559
Bogdanov P, Corraliza L, Villena JA et al (2014) The db/db mouse: a useful model for the study of diabetic retinal neurodegeneration. PLoS One 9(5):e97302. https://doi.org/10.1371/journal.pone.0097302
Ramos-Rodriguez JJ, Jimenez-Palomares M, Murillo-Carretero MI et al (2015) Central vascular disease and exacerbated pathology in a mixed model of type 2 diabetes and Alzheimer’s disease. Psychoneuroendocrinology 62:69–79. https://doi.org/10.1016/j.psyneuen.2015.07.606
Krasemann S, Madore C, Cialic R et al (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47(3):566–581. https://doi.org/10.1016/j.immuni.2017.08.008. (e9)
Mohri I, Taniike M, Taniguchi H et al (2006) Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J Neurosci Off J Soc Neurosci 26(16):4383–4393. https://doi.org/10.1523/JNEUROSCI.4531-05.2006
Shen K, Limon A (2021) Transcriptomic expression of AMPA receptor subunits and their auxiliary proteins in the human brain. Neurosci Lett 755:135938. https://doi.org/10.1016/j.neulet.2021.135938
Landry CF, Ellison JA, Pribyl TM, Campagnoni C, Kampf K, Campagnoni AT (1996) Myelin basic protein gene expression in neurons: developmental and regional changes in protein targeting within neuronal nuclei, cell bodies, and processes. J Neurosci Off J Soc Neurosci 16(8):2452–2462. https://doi.org/10.1523/JNEUROSCI.16-08-02452.1996
Bassani S, Passafaro M (2012) TSPAN7: a new player in excitatory synapse maturation and function. Bioarchitecture 2(3):95–97. https://doi.org/10.4161/bioa.20829
Koh J-Y, Lee S-J (2020) Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol Brain 13(1):116. https://doi.org/10.1186/s13041-020-00654-w
Veyrac A, Besnard A, Caboche J, Davis S, Laroche S (2014) The transcription factor Zif268/Egr1, brain plasticity, and memory. Prog Mol Biol Transl Sci 122:89–129. https://doi.org/10.1016/B978-0-12-420170-5.00004-0
Davis KN, Tao R, Li C et al (2016) GAD2 Alternative transcripts in the human prefrontal cortex, and in schizophrenia and affective disorders. PLoS One 11(2):e0148558. https://doi.org/10.1371/journal.pone.0148558
Michalik KM, You X, Manavski Y et al (2014) Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 114(9):1389–1397. https://doi.org/10.1161/CIRCRESAHA.114.303265
Levy E, Jaskolski M, Grubb A (2006) The role of cystatin C in cerebral amyloid angiopathy and stroke: cell biology and animal models. Brain Pathol 16(1):60–70. https://doi.org/10.1111/j.1750-3639.2006.tb00562.x
Mayanagi T, Sobue K (2011) Diversification of caldesmon-linked actin cytoskeleton in cell motility. Cell Adh Migr 5(2):150–159. https://doi.org/10.4161/cam.5.2.14398
Reyes SD, Campagnoni AT (2002) Two separate domains in the golli myelin basic proteins are responsible for nuclear targeting and process extension in transfected cells. J Neurosci Res 69(5):587–596. https://doi.org/10.1002/jnr.10319
Li B, Yamamori H, Tatebayashi Y et al (2008) Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol 67(1):78–84. https://doi.org/10.1097/nen.0b013e318160c5db
Orvoen S, Pla P, Gardier AM, Saudou F, David DJ (2012) Huntington’s disease knock-in male mice show specific anxiety-like behaviour and altered neuronal maturation. Neurosci Lett 507(2):127–132. https://doi.org/10.1016/j.neulet.2011.11.063
Liu Y, Chen D, Smith A, Ye Q, Gao Y, Zhang W (2021) Three-dimensional remodeling of functional cerebrovascular architecture and gliovascular unit in leptin receptor-deficient mice. J Cereb blood flow Metab Off J Int Soc Cereb Blood Flow Metab 41(7):1547–1562. https://doi.org/10.1177/0271678X211006596
Lee S-J, Seo B-R, Koh J-Y (2015) Metallothionein-3 modulates the amyloid β endocytosis of astrocytes through its effects on actin polymerization. Mol Brain 8(1):84. https://doi.org/10.1186/s13041-015-0173-3
Mou Y, Dong Y, Chen Z et al (2020) Impaired lipid metabolism in astrocytes underlies degeneration of cortical projection neurons in hereditary spastic paraplegia. Acta Neuropathol Commun 8(1):214. https://doi.org/10.1186/s40478-020-01088-0
Liu C-Y, Yang Y, Ju W-N, Wang X, Zhang H-L (2018) Emerging roles of astrocytes in neuro-vascular unit and the tripartite synapse with emphasis on reactive gliosis in the context of Alzheimer’s disease. Front Cell Neurosci 12:1–12. https://doi.org/10.3389/fncel.2018.00193
Zhang J, Sun X, Zheng S et al (2014) Myelin basic protein induces neuron-specific toxicity by directly damaging the neuronal plasma membrane. PLoS One 9(9):e108646. https://doi.org/10.1371/journal.pone.0108646
Fernandez CG, Hamby ME, McReynolds ML, Ray WJ (2019) The role of APOE4 in disrupting the homeostatic functions of astrocytes and microglia in aging and Alzheimer’s disease. Front Aging Neurosci 11:14. https://doi.org/10.3389/fnagi.2019.00014
Shen L, Tso P, Wang DQ-H et al (2009) Up-regulation of apolipoprotein E by leptin in the hypothalamus of mice and rats. Physiol Behav 98(1–2):223–228. https://doi.org/10.1016/j.physbeh.2009.05.013
Simó R, Stitt AW, Gardner TW (2018) Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61(9):1902–1912. https://doi.org/10.1007/s00125-018-4692-1
Shen Q, Zhang G (2021) Depletion of microglia mitigates cerebrovascular dysfunction in diet-induced obesity mice. Am J Physiol Metab 321(3):E367–E375. https://doi.org/10.1152/ajpendo.00086.2021
Simó R, Bogdanov P, Ramos H, Huerta J, Simó-Servat O, Hernández C (2021) Effects of the topical administration of semaglutide on retinal neuroinflammation and vascular leakage in experimental diabetes. Biomedicines 9(8):1–10. https://doi.org/10.3390/biomedicines9080926
Hammes HP, Feng Y, Pfister F, Brownlee M (2011) Diabetic retinopathy: targeting vasoregression. Diabetes 60(1):9–16. https://doi.org/10.2337/db10-0454
Traxler L, Herdy JR, Stefanoni D et al (2022) Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab 34(9):1248–1263. https://doi.org/10.1016/j.cmet.2022.07.014. (e6)
Avram VF, Merce AP, Hâncu IM et al (2022) Impairment of mitochondrial respiration in metabolic diseases: an overview. Int J Mol Sci 23(16):1–25. https://doi.org/10.3390/ijms23168852
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Acknowledgements
The authors wish to thank K. Harkin (Queen’s University Belfast) for assistance with single-cell dissociation experiments. The authors would also like to acknowledge the support provided by Genomics Core Technology Unit (GCTU), as well as resources and consultancy provided by High Performance Computational Services (Kelvin Cluster) team and the Advanced Imaging Core Technology Unit (AICTU), Queen’s University Belfast. The authors thank the Animal Facility of the University of Cadiz and its personnel for their help and support and thank Servicios Centrales de Investigacion en Biomedicina (SC-IBM) from Universidad de Cadiz for the resources and technical support. Schematics were created with BioRender.com.
Data availability
The single-cell sequencing data that support this study are available at GEO accession GSE217665 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE217665)
Funding
This work is supported by the RECOGNISED consortium grant (EU GA 847749) to MGA and AWS and by the Deutsche Forschungsgemeinschaft TI 799/1-3 and Novo Nordisk Foundation P3110103 grants to VKT. MLS is funded by Maria Zambrano fellowship from Spanish Ministry of Science, Innovation and Universities, funded by European Union ‘Next Generation EU’ (Universitat Autònoma de Barcelona). The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Authors’ relationships and activities
AWS is an associate editor for Diabetologia. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work
Contribution statement
KL, AS and ADM contributed equally to experiments, analysis and writing of the manuscript. Authorship order in the manuscript was determined based on date of joining the project. MLS, MVS, MTA, MS, NB and SS contributed to data collection and analysis, and revision of the article drafts. JXC, IK, RS, MGA, VKT and AWS contributed to discussion, and reviewed/edited the manuscript. All authors approved the final version of the article. AWS is the guarantor of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Karis Little, Aditi Singh and Angel del Marco contributed equally; authorship order was determined based on date of joining the project.
Monica Garcia-Alloza, Vijay K. Tiwari and Alan W. Stitt share senior authorship.
Supplementary Information
ESM 1
(PDF 1.66 mb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Little, K., Singh, A., Del Marco, A. et al. Disruption of cortical cell type composition and function underlies diabetes-associated cognitive decline. Diabetologia 66, 1557–1575 (2023). https://doi.org/10.1007/s00125-023-05935-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05935-2