Abstract
Monitoring of glucose plays an essential role in the management of diabetes. However, to fully understand and meaningfully interpret glucose levels, additional information on context is necessary. Important contextual factors include data on behaviours such as eating, exercise, medication-taking and sleep, as well as data on mental health aspects such as stress, affect, diabetes distress and depressive symptoms. This narrative review provides an overview of the current state and future directions of precision monitoring in diabetes. Precision monitoring of glucose has made great progress over the last 5 years with the emergence of continuous glucose monitoring (CGM), automated analysis of new glucose variables and visualisation of CGM data via the ambulatory glucose profile. Interestingly, there has been little progress in the identification of subgroups of people with diabetes based on their glycaemic profile. The integration of behavioural and mental health data could enrich such identification of subgroups to stimulate precision medicine. There are a handful of studies that have used innovative methodology such as ecological momentary assessment to monitor behaviour and mental health in people’s everyday life. These studies indicate the importance of the interplay between behaviour, mental health and glucose. However, automated integration and intelligent interpretation of these data sources are currently not available. Automated integration of behaviour, mental health and glucose could lead to the identification of certain subgroups that, for example, show a strong association between mental health and glucose in contrast to subgroups that show independence of mental health and glucose. This could inform precision diagnostics and precision therapeutics. We identified just-in-time adaptive interventions as a potential means by which precision monitoring could lead to precision therapeutics. Just-in-time adaptive interventions consist of micro-interventions that are triggered in people’s everyday lives when a certain problem is identified using monitored behaviour, mental health and glucose variables. Thus, these micro-interventions are responsive to real-life circumstances and are adaptive to the specific needs of an individual with diabetes. We conclude that, with current developments in big data analysis, there is a huge potential for precision monitoring in diabetes.
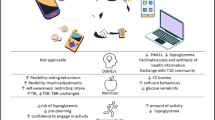
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Diabetes is a condition for which monitoring is essential for diagnosis and therapy [1, 2]. Particularly with the introduction of insulin therapy a hundred years ago, it became increasingly important to monitor glucose levels to dose insulin accordingly [3]. However, it became apparent that attaining treatment targets and simultaneously avoiding hypoglycaemia requires the consideration of contextual factors such as eating and exercise. Historically, this was achieved by logbooks, in which glucose levels, insulin doses, food intake and exercise were recorded by hand [4]. In addition, factors such as sleep [5], mood [6], emotional stress [5], diabetes distress [7] and psychological comorbidities such as depression and eating disorders [7,8,9] proved to be relevant for diabetes outcomes.
Technological advances over the last decade made monitoring of blood glucose levels in the context of the above-mentioned factors easier. This can be seen by a rapid development of glucose sensors for continuous glucose monitoring (CGM) and the increasing availability of new devices, apps and additional sensors, which are able to monitor biological markers, self-management behaviour, sleep, eating patterns and psychological variables. In addition, the ability to integrate large datasets from these different sources and the intelligent processing and interpretation thereof is developing.
By combining CGM data with the monitoring of these contextual variables, precision monitoring in diabetes can be achieved. This is in line with the current ADA and EASD consensus statement that calls for precision monitoring. Based on the consensus statement, precision monitoring is defined as the multimodal assessment of glucose, behaviours, diet, sleep and psychophysiological stress [10].
In this narrative review, we provide an overview of precision monitoring in diabetes by monitoring of glycaemic control, self-care behaviour and mental health. Specifically, we want to address the following points: (1) how precision monitoring could be used to improve prediction of diabetes outcomes (precision prognostics); and (2) what intervention strategies can be derived by the insights gained from precision monitoring (precision therapeutics). Lastly, we discuss current gaps in knowledge, suggest future directions for precision medicine in diabetes, and present a possible roadmap to precision monitoring in diabetes.
Precision monitoring in diabetes
Currently, there are three different methods for monitoring glucose: laboratory testing of blood glucose or HbA1c; self-monitoring of blood glucose (SMBG); and CGM.
Laboratory tests are the most precise measurement of blood glucose; they are usually performed for diagnostic or monitoring purposes and for adjustment or advancements of medical treatments.
SMBG became available in the 1970s and enabled people with diabetes to perform an intensified insulin therapy by autonomously adjusting insulin doses to their lifestyle (e.g. flexibility regarding time and size of their meals and exercise), leading to improved glycaemic control and better health outcomes [11]. Compared with laboratory tests, SMBG allows assessment of distribution and variation of glucose in addition to the level. However, the number of daily SMBG episodes, testing intervals and timings are decisive factors determining the potential of SMBG to identify glucose patterns.
These limitations of SMBG can be overcome by CGM, which provides interstitial glucose values every 5–15 min. This leads to a more complete picture of glucose control, especially during the night and after meals. The percentage of glucose values in the hypoglycaemic and hyperglycaemic ranges, as well as within a normal range, and glucose variability can be validly assessed. A consensus statement recommends certain thresholds for low and high glucose values as well as the percentage of values in the normoglycaemic range [12]. The beneficial effects of monitoring personal glucose levels are highlighted by the emergence and widespread adoption of CGM [13,14,15]. The in-depth glycaemic insights and direct feedback provided by CGM systems have proven efficacious in lowering elevated HbA1c values and reducing the incidence of biochemical and clinical hypoglycaemia [16,17,18]. Further, research using CGM can either adopt a blinded protocol, in which the participant has no access to the recorded values, or open CGM, in which the participant can view and respond to glucose levels.
Kovatchev et al developed the low blood glucose index (LBGI), which can be used for SMBG and CGM data, a logarithmically weighted measure of low glucose values [19]. By combining the LBGI with clinical data on severe hypoglycaemic episodes, subgroups of people with type 1 diabetes and different risk levels for future hypoglycaemia were identified [20]. This might be a first contribution to precision prognostics, allowing the response of avoidance of low glucose values in subgroups with elevated LBGI. To simplify interpretation of SMBG data, Mazze et al developed the Ambulatory Glucose Profile (AGP) [21]. The AGP is able to create individually meaningful displays of glucose levels over the day, to provide information about the individual course of glucose during the day and night. However, clinically relevant subgroups of people with diabetes, or specific therapeutic interventions, were not identified using the AGP because of the lack of important context variables, such as timing of meals, exercise and medication intake/insulin injection.
Diabetes therapies and prognosis are highly dependent on self-management [22, 23]. To achieve near-normal glucose values, people with diabetes are required to keep track of much more than just glucose levels (e.g. administering insulin and/or taking medications, eating habits, exercise, foot care and maintaining general healthy lifestyle habits) [23]. These never-ending requirements of diabetes therapy and constant self-management can also be perceived as a burden and negatively affect mental health [9]. Subsequently, mental health problems, such as elevated distress due to diabetes [7, 24, 25], depressive symptoms [7, 26, 27] and overall reduced quality of life, are common in diabetes [28]. Quality of life is an important endpoint of diabetes, besides morbidity and mortality. Impaired mental health can also negatively affect self-care behaviours and quality of life, which all can be related to unfavourable prognosis [7, 29,30,31,32]. The important impact of mental health is also reflected in the most recent consensus statement of the EASD and ADA [33]. Thus, maintaining an optimal quality of life and preventing acute as well as long-term complications of diabetes require not only monitoring of glucose but also the simultaneous assessment and integration of contextual factors such as mental health, self-care behaviour and sleep. Questionnaires have long been the standard in assessing and monitoring mental health or behaviours. However, questionnaires have considerable limitations that can bias the whole assessment period; these include recall bias (e.g. trying to remember the last 2 weeks) and salient effects when a specific symptom, mood or emotion were particularly relevant [34]. Furthermore, ongoing precision monitoring is not easily possible with questionnaires since the automated integration of different data sources is currently not possible. These limitations can be overcome by ecological momentary assessment (EMA), a methodology that allows the repeated daily sampling of participants’ experiences and behaviours in their everyday lives [35]. EMA allows monitoring of mental health states and self-care behaviours as an important context of continuously measured glucose values, as current mood, affect, sleep quality and diabetes distress as well as other behaviours can be simultaneously assessed on a daily level and multiple times daily. Table 1 provides an overview of current monitoring methods and considerations for a precision monitoring approach.
In the following section, studies that investigate the associations between mental health, self-care behaviour, sleep and glycaemic control are described. An overview of the reviewed studies, including key study and methodological characteristics, is provided in Table 2. All, but one of the studies were observational, sharing the limitations of observational studies. Most studies used open CGM or SMBG.
Mental health and glycaemic control
A study by Cox et al demonstrated postprandial effects of glucose on mood, with the rate of rise in postprandial glucose excursions being directly associated with negative mood (depressive and anxiety symptoms) but not with positive mood [36]. An early study by Hermanns et al, using blinded CGM and multiple mood assessment over 2 days, demonstrated that glucose levels collected 60 min prior to mood ratings showed significant negative associations with hedonic tone (i.e. happy) as well as positive associations with energetic arousal (i.e. active) and tension (i.e. anxious) [6]. Interestingly, this study did not find significant associations between glycaemic variability and mood. This matches with recent findings by Wagner et al in which glycaemic variability, assessed by blinded CGM over 7 days, showed no associations with positive nor negative affect [37]. Overall, Wagner et al only found associations between glycaemic variables (e.g. mean glucose, per cent hypo- or hyperglycaemic values) and negative affect, but not positive affect [37]. These findings indicate that higher glucose levels and negative mood appear to show a stronger association than glucose and positive mood. Support for this comes from Skaff et al, who demonstrated that, in people with type 2 diabetes, negative mood on one day was predictive of higher fasting glucose on the next day, while positive mood had no effect [38].
In teenagers with type 1 diabetes, however, a recent study by Shapira et al found that positive affect was associated with a higher frequency of in-range blood glucose levels and lower likelihood of hypoglycaemic values, as well as less glucose fluctuation. It should be noted that these findings were specific for those teenagers with HbA1c ≤8% (63.9 mmol/mol) [39]. In adults with type 1 diabetes, Polonsky and Fortman demonstrated that over a period of 2 weeks, increases in daily time-in-range were significantly associated with better mood ratings in the evening [40]. However, mood ratings were not associated with daily changes in time-in-hypoglycaemia or with glycaemic variability.
A recent systematic review also concluded that associations between mood and glucose variability could not be convincingly shown. However, this review indicated a significant direct relationship between postprandial glucose and negative mood in people with type 2 diabetes and a potential positive effect of lower glucose variability on depressive mood in adults with type 1 diabetes [41].
In summary, there seems to be growing evidence that negative mood states are associated with elevated or low glucose values, while glucose values within a normal range are rather coincident with positive mood states and less negative affect. The causality and directionality of these relationships require additional study (e.g. by the use of vector autoregression methods [42, 43]). Further analysis of the relationship between mental health aspects and glucose levels, as well as excursions, is needed [44]. Nice examples of such analyses are current studies combing EMA and CGM: the FEEL-T1D study in the USA [45]; the DIA-LINK studies in Germany [44]; and the international Hypo-METRICS study within the Hypo-RESOLVE Research Consortium [46]. Such work could support the identification of subgroups of individuals whose mental health might be more strongly influenced by the course of glucose compared with others whose glycaemic control might not be strongly associated with mental health. From the point of view of ‘precision therapeutics’, different therapy strategies might be offered to the two subgroups: for the first subgroup interventions to improve mental health could be more effective when including the diabetes context; and for the latter subgroup improvement of glycaemic control and mental health could be addressed independently.
Behaviour and glycaemic control
EMA has also been used to study the effects of behaviour on glycaemic control. Wagner et al used blinded CGM for 7 days in Latinos with type 2 diabetes and showed that higher variability in self-care behaviour was associated with a higher percentage of glucose values out of range (either <3.9 mmol/l or >10 mmol/l, particularly values in the hyperglycaemic range (>180 mmol/l) [37]. The consequence was an association between greater self-care behaviour and less glucose variability. This is corroborated by Shapira et al, who demonstrated that stronger negative affect was associated with less optimal self-care (fewer blood glucose checks), especially for teenagers with HbA1c levels >8% (63.9 mmol/mol) [39].
In a study analysing binge eating in type 1 diabetes, people with higher negative affect, guilt, frustration or diabetes distress had a higher risk for binge-eating episodes. This, in turn, led to higher postprandial glucose excursions [47]. In a further step, it might be worth examining whether addressing these negative emotions in people prone to binge eating could help to prevent binge-eating episodes, thereby positively influencing glycaemic control. Another variable playing a potential role is that of executive function problems, which have been found to be associated with disordered eating behaviour [48].
An interesting study reports on digitally phenotyping the monitoring of self-care behaviour in people with type 2 diabetes [49]. Based on participants’ engagement with multiple mHealth devices used for monitoring, the authors found three distinct digital phenotypes: 33% had low and waning engagement, while the rest had either medium engagement or consistently high engagement. The authors found that being in the low and waning engagement group was associated with younger age, female sex, non-white race, lower income and higher baseline HbA1c [49].
The automated assessment and integration of key self-care behaviours such as medication intake and administration of injections, and physical activity and eating, could facilitate consideration of these key behaviours in diabetes therapy. Monitoring of behaviours shows that there are direct links between self-care and glycaemic control [37, 47]. Identification of people with problems in self-care behaviours might enhance precision therapeutics since these people can then be offered specific support. Identification of subgroups of people who show a strong association between mental health problems and problematic self-care behaviours may allow for early intervention before deficits of self-care become obvious and damaging. However, it must be considered that subtyping of groups solely based on self-care behaviour might be difficult due to high variability of behaviour, often dependent upon changing life circumstances.
Sleep and glycaemic control
Sleep is one of the most influential factors in hormonal regulation and circadian rhythm. Unsurprisingly, sleep disturbances are associated with a wide range of physiological and psychological problems (e.g. diabetes, depression). Notably, diabetes self-management efforts using modern devices can also disrupt sleep with, for instance, CGM glucose alerts or pump alarms. In addition, nocturnal hypoglycaemia can also negatively affect sleep quality [50]. Observational data suggest that sleep disturbances or impaired sleep quality are more prevalent in people with diabetes [51]. Indeed, there is evidence that reduced sleep is related to the occurrence of type 2 diabetes, heightened inflammation, insulin resistance and appetite and weight gain [52,53,54]. Furthermore, insomnia and obstructive sleep apnoea are more prevalent in people with vs without diabetes (prevalence estimates of about 50% in those with diabetes) [55, 56]. Meta-analyses suggest that shorter sleep duration and lower sleep quality in particular are associated with less-than-optimal glycaemic control [56, 57]. In adults with type 1 diabetes, those who had poor self-reported sleep quality or who slept for ≤6 h had higher HbA1c [56]. In a meta-analysis of adults with diabetes, a U-shaped association was found, with shorter sleep duration (<6 h) and longer sleep duration (>8–9 h) being associated with higher HbA1c [57].
Wearables can be used to assess sleep behaviour and are especially useful for assessing sleep duration and number of awakenings, as well as duration of rapid-eye-movement (REM) and non-REM sleep [58]. Thus, monitoring of sleep patterns could also inform therapy adjustments by identifying people with disordered sleep.
In general, the literature shows that sleep is an important determinant of the course of diabetes. Sleep quality is associated with less-than-optimal glycaemic control. Subtyping of people with diabetes according to their sleep characteristics might enhance precision prognostics. Monitoring of sleep in the context of glycaemic control as well as mental health might also indicate therapeutic starting points.
Precision prognostics and diagnosis
Monitoring of glucose levels, (self-care) behaviours and mental health can provide a near-complete picture of glucose patterns, corresponding behaviours and the subjective mental states of people with diabetes at a certain point in time. Combining and synthesising these three data sources could contribute to a better prediction of long-term diabetes outcomes such as morbidity, mortality and quality of life.
Figure 1 offers a conceptual representation of different aspects of precision monitoring in diabetes. Combination of all three or two of the monitoring areas may encompass precision monitoring [10]. Of course, the approach taken to precision monitoring should be tailored to the individual and may not be entirely perfect for each person with diabetes nor suitable for all subgroups. For people without indication of impaired mental health, monitoring of behaviour and glucose could lead to therapy adjustments regarding medication or behavioural changes. For people with a strong association between mental health variables, such as diabetes distress, and glycaemic outcomes, a different prognosis regarding the future course of glycaemic control or intervention might result when compared with people with a weak or no association between glucose and diabetes distress. This is exemplified by the simultaneous monitoring of hypoglycaemia-related distress and exposure to low glucose values. Figure 2 depicts data from three people with different associations between perceived hypoglycaemia distress (assessed via EMA) and exposure to low glucose values (assessed by CGM). In the individual shown in Fig. 2a, the pattern of perceived subjective distress closely mirrors that of low glucose levels. A promising strategy for reducing perceived distress might be to avoid low glucose values by medical interventions such as adjustment of glycaemic targets, change of medication dose, the use of insulin pump therapy or automated insulin delivery (AID) systems, or diabetes education. The individual whose data are shown in Fig. 2b might not profit from these medical interventions regarding hypoglycaemia distress, since subjective distress seems unrelated to the actual exposure to low glucose values. Here, the identification of different triggers of hypoglycaemic distress (e.g. thoughts, high level of general anxiety) might be more appropriate. Figure 2c shows data from an individual who has high exposure to low glucose values but no subjective hypoglycaemia distress. This person might benefit from a different risk appraisal regarding hypoglycaemia. These examples demonstrate how precision monitoring can inform precision diagnostics and may help to suggest differential therapeutical targets and starting points.
Conceptual model of multidimensional monitoring in diabetes (glucose, self-care behaviour, mental health). Methods of monitoring glycaemic variables (green), behaviour and physiological variables (orange) and psychological variables (blue) are shown. Combination of two or all of these monitoring areas can contribute to precision monitoring in diabetes, as shown by overlap of the circles. Rectangular boxes show precision monitoring areas (associations among glucose control, mental health and behaviour, which can affect psychosocial and metabolic responsiveness, lead to self-management strain and provide opportunities for diabetes therapy adjustments). This figure is available as part of a downloadable slideset
The association between perceived hypoglycaemia distress and exposure to low glucose values. Data arising from three different case studies are shown. Low blood glucose, assessed by CGM, was defined as <3.9 mmol/l; perceived hypoglycaemia distress was assessed by EMA 0–10. Data are taken from three individual participants in the DIA-LINK study led by NH, DE, ASc and BK (unpublished). This figure is available as part of a downloadable slideset
Thus, the longitudinal monitoring of variables from key areas (behaviour, glycaemic control and mental health) allows the aggregation of these variables at a within-person level and the statistical analysis of individual courses. Assessment of such idiosyncratic associations between variables of the three key areas may also enhance precision diagnostics and prognostics in diabetes at an individual level. An individual longitudinal neural network analysis with multiple assessments over time or latent class growth analysis [59] may provide such idiosyncratic courses over time. At a between-person level, the magnitude of the idiosyncratic associations can also be combined with genetic, metabolic or other biological–medical factors by cluster analytical methods. This would allow subtyping of people with diabetes based on precision monitoring and provide individual targets for interventions.
Precision therapeutics in diabetes
The identification of ‘glycaemic–behavioural–mental health patterns’ via precision monitoring can lead to different treatment approaches and better outcomes as described above. An intriguing possibility is the automated integration of monitoring results into treatment decisions, a concrete example being the use of AID systems in type 1 diabetes. Studies showed that AID systems could increase time-in-range (glucose levels of 3.9–10 mmol/l) by approximately ten percentage points [60,62,62]. However, in these studies time-in-range usually hovered around 70% [60,62,62], possibly indicating that the full potential of AID control is not yet achieved. One problem might be that relevant contextual factors such as eating behaviour, exercise and experience of stress are not sufficiently considered in the current systems. The automated integration of monitoring results regarding stress, exercise and amount of carbohydrates might help to inform the algorithms of AID systems about upcoming glucose excursions, allowing earlier response. Integration of this contextual information might also help to improve outcomes in people with type 2 or type 1 diabetes who are using less-complex therapies (e.g. multiple daily insulin injections) or other decision support systems (e.g. smart pens, bolus calculators).
Precision monitoring of glucose, behaviour and mental health could also be used to trigger interventions automatically. One possible approach involves so-called just-in-time adaptive interventions [63]. This comprises micro-interventions that are automatically triggered when a specific problem is identified by precision monitoring. Thus, just-in-time adaptive interventions could be tailored to a specific person by being adaptive to the current circumstances (e.g. high glucose, low adherence, high psychological stress) and responsive to the moment a problem is identified (e.g. suggestion of a micro-intervention on a person’s smartphone). These micro-interventions could not only target glycaemic control but also self-care behaviour and mental health.
In severe mental illness with a cyclic course (e.g. bipolar depression, psychosis), which often presents insurmountable hurdles to optimal glycaemic control, precision monitoring of symptoms or other variables such as activity, mobility or communication behaviour could support early detection of a relapse. This would inform ‘in-time’ (pharmacological) treatment intensification or psychological interventions such as cognitive behaviour therapy or mindfulness-based cognitive therapies, which have proven to be effective in treating severe mental illness and preventing deterioration of glycaemic control [64, 65].
Roadmap to precision monitoring
There are several knowledge gaps that need to be addressed in order to achieve precision monitoring in diabetes:
-
1.
More information is needed regarding the measurement performance, accuracy and reliability of sensor data yielded by monitoring of glucose, behaviour and mental health variables. Using EMA in routine care also raises the question of the clinical validity of EMA results compared with classical psychometric measurements by questionnaire or interview.
-
2.
The stability and directionality of associations between glucose, behaviour and mental health is as unresolved as the question of directionality. Another open question is the potential of clustering people based on individualised associations between these key monitoring areas into meaningful subgroups regarding prognosis and treatment. Further, more information is needed on whether all three key monitoring areas (behaviour, mental health, glucose outcomes) should be weighted equally or differentially.
-
3.
Mechanisms of change that can be targeted by interventions need to be identified. The impact of therapeutic use of feedback regarding individual associations also needs to be addressed in pilot-studies and tailored algorithms to control such feedback needs to be developed. Avoidance of potential information overload and assessment burden must be taken into account when developing such interventions based on precision monitoring.
-
4.
There is a need for rigorous testing of newly developed interventions in controlled studies (ideally in randomised controlled studies). Factorial and platform trials may provide a greater cost-effectiveness of such testing.
-
5.
Medical, demographic and social variables (e.g. age, sex, diabetes type, socioeconomic status, comorbidities, cognitive decline) often interact with the readiness to monitor certain aspects of living with diabetes as well as with the readiness to take part in intervention studies. Real-world studies and healthcare research can provide data about the efficacy of the newly developed interventions under conditions of routine care and also about cost-effectiveness. Particularly, these studies can provide a clearer picture of which subgroups of people can profit from precision monitoring in diabetes.
Precision monitoring in diabetes is a new and developing field of research and clinical care. A possible roadmap towards precision monitoring, addressing the identified gaps, is depicted in Fig. 3. We suggest five evaluative milestones in the Text box, above. This roadmap may inform the way to precision diagnostics and precision therapeutics that are based on precision monitoring.
Roadmap suggesting studies necessary for achieving precision monitoring in diabetes. This figure is available as part of a downloadable slideset
Conclusion
Taken together, monitoring of self-care behaviour and mental health can significantly enrich glucose data by providing context to the glucose values. Interpreting glucose while considering self-care behaviours and mental health issues will become more precise and could facilitate clinically meaningful inferences and opportunities for therapy adjustments that match the specific needs of an individual. Use of precision monitoring could allow identification of psychobehavioural glucotypes, each of which could then benefit from a precision medicine approach to treatment. To achieve this, standards for monitoring and interpretation of self-care behaviours and mental health must be developed, based on the example of CGM [66]. Furthermore, there is need for the automated combination and integration of the data sources using technological as well as artificial intelligence solutions. While precision monitoring is not yet established, it is a next step towards giving people with diabetes and healthcare professionals the tools to better understand the intricacies of diabetes therapy and help inform appropriate management.
Abbreviations
- AGP:
-
Ambulatory Glucose Profile
- AID:
-
Automated insulin delivery
- CGM:
-
Continuous glucose monitoring
- EMA:
-
Ecological momentary assessment
- LBGI:
-
Low blood glucose index
- REM:
-
Rapid-eye-movement
- SMBG:
-
Self-monitoring of blood glucose
References
American Diabetes Association (2021) 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 44(Suppl 1):S15–S33. https://doi.org/10.2337/dc21-S002
American Diabetes Association (2021) 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2021. Diabetes Care 44(Suppl 1):S40–S52. https://doi.org/10.2337/dc21-S004
Polonsky KS (2012) The past 200 years in diabetes. N Engl J Med 367(14):1332–1340. https://doi.org/10.1056/NEJMra1110560
Barnett D (1999) Elliott P. Joslin MD: A Centennial Portrait. Joslin Diabetes Clinic, Boston MA
Gonder-Frederick LA, Grabman JH, Kovatchev B et al (2016) Is Psychological Stress a Factor for Incorporation Into Future Closed-Loop Systems? J Diabetes Sci Technol 10(3):640–646. https://doi.org/10.1177/1932296816635199
Hermanns N, Scheff C, Kulzer B et al (2007) Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia 50(5):930–933. https://doi.org/10.1007/s00125-007-0643-y
Snoek FJ, Bremmer MA, Hermanns N (2015) Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol 3(6):450–460
Petrak F, Herpertz S, Albus C, Hirsch A, Kulzer B, Kruse J (2004) Evidence-based guidelines of the German Diabetes Association - Psychosocial factors and diabetes mellitus. J of Psychosom Res 56(Suppl):672
Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M (2016) Psychosocial Care for People With Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 39(12):2126–2140. https://doi.org/10.2337/dc16-2053
Chung WK, Erion K, Florez JC et al (2020) Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 63(9):1671–1693. https://doi.org/10.1007/s00125-020-05181-w
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N Engl J Med 329(14):977–986
Battelino T, Danne T, Bergenstal RM et al (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42(8):1593–1603
Roos T, Hochstadt S, Keuthage W et al (2020) Level of Digitalization in Germany: Results of the Diabetes Digitalization and Technology (D.U.T) Report 2020. J Diabetes Sci Technol 16(1):144–151. https://doi.org/10.1177/1932296820965553
Foster NC, Beck RW, Miller KM et al (2019) State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 21(2):66–72. https://doi.org/10.1089/dia.2018.0384
van den Boom L, Karges B, Auzanneau M et al (2019) Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care 42(11):2050–2056
Beck RW, Riddlesworth TD, Ruedy KJ et al (2017) Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 317(4):371–378. https://doi.org/10.1001/jama.2016.19975
Lind M, Polonsky W, Hirsch IB et al (2017) Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA 317(4):379–387. https://doi.org/10.1001/jama.2016.19976
Heinemann L, Freckmann G, Ehrmann D et al (2018) Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. The Lancet 391(10128):1367–1377. https://doi.org/10.1016/S0140-6736(18)30297-6
Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W (1997) Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care 20(11):1655–1658
Kovatchev B, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke WL (1998) Assessment of risk of severe hypoglycemia among adults with IDDM. Diabetes Care 21:1870–1875
Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D (1987) Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care 10(1):111–117. https://doi.org/10.2337/diacare.10.1.111
Surwit RS, Feinglos MN, Scovern AW (1983) Diabetes and behavior. A paradigm for health psychology. Am Psychol 38:255–262
American Diabetes Association (2021) 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes-2021. Diabetes Care 44(Suppl 1):S53–S72. https://doi.org/10.2337/dc21-S005
Skinner TC, Joensen L, Parkin T (2020) Twenty-five years of diabetes distress research. Diabet Med 37(3):393–400. https://doi.org/10.1111/dme.14157
Perrin NE, Davies MJ, Robertson N, Snoek FJ, Khunti K (2017) The prevalence of diabetes-specific emotional distress in people with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med 34(11):1508–1520. https://doi.org/10.1111/dme.13448
Roy T, Lloyd CE (2012) Epidemiology of depression and diabetes: a systematic review. J Affect Disord 142(Suppl. 1):S8–S21
Khaledi M, Haghighatdoost F, Feizi A, Aminorroaya A (2019) The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetologica 56(6):631–650. https://doi.org/10.1007/s00592-019-01295-9
Schunk M, Reitmeir P, Schipf S et al (2012) Health-related quality of life in subjects with and without Type 2 diabetes: pooled analysis of five population-based surveys in Germany. Diabet Med 29(5):646–653
Black SA, Markides KS, Ray LA (2003) Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care 26(10):2822–2828
van Dooren FE, Nefs G, Schram MT, Verhey FR, Denollet J, Pouwer F (2013) Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PLoSOne 8(3):e57058
Nouwen A (2015) Depression and diabetes distress. Diabet Med 32(10):1261–1263
Nouwen A, Adriaanse M, van Dam K et al (2019) Longitudinal associations between depression and diabetes complications: A systematic review and meta-analysis. Diabet Med 36(12):1562–1572
Holt RI, DeVries JH, Hess-Fischl A et al (2021) The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 44(11):2589–2625
Fredrickson BL (2000) Extracting meaning from past affective experiences: The importance of peaks, ends, and specific emotions. Cogn Emot 14(4):577–606
Shiffman S, Stone AA, Hufford MR (2008) Ecological momentary assessment. Annu Rev Clin Psychol 4:1–32
Cox DJ, McCall A, Kovatchev B, Sarwat S, Ilag LL, Tan MH (2007) Effects of blood glucose rate of changes on perceived mood and cognitive symptoms in insulin-treated type 2 diabetes. Diabetes Care 30(8):2001–2002. https://doi.org/10.2337/dc06-2480
Wagner J, Armeli S, Tennen H, Bermudez-Millan A, Wolpert H, Perez-Escamilla R (2017) Mean Levels and Variability in Affect, Diabetes Self-Care Behaviors, and Continuously Monitored Glucose: A Daily Study of Latinos With Type 2 Diabetes. Psychosom Med 79(7):798–805. https://doi.org/10.1097/PSY.0000000000000477
Skaff MM, Mullan JT, Almeida DM et al (2009) Daily negative mood affects fasting glucose in type 2 diabetes. Health Psychol 28(3):265–272. https://doi.org/10.1037/a0014429
Shapira A, Volkening LK, Borus JS, Laffel LM (2021) Ecological Momentary Assessment (EMA) of Positive and Negative Affect and Associations with Blood Glucose (BG) in Teens with Type 1 Diabetes (T1D). J Diabetes Sci Technol 30 July. https://doi.org/10.1177/19322968211035451
Polonsky WH, Fortmann AL (2020) The influence of time in range on daily mood in adults with type 1 diabetes. J Diabetes Complications 34(12):107746. https://doi.org/10.1016/j.jdiacomp.2020.107746
Muijs LT, Racca C, de Wit M et al (2021) Glucose variability and mood in adults with diabetes: A systematic review. Endocrinol Diabetes Metab 4(1):e00152
van der Krieke L, Blaauw FJ, Emerencia AC et al (2017) Temporal dynamics of health and well-being: A crowdsourcing approach to momentary assessments and automated generation of personalized feedback. Psychosom Med 79(2):213–223
van der Krieke L, Emerencia AC, Bos EH et al (2015) Ecological momentary assessments and automated time series analysis to promote tailored health care: a proof-of-principle study. JMIR Res Protoc 4(3):e100
Ehrmann D, Priesterroth L, Schmitt A, Kulzer B, Hermanns N (2021) Associations of Time in Range and Other Continuous Glucose Monitoring-Derived Metrics With Well-Being and Patient-Reported Outcomes: Overview and Trends. Diabetes Spectr 34(2):149–155. https://doi.org/10.2337/ds20-0096
Pyatak EA, Hernandez R, Pham LT et al (2021) Function and Emotion in Everyday Life With Type 1 Diabetes (FEEL-T1D): Protocol for a Fully Remote Intensive Longitudinal Study. JMIR Res Protoc 10(10):e30901. https://doi.org/10.2196/30901
de Galan B, McCrimmon R, Ibberson M et al (2020) Reducing the burden of hypoglycaemia in people with diabetes through increased understanding: design of the Hypoglycaemia REdefining SOLutions for better liVEs (Hypo-RESOLVE) project. Diabet Med 37(6):1066–1073
Moskovich AA, Dmitrieva NO, Babyak MA et al (2019) Real-time predictors and consequences of binge eating among adults with type 1 diabetes. J Eat Disord 7:7. https://doi.org/10.1186/s40337-019-0237-3
Cecilia-Costa R, Hansmann M, McGill DE, Volkening LK, Laffel LM (2021) Association of executive function problems and disordered eating behaviours in teens with type 1 diabetes. Diabet Med 38(11):e14652
Yang Q, Hatch D, Crowley MJ et al (2020) Digital Phenotyping Self-Monitoring Behaviors for Individuals With Type 2 Diabetes Mellitus: Observational Study Using Latent Class Growth Analysis. JMIR Mhealth Uhealth 8(6):e17730. https://doi.org/10.2196/17730
Frier BM (2014) Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 10(12):711–722. https://doi.org/10.1038/nrendo.2014.170
Nefs GM, Bazelmans E, Donga E, Tack CJ, de Galan BE (2020) Sweet dreams or bitter nightmare: a narrative review of 25 years of research on the role of sleep in diabetes and the contributions of behavioural science. Diabet Med 37(3):418–426. https://doi.org/10.1111/dme.14211
Reutrakul S, Van Cauter E (2014) Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci 1311:151–173. https://doi.org/10.1111/nyas.12355
Knutson KL, Spiegel K, Penev P, Van Cauter E (2007) The metabolic consequences of sleep deprivation. Sleep Med Rev 11(3):163–178. https://doi.org/10.1016/j.smrv.2007.01.002
Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS (2011) Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care 34(5):1171–1176
Doumit J, Prasad B (2016) Sleep Apnea in Type 2 Diabetes. Diabetes Spectr 29(1):14–19. https://doi.org/10.2337/diaspect.29.1.14
Reutrakul S, Thakkinstian A, Anothaisintawee T et al (2016) Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med 23:26–45
Lee SWH, Ng KY, Chin WK (2017) The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med Rev 31:91–101. https://doi.org/10.1016/j.smrv.2016.02.001
Guillodo E, Lemey C, Simonnet M et al (2020) Clinical applications of mobile health wearable–based sleep monitoring: systematic review. JMIR mHealth and uHealth 8(4):e10733
Jung T, Wickrama KA (2008) An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass 2(1):302–317
Brown SA, Kovatchev BP, Raghinaru D et al (2019) Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med 381(18):1707–1717. https://doi.org/10.1056/NEJMoa1907863
Breton MD, Kanapka LG, Beck RW et al (2020) A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 383(9):836–845
Benhamou P-Y, Franc S, Reznik Y et al (2019) Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. The Lancet Digital Health 1(1):e17–e25
Nahum-Shani I, Hekler EB, Spruijt-Metz D (2015) Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychol 34S:1209–1219. https://doi.org/10.1037/hea0000306
Baumeister H, Hutter N, Bengel J (2012) Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev 12:CD008381
Lean M, Fornells-Ambrojo M, Milton A et al (2019) Self-management interventions for people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry 214(5):260–268
Danne T, Nimri R, Battelino T et al (2017) International consensus on use of continuous glucose monitoring. Diabetes Care 40(12):1631–1640
Cecilia-Costa R, Hansmann M, McGill DE, Volkening LK, Laffel LM (2021) Association of executive function problems and disordered eating behaviours in teens with type 1 diabetes. Diabet Med 38(11): e14652. https://doi.org/10.1111/dme.14652
Reutrakul S, Hood MM, Crowley SJ et al (2013) Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care 36(9):2523–2529
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
NH, DE and ASh wrote the manuscript and reviewed studies. BK, ASh and LL revised the manuscript and contributed to the discussion. All authors contributed to the conception of this review and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Work in the authors’ laboratory is supported by the German Center for Diabetes Research (DZD) grant no. 82DZD11A02.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM
(PPTX 623 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hermanns, N., Ehrmann, D., Shapira, A. et al. Coordination of glucose monitoring, self-care behaviour and mental health: achieving precision monitoring in diabetes. Diabetologia 65, 1883–1894 (2022). https://doi.org/10.1007/s00125-022-05685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05685-7