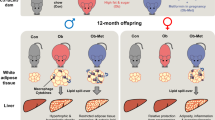
Abstract
Aims/hypothesis
Obesity and hepatic steatosis are risk factors for gestational diabetes mellitus (GDM), a common complication of pregnancy. Adiponectin is a fat-derived hormone that improves hepatic steatosis and insulin sensitivity. Low levels of circulating adiponectin are associated with GDM development. We hypothesised that adiponectin deficiency causes fatty liver during pregnancy, contributing to the development of GDM.
Methods
To determine the role of adiponectin in fatty liver development during pregnancy, we compared pregnant (third week of pregnancy) adiponectin knockout (KO) mice (strain B6;129-Adipoqtm1Chan/J) with wild-type mice and assessed several variables of hepatic lipid metabolism and glucose homeostasis. The impact of adiponectin supplementation was measured by administering adenovirus-mediated full-length adiponectin at the end of the second week of pregnancy and comparing with green fluorescent protein control.
Results
In the third week of pregnancy, fasted pregnant adiponectin KO mice were hyperglycaemic on a low-fat diet (9.2 mmol/l vs 7.7 mmol/l in controls, p<0.05) and were glucose and pyruvate intolerant relative to wild-type mice. Pregnant adiponectin KO mice developed hepatic steatosis and a threefold elevation in hepatic triacylglycerols (p<0.05) relative to wild-type mice. Gestational weight gain and food consumption were similar in KO and wild-type mice. Adenoviral-mediated adiponectin supplementation to pregnant adiponectin KO mice improved glucose tolerance, prevented fasting hyperglycaemia and attenuated fatty liver development.
Conclusions/interpretation
Adiponectin deficiency increased hepatic lipid accumulation during the period of pregnancy associated with increased fat utilisation. Consequently, adiponectin deficiency contributed to glucose intolerance, dysregulated gluconeogenesis and hyperglycaemia, all of which are characteristic of GDM. Increasing adiponectin in the last week of pregnancy alleviated hepatic steatosis and restored normal glucose homeostasis during pregnancy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Gestational diabetes mellitus (GDM) is on the rise and remains the most common complication of pregnancy [1]. Mothers with GDM are seven times more likely to develop type 2 diabetes postpartum [1]. GDM also has implications for the offspring, including a higher risk of delivery complications as well as obesity and type 2 diabetes later in life [1, 2].
Research is beginning to clarify mechanisms that lead to the development of GDM. It is increasingly recognised that hepatic fat and abdominal adiposity in early pregnancy predict glucose intolerance in mid-pregnancy [3]. Prospective cohort studies have shown that the presence of elevated visceral adiposity together with sonographically detectable hepatic fat predicts GDM, independent of maternal age, ethnicity and BMI [3, 4]. The prevalence of non-alcoholic fatty liver disease (NAFLD) among women of childbearing age is estimated to be 10% [5]; however, the link between NAFLD and the development of GDM remains to be determined.
Adiponectin is a hormone secreted by adipocytes and controls the metabolism of glucose and lipids by decreasing gluconeogenesis and stimulating glycolysis and fatty acid oxidation [6]. The degree of adiponectin secretion is reduced with elevated adiposity and even further following a diagnosis of dysglycaemia [6]. Adiponectin knockout (KO) mice develop more severe high-fat-diet-induced hepatic steatosis, when compared with wild-type (WT) mice [7]. Increasing adiponectin levels improves glucose tolerance, and suppresses hepatic glucose production and steatosis [8, 9]. Low adiponectin levels in the first week of pregnancy are an independent predictor for the development of GDM in women [10]. In line with its role in regulating insulin sensitivity, low adiponectin levels are observed in women diagnosed with GDM, independent of pre-pregnancy BMI or insulin sensitivity [11]. Interestingly, maternal plasma adiponectin correlates with both the severity of NAFLD and the risk of developing GDM [3]. Previously, we showed that GDM, characterised by mid-gestational hyperglycaemia, excess gestational weight gain and low circulating adiponectin, was induced by a high-fat-and-sucrose diet in rats [12]. Recently, Qiao et al showed that pregnant adiponectin KO mice developed glucose intolerance and hyperlipidaemia [13], although hepatic steatosis was not investigated.
Based on this, we hypothesised that adiponectin deficiency caused fatty liver during pregnancy and contributed to the development of GDM. Our objective was to determine how adiponectin regulates glucose and lipid metabolism in the liver during pregnancy.
Methods
Animal care
All procedures performed were approved by the Animal Welfare Committee of the University of Manitoba, in adherence with the Canadian Council on Animal Care and the Council for International Organizations of Medical Sciences. Adiponectin KO (B6;129-Adipoqtm1Chan/J) and WT (C57/BL6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) to establish a breeding colony maintained by the University of Manitoba Central Animal Care. Three-week-old mice were placed on either a low-fat (LF) diet (20% protein, 70% carbohydrate, 10% fat; 16.11 kJ/g; Research Diets, New Brunswick, NJ, USA) D12450B), a control diet or a high-fat-and-sucrose (HFS) diet (20% protein, 35% carbohydrate, 45% fat; 19.79 kJ/g; Research Diets D12451). Mice were fed ad libitum for a minimum of 6 weeks prior to breeding and throughout pregnancy. Female adiponectin KO and WT mice were mated with male mice of the same genotype. Four experimental groups were generated: lean WT controls (LF WT); a diet-induced obesity model of gestational diabetes (HFS WT); lean adiponectin KO mice (LF KO); and adiponectin KO mice with pre-existing obesity (HFS KO). To determine the effect of adiponectin supplementation in pregnancy, replication-deficient adenovirus containing full-length adiponectin (Ad-APN, 1 × 1011 plaque-forming units [PFU]/ml) or green fluorescent protein (GFP) (Ad-GFP, 1 × 1011 PFU/ml) was administered to pregnant mice at embryonic day (e)14.5 by tail-vein injection (see ESM Methods [Adenoviral supplementation] for details). The food consumption and body weight of female mice were monitored weekly throughout gestation. GTTs and pyruvate tolerance tests (PTTs) were performed as previously described [12, 14].
At the end of gestation (e18.5) mice were euthanised with i.p. injection of an overdose of sodium pentobarbital and terminated with blood collection via cardiac puncture. Some mice received an i.p. injection of insulin (1 mU/kg body weight) 10 min before being euthanised for the assessment of gene expression responses to insulin. In some studies, primary hepatocytes were isolated using the collagenase perfusion technique [15] (see ESM Methods [Primary hepatocyte isolation] for details). Liver tissue and gonadal white adipose tissue (GWAT) were collected at the time of death. Detailed methods for histology (H&E and Oil Red O staining) and immunofluorescence are available in ESM Methods.
Circulating factors, gene expression and immunoblots
Blood collected by cardiac puncture was stored on ice and serum was separated by centrifugation at 2000 g for 10 min at 4°C and stored at −80°C for further analysis. Serum adiponectin and insulin concentrations were determined by ELISA (ALPCO, Salem, NH, USA; Mercodia, Uppsala, Sweden) according to the manufacturers’ instructions. Serum NEFA, cholesterol and β-hydroxybutyrate (Cayman Chemical, Ann Arbor, MI, USA) and triacylglycerols (WAKO Diagnostics, Mountain View, CA, USA) were measured using colorimetric assays. Liver tissue was processed for immunoblots as described previously [14], using antibodies from Cell Signaling Technologies (Danvers, MA, USA) and Abcam (Cambridge, MA, USA) (ESM Table 1). RNA was isolated using the PureLink RNA Mini Kit according to the manufacturer’s instructions (Ambion, Carlsbad, CA). Primers were acquired from IDT (Coralville, IA, USA) (ESM Table 2). Amplification of cDNA was performed using the CFX Real Time PCR (BioRad, CA, USA) (further details are available in ESM Methods [RNA isolation and qPCR]). Relative gene expression was determined using the method [14]. Data were normalised to the LF WT group and presented as fold change.
Mitochondrial analysis
Hepatocellular metabolism using glucose was determined using Seahorse XF-24 Extracellular Flux analyzer and a Mito Stress Test kit (Agilent, Santa Clara, CA, USA). For assessment of oxygen consumption rate (OCR) during glucose metabolism, hepatocytes were plated into media containing 1 mmol/l pyruvate and 25 mmol/l glucose. Fatty acid oxidation by hepatocytes was determined using an XF Palmitate-BSA FAO Substrate (Agilent) in combination with etomoxir (4 μmol/l) and BSA control according to manufacturer’s instructions. For assessment of fatty acid metabolism, hepatocytes were plated into media containing 0.5 mmol/l carnitine, 2.5 mmol/l glucose and 0.175 mmol/l palmitate-BSA and data were normalised to cellular protein.
Statistical analyses
Data are presented as mean ± SEM. Differences in measurements performed among four groups of dams were analysed using two-way ANOVA to assess the main effect for each source of variation between the genotype and diet. An ANOVA with a repeated measures test was performed when time was an additional variable. For analyses using repeated measures or multiple comparisons, a Bonferroni post hoc correction was performed. GraphPad Prism 7 Software (La Jolla, CA, USA) was used for statistical analyses. p<0.05 was regarded as statistically significant for all analyses.
Results
Adiponectin deficiency does not affect gestational weight gain
Figure 1a shows the effect of the HFS diet on the pattern of circulating adiponectin in pregnant WT mice. Entering pregnancy in an overweight or obese state increases the risk of developing GDM. Adiponectin deficiency is not a significant determinant of body-weight gain prior to mating and the HFS diet caused similar gestational weight gain in WT and adiponectin KO mice (Fig. 1b). Thus, adiponectin deficiency in pregnancy did not appear to contribute to excess gestational weight gain or excess food consumption when compared with WT control mice (ESM Table 3).
(a) Serum total adiponectin in WT mice throughout gestation (n = 6 LF WT, n = 5 HFS WT at e7.5; n = 5 LF WT, n = 6 HFS WT at e12.5; n = 5 LF WT, n = 4 HFS WT at e18.5). (b) Body weight over time, prior to breeding and during gestation (n = 13 LF WT, n = 15 LF KO, n = 12 HFS WT, n = 14 HFS KO). (c) Non-pregnant fasting blood glucose prior to breeding (n = 12 LF WT, n = 10LF KO, n = 10 HFS WT, n = 14 HFS KO) and pregnant fasting blood glucose at e18.5 (n = 9 LF WT, n = 9 LF KO, n = 11 HFS WT, n = 9 HFS KO). (d, e) GTTs performed at e16.5 on LF-fed mice (n = 8 LF WT, n = 8 LF KO) (d) and HFS-fed mice (n = HFS WT, n = 9 HFS KO) (e). AUC calculation for GTTs is shown in inset. (f) Fasting serum insulin at e18.5 (n = 9 LF WT, n = 7 LF KO, n = 8 HFS WT, n = 9 HFS KO). *p<0.05 LF vs HFS, †p<0.05 KO vs WT and ‡p<0.05 for NP vs PG (two-way ANOVA). NP, non-pregnant, PG, pregnant
An important adaptation of pregnancy is the ability of white adipose tissue to expand early in pregnancy. HFS diet consumption (relative to LF diet) led to increased GWAT mass and increased serum leptin in both WT and adiponectin KO mice in the third week of pregnancy (Table 1). Non-pregnant adiponectin KO and WT mice showed similar visceral fat deposition (Table 1). While HFS-diet feeding increased perirenal white adipose tissue (PWAT) and GWAT mass, there were no discernible genotype effects on fat pad mass in pregnancy (Table 1). Interestingly, pregnant LF-diet fed adiponectin KO mice had larger adipocytes (ESM Fig. 1b), which may have implications for adipose tissue function.
Adiponectin deficiency in pregnancy causes hyperglycaemia and glucose intolerance
Non-pregnant adiponectin KO mice were not hyperglycaemic (Fig. 1c). During pregnancy, WT mice fed an HFS diet displayed fasting hyperglycaemia relative to WT mice fed an LF diet. Both LF- and HFS-fed pregnant adiponectin KO mice fed an LF diet had higher fasted blood glucose levels (9.2 mmol/l vs 7.7 mmol/l in controls) and HFS-fed adiponectin KO mice in the third week of pregnancy were also hyperglycaemic, relative to HFS-fed WT control mice (Fig. 1c). In the last week of pregnancy, GTTs showed that LF-fed adiponectin KO mice had impaired glucose tolerance relative to WT mice (Fig. 1d) and HFS-fed adiponectin KO mice had more severe impairments (Fig. 1e). Notably, we did not observe differences in circulating insulin between adiponectin KO and WT mice in late gestation (Fig. 1f).
Dysregulation of gluconeogenesis in pregnant adiponectin KO mice
To understand how adiponectin deficiency during pregnancy worsened hyperglycaemia, a PTT was used to evaluate hepatic glucose production. While adiponectin deficiency had no impact on the pyruvate tolerance in non-pregnant females (Fig. 2a), pregnant adiponectin KO mice showed elevated glucose production from pyruvate relative to WT controls, indicating dysregulation of gluconeogenesis (Fig. 2b). To evaluate the regulation of gluconeogenesis and glycolysis, we measured mRNA expression of genes responsible for key steps in these pathways in pregnant mice that were administered insulin. Due to the natural insulin resistance of late pregnancy, there was minimal suppression of gluconeogenic genes in WT mice following insulin administration (Fig. 2c). However, gluconeogenic genes such as those encoding PEPCK (Pck1 and Pck2) were upregulated in the livers of adiponectin KO mice, most notably after insulin administration when suppression of these genes would be expected. The expression of genes coding for glucose-6-phosphatase (G6pc) and pyruvate carboxylase (Pcx) were similar in all groups in pregnancy. Moreover, the mRNA expression of the hepatic isoform of pyruvate kinase (Pklr) and glucokinase (Gck) was similar in the livers of pregnant adiponectin KO mice relative to WT mice. This may indicate dysregulation of glycolytic flux, given that even in the presence of hyperglycaemia in adiponectin KO mice, insulin did not increase their expression.
(a) PTT performed prior to conception on LF-fed mice; inset shows AUC (n = 5 LF WT, n = 7 LF KO). (b) PTT performed on LF-fed pregnant mice (e16.5); inset shows AUC (n = 8 LF WT, n = 6 LF KO). (c) Relative fold change (ΔCt) of hepatic gene expression (log10 scale) in e18.5 pregnant mice administered saline or insulin (1 mU/kg body weight) 20 min prior to being euthanised: Pck1 (n = 6 WT SAL n = 7 WT INS, n = 7 KO SAL, n = 7 KO INS); Pck2 (n = 7 WT SAL n = 8 WT INS, n = 7 KO SAL, n = 6 KO INS); G6pc (n = 7 WT SAL n = 8 WT INS, n = 7 KO SAL, n = 5 KO INS); Pklr (n = 7 WT SAL n = 7 WT INS, n = 6 KO SAL, n = 6 KO INS); Gck (n = 7 WT SAL n = 6 WT INS, n = 6 KO SAL, n = 5 KO INS); Pcx (n = 8 WT SAL n = 8 WT INS, n = 6 KO SAL, n = 7 KO INS); Foxo1 (n = 5 WT SAL n = 6 WT INS, n = 5 KO SAL, n = 6 KO INS); Dgat1 (n = 8 WT SAL n = 8 WT INS, n = 7 KO SAL, n = 6 KO INS); Fasn (n = 7 WT SAL n = 7 WT INS, n = 6 KO SAL, n = 6 KO INS); Acaca (n = 7 WT SAL n = 6 WT INS, n = 6 KO SAL, n = 6 KO INS); Bdh1 (n = 8 WT SAL n = 7 WT INS, n = 6 KO SAL, n = 6 KO INS); Srebf1 (n = 8 WT SAL n = 7 WT INS, n = 6 KO SAL, n = 6 KO INS); Cpt1a (n = 8 WT SAL n = 8 WT INS, n = 6 KO SAL, n = 7 KO INS); and Gpam (n = 8 WT SAL n = 8 WT INS, n = 7 KO SAL, n = 7 KO INS). mRNA expression is relative to geomean of Rn18s, B2m, Rplp-1 and Hprt1 and normalised to LF WT SAL. (d) Hepatic protein expression in pregnant LF-fed mice, showing p-FOXO1 relative to total FOXO1 and tubulin, normalised to WT SAL (n = 6 LF WT SAL, n = 6 LF WT INS, n = 6 LF KO SAL, n = 5 LF KO INS). (e) Representative images of H&E-stained liver sections from e18.5 pregnant adiponectin KO and WT mice. Magnification ×20. Scale bar, 50 μm. (f) Hepatic triacylglycerol content in LF- and HFS-fed adiponectin KO and WT mice at e18.5 (n = 6 LF WT, n = 6 HFS WT, n = 7 LF KO, n = 5 HFS KO). SAL, saline treated; INS, insulin treated. †p<0.05 for KO vs WT and §p<0.05 for SAL vs INS (two-way ANOVA)
Forkhead box O1 (FOXO1) is a transcription factor that regulates the expression of gluconeogenic genes and adiponectin receptors [16, 17]. Foxo1 expression was dramatically increased in adiponectin KO mice following insulin administration (Fig. 2c). This was accompanied by a decrease in the inhibitory phosphorylation of FOXO1 in adiponectin KO mice (Fig. 2d). These findings could explain the increased Pck1 and Pck2 expression as well as the increased expression of adiponectin receptors (ESM Fig. 2f). Collectively, these results show that adiponectin KO mice have dysregulated hepatic glucose output, which may contribute to hyperglycaemia and GDM in late gestation.
Fatty liver during pregnancy in adiponectin KO mice
Pregnant adiponectin KO mice developed histologically detectable hepatic steatosis during pregnancy after LF as well as HFS feeding (Fig. 2e). Hepatic fat deposition was detectable using H&E (Fig. 2e) and Oil Red O staining in pregnant HFS-fed WT mice (ESM Fig. 3a). HFS-diet feeding in the third week of pregnancy increased the steatosis score relative to LF-diet feeding in WT mice (Table 1). Relative to pregnant WT mice, adiponectin KO mice developed a threefold elevation in hepatic triacylglycerols (p<0.05) relative to WT mice (Fig. 2f). This is consistent with the general upregulation of lipogenic genes in pregnant adiponectin KO mice, including those coding for diacylglycerol transferase-1 (Dgat1), fatty acid synthase (Fasn), acetyl-CoA carboxylase (Acaca) and the mitochondrial isoform of glycerol-3-phosphate acyltransferase (Gpam) (Fig. 2c). Interestingly expression of the gene coding for β-hydroxybutyrate dehydrogenase (Bdh1) was also increased (Fig. 2c), corresponding to elevated serum β-hydroxybutyrate in adiponectin KO mice (Table 2).
Adiponectin deficiency during pregnancy alters hepatocyte lipid metabolism
In late gestation, maternal metabolism shifts to lipid utilisation [18]. Since adiponectin regulates hepatic lipogenesis and fatty acid oxidation [19, 20], we investigated the impact of adiponectin deficiency during pregnancy. We found that adiponectin KO mice had higher rates of 14C-labelled acetate uptake during pregnancy compared with WT control mice (Fig. 3a); this is associated with the development of NAFLD [21]. Next, we aimed to determine the fate of acetate within the cell. Pregnant adiponectin KO mice displayed elevated cellular synthesis of triacylglycerol from acetate, relative to WT mice, but this was not observed prior to pregnancy (Fig. 3b). Secretion of triacylglycerol into the media followed a similar pattern, with increased triacylglycerol secretion observed from pregnant adiponectin KO mice relative to WT mice and to non-pregnant adiponectin KO mice (Fig. 3c). There were no differences in fatty acid uptake in the form of oleate when comparing hepatocytes isolated from adiponectin KO and WT mice, either prior to or during pregnancy (Fig. 3d). There were no genotype differences in the cellular synthesis of triacylglycerols from oleate in non-pregnant mice; however, in hepatocytes isolated in pregnancy, triacylglycerol synthesis from oleate was significantly higher in adiponectin KO mice (Fig. 3e). Interestingly, secretion of 14C-labelled oleate triacylglycerols into the media was similar in pregnant and non-pregnant adiponectin KO and WT mice, relative to hepatocytes from non-pregnant mice (Fig. 3f). Like triacylglycerol synthesis, diacylglycerol synthesis from oleate was increased in hepatocytes from non-pregnant adiponectin KO females compared with non-pregnant WT females (ESM Fig. 3c) and was unaltered in pregnancy.
(a) 14C-labelled acetate uptake over time by primary hepatocytes isolated from LF-fed adiponectin KO and WT mice prior to pregnancy and at e18.5 (n = 3 LF WT NP, n = 3 LF KO NP, n = 3 LF WT PG, n = 3 LF KO PG). (b, c) Cellular triacylglycerol synthesis (b) (n = 2 LF WT NP, n = 3 LF KO NP, n = 3 LF WT PG, n = 5 LF KO PG) and secretion into media (c) over time (n = 3 LF WT NP, n = 4 LF KO NP, n = 3 LF WT PG, n = 4 LF KO PG) from 14C-labelled acetate by primary hepatocytes from LF-fed non-pregnant and pregnant adiponectin KO and WT mice. (d) Fatty acid uptake over time as measured by 14C-labelled oleate incorporation into primary hepatocytes isolated from LF-fed non-pregnant and pregnant adiponectin KO and WT mice (n = 5 LF WT NP, n = 3 LF KO NP, n = 2 LF WT PG, n = 5 LF KO PG). (e, f) Cellular triacylglycerol synthesis (e) (n = 5 LF WT NP, n = 3 LF KO NP, n = 3 LF WT PG, n = 5 LF KO PG) from 14C-labelled oleate and secretion into media (f) (n = 4 LF WT NP, n = 3 LF KO NP, n = 3 LF WT PG, n = 5 LF KO PG) over time by primary hepatocytes isolated from LF-fed non-pregnant and pregnant adiponectin KO and WT mice sacrificed at e18.5. †p<0.05 for WT vs KO and ‡p<0.05 for NP vs PG (two-way ANOVA). NP, non-pregnant; PG, pregnant; TG, triacylglycerol
In non-pregnant female adiponectin KO mice, cellular synthesis of cholesteryl esters from oleate but not acetate was significantly increased (ESM Fig. 3d). Pregnant adiponectin KO mice, relative to WT mice, showed increased synthesis of cholesteryl esters from acetate (ESM Fig. 3d). There was a corresponding increase in the secretion of cholesteryl esters synthesised from acetate but not oleate in pregnant adiponectin KO mice (ESM Fig. 3e).
Dysregulation of mitochondrial energy metabolism in pregnant adiponectin KO mice
Primary hepatocytes from pregnant adiponectin KO mice fed an LF diet showed reduced OCR relative to WT control mice when using glucose as primary energy source (Fig. 4a). While basal respiration was unaffected by adiponectin deficiency (Fig. 4b), significant impairments in maximal respiration (Fig. 4c) and spare capacity (Fig. 4e) were observed in hepatocytes from pregnant adiponectin KO mice relative to WT control mice. ATP production (Fig. 4d) and proton leak (Fig. 4f) were unaltered by adiponectin deficiency.
(a) Representative trace of OCR over time by primary hepatocytes utilising glucose, normalised to cellular protein (n = 5 wells LF WT, n = 6 wells LF KO). (b–f) Basal respiration (b), maximal (FCCP uncoupled) respiration (c), ATP production (oligomycin sensitive) (d), spare capacity (e) and proton leak (f) by primary hepatocytes utilising glucose (n = 5 LF WT, n = 5 LF KO). †p<0.05 for WT vs KO (unpaired t test). FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
To determine the effect of adiponectin deficiency on fatty acid oxidation during pregnancy, OCR was examined in media containing BSA-palmitate as fatty acid substrate and utilising etomoxir to inhibit mitochondrial uptake of fatty acids. When using fatty acids as a substrate (represented as Vehicle–ETOX in Fig. 5), hepatocytes from pregnant adiponectin KO mice showed marked impairments in basal (Fig. 5b) and maximal (Fig. 5c) respiration, and their ability to produce ATP was blunted dramatically (Fig. 5d). Impairments in OCR attributed to fatty acid oxidation were more severe when hepatocytes were treated with etomoxir. Collectively, these findings suggest that primary hepatocytes from pregnant adiponectin KO mice have an impaired capacity to handle increased metabolic stress. This was not a consequence of altered mitochondrial content since citrate synthase was unchanged (Fig. 5f).
(a) Representative trace of OCR over time by primary hepatocytes utilising all substrates (+ Vehicle: BSA-palmitate 0.175 mmol/l) or all substrates except fatty acids (+ ETOX: etomoxir 4 μmol/l) and normalised to cellular protein (n = 3 wells LF WT + Vehicle, n = 3 wells LF WT + ETOX, n = 3 wells LF KO + Vehicle, n = 3 wells LF KO + ETOX). (b–d) Basal respiration (n = 5 LF WT + Vehicle, n = 5 LF WT + ETOX, n = 4 LF KO + Vehicle, n = 4 LF KO + ETOX) (b), maximal (FCCP uncoupled) respiration (n = 4 LF WT + Vehicle, n = 4 LF WT + ETOX, n = 4 LF KO Vehicle, n = 4 LF KO + ETOX) (c), and oligomycin-sensitive OCR (ATP production) (n = 4 LF WT + Vehicle, n = 4 LF WT + ETOX, n = 4 LF KO + Vehicle, n = 4 LF KO + ETOX) (d) by primary hepatocytes when respiring utilising all substrates available (vehicle), all substrates with fatty acid oxidation inhibited (ETOX), and fatty acid oxidation only (vehicle–ETOX; calculated from other groups). (e) Acid-soluble metabolites (n = 5 LF WT NP, n = 3 LF KO NP, n = 2 LF WT PG, n = 3 LF KO PG) synthesised from 14C-labelled oleate and secreted into the media by primary hepatocytes isolated from LF-fed non-pregnant and pregnant adiponectin KO and WT mice (n = 3). (f) Hepatic citrate synthase protein expression normalised to tubulin from livers of adiponectin KO and WT mice (n = 4 LF WT SAL, n = 4 LF WT INS, n = 4 LF KO SAL, n = 4 LF KO INS). †p<0.05 for WT vs KO (unpaired t test). ETOX, etomoxir; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; INS, insulin treated; NP, non-pregnant; PG, pregnant; SAL, saline treated; VEH, vehicle
Increased adiponectin during pregnancy attenuates hepatic steatosis
An adenovirus containing full-length adiponectin (Ad-APN) or GFP (Ad-GFP) as a control was administered to mice (at e14.5 when GDM is initially apparent) to reduce the potential for hyperglycaemia in the final week of pregnancy. Increased circulating total and high-molecular-weight adiponectin levels were observed 5 days post-injection in both LF- and HFS-fed adiponectin KO mice, although these levels were significantly lower than in WT mice (ESM Fig. 2e). Pregnant adiponectin KO mice administered Ad-GFP as a control had undetectable levels of adiponectin.
Ad-APN reduced hepatic lipid accumulation in both WT and adiponectin KO mice fed an LF diet, and this effect was more pronounced in mice that consumed an HFS diet (Fig. 6a, Table 1). Ad-APN also reduced hepatic triacylglycerols in pregnant HFS-fed mice (Fig. 6b). Ad-APN improved fasting blood glucose levels in pregnant LF-fed adiponectin KO mice but did not significantly reduce blood glucose levels in HFS-fed adiponectin KO mice (Fig. 6c). We also found that Ad-APN improved glucose tolerance in pregnant LF-fed adiponectin KO mice compared with GFP control mice (ESM Fig. 2c), although it was not sufficient to significantly improve the glucose tolerance of HFS-fed adiponectin KO mice (ESM Fig. 2d). Ad-APN, relative to Ad-GFP, had no effect on glucose production from pyruvate in LF-fed WT mice (Fig. 6d) but reduced glucose output in adiponectin KO mice (Fig. 6e).
(a) Representative images of H&E-stained liver sections (magnification ×20) from WT and adiponectin KO mice supplemented with adenovirus-mediated adiponectin or GFP. Scale bar, 50 μm. Livers were collected at e18.5. (b) Hepatic triacylglycerol content in LF- and HFS-fed WT and adiponectin KO mice supplemented with adenovirus-mediated adiponectin or GFP at e14.5 and sacrificed at e18.5 (n = 6 LF WT GFP, n = 6 LF WT APN, n = 6 LF KO GFP, n = 9 LF KO APN, n = 4 HFS WT GFP, n = 4 HFS WT APN, n = 4 HFS KO GFP, n = 6 HFS KO APN). (c) Fasting blood glucose at e18.5 (n = 13 LF KO GFP, n = 9 LF KO APN, n = 6 HFS KO GFP, n = 6 HFS KO APN). (d, e) PTT performed between e14.5 and 18.5 on LF-fed WT mice (n = 4 LF WT GFP, n = 5 LF WT APN) (d) and adiponectin KO mice (n = 7 LF KO GFP, n = 5 LF KO APN) (e) after supplementation with adenovirus-mediated GFP or adiponectin. Inset shows AUC. (f) Relative fold change (ΔCt) of hepatic gene expression (log10 scale) of G6pc (n = 8 LF WT GFP, n = 7 LF WT APN, n = 8 LF KO GFP, n = 10 LF KO APN), Pcx (n = 8 LF WT GFP, n = 7 LF WT APN, n = 8 LF KO GFP, n = 10 LF KO APN), Pck1 (n = 8 LF WT GFP, n = 6 LF WT APN, n = 8 LF KO GFP, n = 11 LF KO APN), Pck2 (n = 8 LF WT GFP, n = 7 LF WT APN, n = 9 LF KO GFP, n = 11 LF KO APN), Fasn (n = 9 LF WT GFP, n = 8 LF WT APN, n = 11 LF KO GFP, n = 12 LF KO APN), Dgat1 (n = 8 LF WT GFP, n = 7 LF WT APN, n = 9 LF KO GFP, n = 10 LF KO PN), Acaca (n = 8 LF WT GFP, n = 7 LF WT APN, n = 10 LF KO GFP, n = 11 LF KO APN) and Srebf1 (n = 9 LF WT GFP, n = 8 LF WT APN, n = 10 LF KO GFP, n = 12 LF KO APN) from LF-fed adiponectin KO and WT mice supplemented with adenovirus-mediated adiponectin or GFP and sacrificed at e18.5 for tissue collection. Gene expression is relative to geomean of Rn18s, B2m, Rplp-1 and Hprt1 and normalised to LF WT GFP. *p<0.05 for LF vs HFS; †p<0.05 for WT vs KO and ¶p<0.05 for GFP vs APN injection (two-way ANOVA). APN, adiponectin
Consistent with reduced hepatic steatosis, administration of Ad-APN reduced the expression of several genes involved in hepatic lipogenesis that had been elevated in adiponectin KO mice. The increased expression of Dgat1 and Fasn in pregnant adiponectin KO mice was attenuated by Ad-APN (Fig. 6f). Adiponectin KO mice administered Ad-GFP showed increased expression of Srebf1 relative to WT mice; the increase was reduced by approximately half in the pregnant Ad-APN mice, suggesting that adiponectin suppressed lipogenesis in the liver via reduced Srebf1 (Fig. 6f). Ad-APN increased hepatic Adipoq expression relative to Ad-GFP control but the increase was not statistically significant (ESM Fig. 2g). Consistent with the inhibition of hepatic glucose output, Ad-APN administration also reduced the expression of Pck1 and Pck2 in pregnant adiponectin KO mice (Fig. 6f) when compared with Ad-GFP administration. Taken together these results suggest that increasing adiponectin during pregnancy improves glucose tolerance via reduced hepatic lipid accumulation and gluconeogenesis.
Discussion
The objective of this study was to determine whether adiponectin deficiency contributes to the development of fatty liver and hyperglycaemia in pregnancy. Studies have shown low levels of circulating adiponectin to be a risk factor for GDM [22] but whether hepatic steatosis is involved is unknown. Prior to pregnancy, female adiponectin KO mice were not hyperglycaemic or glucose intolerant relative to WT controls but late in gestation adiponectin KO mice had higher fasting blood glucose levels than WT mice. This suggests that adiponectin deficiency contributes to dysglycaemia in pregnancy, even in the absence of obesity. Our findings are consistent with those of Qiao et al, who observed glucose intolerance and hyperlipidaemia in late pregnancy of adiponectin KO mice [13], although hepatic steatosis and its contribution to dysglycaemia during pregnancy was not evaluated. Notably, we observed that the impaired glucose homeostasis in adiponectin KO mice corresponded with the development of hepatic steatosis in pregnancy.
While there is evidence to implicate ectopic lipid deposition in the pathogenesis of type 2 diabetes and insulin resistance [23], less is known about its contribution to GDM. One study showed that circulating adiponectin level was inversely correlated with increasing severity of NAFLD in pregnant women [3]. Mice lacking adiponectin develop high-fat-diet-induced NAFLD more readily than WT mice, as adiponectin is known to regulate hepatic lipogenesis [24] and β-oxidation [25]. Hepatic steatosis can result from impaired lipid handling, including increased lipid uptake, altered synthesis and secretion of hepatic lipids or disrupted β-oxidation. Increased acetate uptake in adiponectin KO mice relative to WT mice agrees with previous reports that individuals with NAFLD had increased uptake of labelled acetate relative to a healthy control group [21]. Consistent with lipid accumulation in the liver, acetate incorporated into triacylglycerols was increased in hepatocytes from pregnant adiponectin KO mice.
Despite increased hepatic triacylglycerol synthesis, adiponectin KO mice had significantly less circulating triacylglycerol when fed an HFS diet in the last week of pregnancy, relative to WT mice. In the absence of hyperlipidaemia, newly synthesised lipids appear to be stored. Oleate labelling experiments showed elevated diacylglycerol in primary hepatocytes of non-pregnant adiponectin KO mice, with a significant reduction in late gestation that could be due to rapid conversion of diacylglycerol to triacylglycerols in pregnant adiponectin KO mice. Consistent with this, we observed increased expression of lipogenic genes in livers of pregnant adiponectin KO mice; these genes included Dgat1 and Gpam, which are linked to hepatic steatosis [26, 27]. Accumulation of triacylglycerol and diacylglycerol can also impair β-oxidation [28]. The disruption of β-oxidation leads to lipid peroxidation, reactive oxygen species generation, inflammation and insulin resistance [29]. We also observed impaired mitochondrial respiration in primary hepatocytes from pregnant adiponectin KO mice, especially during the oxidation of fatty acids. Reduced spare capacity and maximal respiration imply limited metabolic flexibility of adiponectin KO mice during pregnancy.
Increased lipogenesis and decreased fatty acid oxidation can lead to the development of hepatic steatosis. In the absence of adiponectin, increased lipogenesis via acetyl-CoA carboxylase leads to increased malonyl CoA production, which inhibits carnitine palmitoyl transferase-1 [30, 31]. Carnitine palmitoyl transferase-1 inhibition with etomoxir did not reduce mitochondrial respiration in primary hepatocytes, suggesting that in late pregnancy, fatty acid oxidation did not contribute significantly to hepatic respiration in adiponectin KO mice. A limitation is that these metabolic observations were from hepatocyte cultures rather than the whole animal. Nonetheless, we do show that in the absence of pregnancy hormones, the hepatocytes from adiponectin KO mice display intrinsic alterations in lipid and mitochondrial metabolism.
Women with GDM have elevated hepatic glucose output relative to healthy women [30]. Adiponectin is thought to suppress gluconeogenesis independent of insulin [17]. Consistent with this, we showed that pregnant adiponectin KO mice showed higher rates of hepatic glucose production from pyruvate compared with WT mice. Similarly, Qiao and colleagues observed increased hepatic glucose output at baseline, and impaired suppression of gluconeogenesis by insulin in pregnant adiponectin KO mice [13]. We also showed that Pck1 and Pck2 were upregulated in livers of adiponectin KO mice and their expression was not suppressed by insulin. Elevated hepatic FOXO1 increases gluconeogenic gene expression [31] while inhibition of FOXO1 improves metabolic homeostasis and glucose control [32]. In pregnant adiponectin KO mice, there was a significant increase in Foxo1 gene expression in the liver and insulin did not suppress Foxo1 expression. Collectively, these findings show that adiponectin KO mice develop hyperglycaemia, impaired glucose and insulin tolerance, and excessive hepatic glucose output in pregnancy that are characteristic of GDM.
Partial reconstitution of adiponectin in pregnancy improved fasting hyperglycaemia and glucose tolerance in LF-fed adiponectin KO mice but adiponectin was not sufficiently increased to have a significant effect in HFS-fed adiponectin KO mice. Increasing adiponectin levels in mice inhibits hepatic gluconeogenesis [19, 33,34,35]. Consistent with this, administration of Ad-APN reduced glucose production from pyruvate in pregnant adiponectin KO mice. Adiponectin could also improve blood glucose through increased placental lactogen expression, which stimulates pregnancy-associated increase in beta cell mass [36]. In this study, the adiponectin KO mice still had significantly less circulating adiponectin than WT mice, following Ad-APN administration.
A previous study showed that increased adiponectin reduced markers of fatty liver in a mouse model of alcoholic fatty liver and in ob/ob mice with NAFLD [9]. We showed that partial reconstitution of adiponectin improved hepatic fat deposition in WT and adiponectin KO mice. Surprisingly, modest increases in serum adiponectin levels in WT mice dramatically reduced hepatic steatosis. The viral gene therapy we delivered hydrodynamically through tail-vein injection has been shown to be targeted to and primarily sequestered in the liver [37, 38]. The full-length adiponectin used in our study binds with highest specificity to adipoR2, the predominant adiponectin receptor in the liver [39]. Given the marked improvements in hepatic steatosis, it is possible that many of the effects we observed were a consequence of paracrine adiponectin signalling rather than systemic effects (ESM Fig. 4).
Studies using transgenic adiponectin overexpression in mice showed reduced hepatic lipogenic gene expression [20]. We found reduced Fasn and Acaca expression in adiponectin KO mice and a fivefold reduction in Srebf1 expression in pregnant adiponectin KO mice administered Ad-APN, consistent with previously reported suppression of Srebf1 by adiponectin [24]. These findings suggest that reduction in hepatic lipogenesis could be a consequence of adiponectin-induced changes in lipogenic gene expression, which may be part of the mechanism by which Ad-APN reduces hepatic steatosis.
Overall, we found that pregnant adiponectin KO mice develop fasting hyperglycaemia and glucose intolerance characteristic of GDM and readily develop hepatic steatosis associated with dysregulated gluconeogenesis. Partial reconstitution of adiponectin in pregnancy reduced hepatic glucose output and improved fasting blood glucose in adiponectin KO mice. Further, partial reconstitution of adiponectin suppressed lipogenic gene expression and dramatically reduced hepatic lipid accumulation in pregnant WT and adiponectin KO mice. Our findings implicate adiponectin deficiency in the development of hepatic steatosis and diabetes during pregnancy.
Data availability
Data are available upon request to the corresponding author.
Abbreviations
- Ad-APN:
-
Adenovirus containing full-length adiponectin
- Ad-GFP:
-
Adenovirus containing GFP
- e:
-
Embryonic day
- FOXO1:
-
Forkhead box O1
- GDM:
-
Gestational diabetes mellitus
- GFP:
-
Green fluorescent protein
- GWAT:
-
Gonadal white adipose tissue
- HFS:
-
High-fat-and-sucrose
- KO:
-
Knockout
- LF:
-
Low-fat
- NAFLD:
-
Non-alcoholic fatty liver disease
- OCR:
-
Oxygen consumption rate
- PTT:
-
Pyruvate tolerance test
- PWAT:
-
Perirenal white adipose tissue
- WT:
-
Wild-type
References
Reece EA, Leguizamón G, Wiznitzer A (2009) Gestational diabetes: the need for a common ground. Lancet 373(9677):1789–1797. https://doi.org/10.1016/s0140-6736(09)60515-8
Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW (2018) Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci 55(2):71–101. https://doi.org/10.1080/10408363.2017.1422109
Lee S, Kwak S, Koo J et al (2019) Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia 62(2):238–248. https://doi.org/10.1007/s00125-018-4779-8
De Souza LR, Berger H, Retnakaran R et al (2016) Hepatic fat and abdominal adiposity in early pregnancy together predict impaired glucose homeostasis in mid-pregnancy. Nutr Diabetes 6(9):e229–e229. https://doi.org/10.1038/nutd.2016.39
Hershman M, Mei R, Kushner T (2019) Implications of nonalcoholic fatty liver disease on pregnancy and maternal and child outcomes. Gastroenterol Hepatol 15(4):221–228
Turer AT, Scherer PE (2012) Adiponectin: mechanistic insights and clinical implications. Diabetologia 55(9):2319–2326. https://doi.org/10.1007/s00125-012-2598-x
Asano T, Watanabe K, Kubota N et al (2009) Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J Gastroenterol Hepatol 24(10):1669–1676. https://doi.org/10.1111/j.1440-1746.2009.06039.x
Brooks NL, Trent CM, Raetzsch CF et al (2007) Low utilization of circulating glucose after food withdrawal in Snell dwarf mice. J Biol Chem 282(48):35069–35077. https://doi.org/10.1074/jbc.M700484200
Xu A, Wang Y, Keshaw H, Xu LY, Lam KSL, Cooper GJS (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112(1):91–100. https://doi.org/10.1172/JCI17797
Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA (2004) Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab 89(5):2306–2311. https://doi.org/10.1210/jc.2003-031201
Retnakaran R, Connelly PW, Maguire G, Sermer M, Zinman B, Hanley AJG (2007) Decreased high-molecular-weight adiponectin in gestational diabetes: implications for the pathophysiology of type 2 diabetes. Diabet Med 24(3):245–252. https://doi.org/10.1111/j.1464-5491.2007.02077.x
Pereira TJ, Fonseca MA, Campbell KE et al (2015) Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J Physiol 593(14):3181–3197. https://doi.org/10.1113/JP270429
Qiao L, Wattez J-S, Lee S et al (2017) Adiponectin deficiency impairs maternal metabolic adaptation to pregnancy in mice. Diabetes 66(5):1126–1135. https://doi.org/10.2337/db16-1096
Brawerman GM, Kereliuk SM, Brar N et al (2019) Maternal resveratrol administration protects against gestational diabetes-induced glucose intolerance and islet dysfunction in the rat offspring. J Physiol 597(16):4175–4192. https://doi.org/10.1113/JP278082
Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF (1981) Mouse liver cell culture. I. Hepatocyte isolation. In Vitro 17(10):913–925. https://doi.org/10.1007/bf02618288
Tsuchida A, Yamauchi T, Ito Y et al (2004) Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 279(29):30817–30822. https://doi.org/10.1074/jbc.M402367200
Zhou H, Song X, Briggs M et al (2005) Adiponectin represses gluconeogenesis independent of insulin in hepatocytes. Biochem Biophys Res Commun 338(2):793–799. https://doi.org/10.1016/j.bbrc.2005.10.007
Herrera E (2002) Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 19(1):43–55. https://doi.org/10.1385/endo:19:1:43
Yamauchi T, Kamon J, Minokoshi Y et al (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8(11):1288–1295. https://doi.org/10.1038/nm788
Shetty S, Ramos-Roman MA, Cho Y-R et al (2012) Enhanced fatty acid flux triggered by adiponectin overexpression. Endocrinology 153(1):113–122. https://doi.org/10.1210/en.2011-1339
Nejabat M, Leisser A, Karanikas G et al (2018) [11C]acetate PET as a tool for diagnosis of liver steatosis. Abdom Radiol 43(11):2963–2969. https://doi.org/10.1007/s00261-018-1558-4
Retnakaran R, Hanley AJG, Raif N, Connelly PW, Sermer M, Zinman B (2004) Reduced adiponectin concentration in women with gestational diabetes. A potential factor in progression to type 2 diabetes. J Diabetes Care 27(3):799–800. https://doi.org/10.2337/diacare.27.3.799
Snel M, Jonker JT, Schoones J et al (2012) Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol 2012:983814. https://doi.org/10.1155/2012/983814
Awazawa M, Ueki K, Inabe K et al (2009) Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 382(1):51–56. https://doi.org/10.1016/j.bbrc.2009.02.131
Stern JH, Rutkowski JM, Scherer PE (2016) Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab 23(5):770–784. https://doi.org/10.1016/j.cmet.2016.04.011
Villanueva CJ, Monetti M, Shih M et al (2009) Specific role for acyl CoA:diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology 50(2):434–442. https://doi.org/10.1002/hep.22980
Monetti M, Levin MC, Watt MJ et al (2007) Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6(1):69–78. https://doi.org/10.1016/j.cmet.2007.05.005
Lindén D, William-Olsson L, Ahnmark A et al (2006) Liver-directed overexpression of mitochondrial glycerol-3-phosphate acyltransferase results in hepatic steatosis, increased triacylglycerol secretion and reduced fatty acid oxidation. FASEB J 20(3):434–443. https://doi.org/10.1096/fj.05-4568com
Verna EC, Berk PD (2008) Role of fatty acids in the pathogenesis of obesity and fatty liver: impact of bariatric surgery. Semin Liver Dis 28(04):407–426. https://doi.org/10.1055/s-0028-1091985
Catalano PM, Huston L, Amini SB, Kalhan SC (1999) Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 180(4):903–916. https://doi.org/10.1016/S0002-9378(99)70662-9
Wang Y, Zhou Y, Graves DT (2014) FOXO transcription factors: their clinical significance and regulation. Biomed Res Int 2014:925350. https://doi.org/10.1155/2014/925350
Xie X, Yan D, Li H et al (2018) Enhancement of adiponectin ameliorates nonalcoholic fatty liver disease via inhibition of FoxO1 in type I diabetic rats. J Diabetes Res 2018:6254340. https://doi.org/10.1155/2018/6254340
Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 108(12):1875–1881. https://doi.org/10.1172/jci14120
Shklyaev S, Aslanidi G, Tennant M et al (2003) Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci U S A 100(24):14217–14222. https://doi.org/10.1073/pnas.2333912100
Ma Y, Liu D (2013) Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene Ther 20(8):846–852. https://doi.org/10.1038/gt.2013.8
Qiao L, Saget S, Lu C, Hay WW Jr, Karsenty G, Shao J (2021) Adiponectin promotes maternal β-cell expansion through placental Lactogen expression. Diabetes 70(1):132–142. https://doi.org/10.2337/db20-0471
Shayakhmetov DM, Li Z-Y, Ni S, Lieber A (2004) Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of Fiber-modified vectors. J Virol 78(10):5368. https://doi.org/10.1128/JVI.78.10.5368-5381.2004
Turner PV, Brabb T, Pekow C, Vasbinder MA (2011) Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50(5):600–613
Yamauchi T, Kamon J, Waki H et al (2003) Globular adiponectin protected Ob/Ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278(4):2461–2468. https://doi.org/10.1074/jbc.M209033200
Acknowledgements
The authors acknowledge the excellent technical support given by M. Vandal, G. Brawerman and O. Kotova of the University of Manitoba. Some of these data were previously presented as an abstract at the 15th annual Child Health Research Days meeting in 2019.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
BLMG was the recipient of a CHRIM/Research Manitoba studentship. LKC was the recipient of a CIHR/HSFC IMPACT Fellowship. CAD is the Dr. J. A. Moorhouse Fellow of the Diabetes Foundation of Manitoba. VWD is the Allen Rouse-Manitoba Medical Services Foundation Basic Scientist. This research is supported by a Canadian Institutes for Health Research Grant MOP no.136885 and funding from Research Manitoba.
Author information
Authors and Affiliations
Contributions
VWD conceptualised the study and was responsible for funding acquisition. Investigations were primarily designed and carried out by BLMG. BLMG carried out data curation, formal analysis and visualisations. LKC performed cell culture, thin-layer chromatography and Seahorse XF-24 experiments on primary hepatocytes isolated by BLMG and assisted with preliminary data analysis. BX and BLMG performed glucose and insulin tolerance tests, tail-vein injection in pregnant mice and ultrasound detection of pregnancy. MAF performed data acquisition. JK performed blinded histopathological analysis of liver samples and assessed degree of steatosis. CAD and GMH performed analysis and interpretation of the data and contributed to writing. BLMG and VWD were responsible for writing the paper and all authors reviewed, edited and approved the manuscript. VWD is responsible for the integrity of the work as a whole.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 5.31 MB)
Rights and permissions
About this article
Cite this article
Moyce Gruber, B.L., Cole, L.K., Xiang, B. et al. Adiponectin deficiency induces hepatic steatosis during pregnancy and gestational diabetes in mice. Diabetologia 65, 733–747 (2022). https://doi.org/10.1007/s00125-021-05649-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05649-3