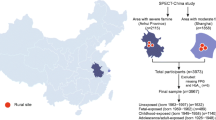
Abstract
Aims/hypothesis
Early famine exposure has been related to the development of type 2 diabetes; however, little is known about whether the genetic background modifies this association. We aimed to investigate the joint effects of famine exposure at different stages of early life and genetic susceptibility on diabetes risk in adulthood.
Methods
The study included 8350 participants from the Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China) who were born around the time of the Chinese Great Famine. We determined famine exposure subgroups according to the birth year as nonexposed (1963–1974), fetal-exposed (1959–1962), childhood-exposed (1949–1958), and adolescence-exposed (1941–1948). We developed a genetic risk score of 21 variants previously associated with type 2 diabetes in East Asians. Hierarchical logistic models were used to examine the association of famine exposure and genetic risk with diabetes.
Results
The age-standardised prevalence of diabetes in nonexposed, fetal-exposed, childhood-exposed and adolescence-exposed subgroups was 13.0%, 18.2%, 15.1% and 13.2%, respectively. Compared with nonexposed participants, fetal-exposed participants showed an increased risk of diabetes in adulthood (OR 1.47; 95% CI 1.13, 1.93). A higher genetic risk score was associated with an increased risk of diabetes (OR 1.23; 95% CI 1.15, 1.31 per SD increment). The association between famine exposure and diabetes was consistent across genetic risk strata (all p for interaction >0.05). When considered jointly, fetal- or childhood-exposed participants at high genetic risk (highest tertile of genetic risk score) had 2.60-fold (95% CI 1.71, 3.93) and 1.95-fold (95% CI 1.24, 3.05) higher risks of diabetes, respectively, compared with nonexposed participants at low genetic risk (lowest tertile).
Conclusions/interpretations
Prenatal exposure to famine was associated with an increased risk of type 2 diabetes in Chinese adults independent of genetic risk score using 21 variants common in the East Asian population. Famine exposure and genetic susceptibility may exhibit an additive effect on diabetes development.
Graphical abstract

Similar content being viewed by others

Introduction
Type 2 diabetes poses a major public health threat worldwide. An estimated 463 million people were living with type 2 diabetes in 2019, and China has become the epicentre of the diabetes epidemic [1]. The increasing burden of diabetes is primarily attributed to drastic shifts in lifestyle toward sedentary behaviours and Western-pattern diets [2]. Additionally, the developmental origins hypothesis postulates that malnutrition during fetal development may also predispose to type 2 diabetes in later life [3, 4]. Previous studies of the Dutch famine and the Ukraine famine reported that intrauterine exposure to starvation was associated with an elevated risk of high blood glucose and diabetes in adulthood [5, 6]. The Chinese Great Famine of 1959–1962 is regarded as one of the largest famines in human history and caused millions of deaths [7]. Early-life exposure to the Chinese famine has also been associated with an increased adulthood risk of type 2 diabetes [8,9,10].
Type 2 diabetes is a multifactorial disease driven by both genetic and environmental factors [11]. Several studies have examined the interaction between low birthweight (a surrogate measure for fetal growth retardation) and high-risk phenotypes in the development of diabetes, but have yielded mixed results [12, 13]. Data from the Dutch Famine Birth Cohort showed that certain genetic variants may interact with fetal exposure to famine to affect glucose metabolism or diabetes risk [14,15,16]. However, such work has been based on a relatively small sample that target specific genes. A comprehensive assessment of the impact of genetic susceptibility on type 2 diabetes in relation to famine exposure is lacking. Over the past decade, genome-wide association studies (GWAS) have identified a series of variants associated with type 2 diabetes. Combining these identified risk alleles for analysis facilitates a quantitative measure of overall genetic susceptibility [17]. In addition, considering racial difference in genetic composition, evidence derived from populations of European ancestry might not be applied to other ethnic populations. Thus, the interplay between genetic susceptibility and famine exposure on type 2 diabetes in Chinese adults remains to be determined.
In this study, we analysed data from the Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China) to evaluate whether exposure to the Chinese Great Famine in early life could interact with genetic predisposition to increase the risk of type 2 diabetes in adulthood. We then examined the joint association of famine exposure and genetic predisposition with type 2 diabetes.
Methods
Study population
The SPECT-China study is a population-based study investigating the prevalence of metabolic diseases and related risk factors in East China. Details of the study design and survey methods have been described elsewhere [18, 19]. Briefly, a stratified cluster sampling method was used to select a sample from the general population at 23 sites across Shanghai, Zhejiang, Jiangsu, Anhui and Jiangxi Province. The sampling process was stratified by urban/rural areas and economic status. Generally, in urban areas, one city with low economic status and one with high economic status were randomly selected. Three districts were randomly selected from each city, and one community was then randomly sampled from each of the districts. In rural areas, six villages with low economic status and six with high economic status were randomly selected. All eligible participants in each region were identified through local residential records and were invited during house visits by community health workers, following extensive advertising campaigns. A total of 12,666 Chinese adults aged ≥18 years were recruited for the study during 2014–2016 (response rate > 90%). Valid data included a completed questionnaire and physical measurements and biological samples were required for genetic testing. The study protocol was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. All participants provided written informed consent.
For the present study, we restricted the analysis to participants who were born between 1 January 1941 and 31 December 1974 (n = 10,211). We excluded 1652 participants because sufficient blood samples were not available for genotyping. We further excluded participants with missing data on more than two SNP genotypes (n = 205) and plasma glucose measures (n = 4), leaving 8350 eligible participants for the current analysis (Fig. 1).
Data collection and measurements
Questionnaires were administered and anthropometric measurements were taken by the same trained staff according to a standard protocol at each study site. Information on sociodemographic characteristics, lifestyle factors (e.g. smoking, alcohol consumption and physical activity), and medical history was obtained from a questionnaire administered through personal interviews. Regional economic status was assessed by the gross domestic product per capita at each site and categorised into high and low economic status based on the national level in 2013 ($6807 per capita from the World Bank) [19]. Body weight, height, waist circumference and BP were measured by trained technicians using calibrated instruments with standard procedures. BMI was calculated as body weight in kilograms divided by height in metres squared (kg/m2).
Venous blood samples were drawn after an overnight fast of at least 8 h. After centrifugation on site within 2 h of collection, blood specimens were shipped in dry ice to a central laboratory certified by the College of American Pathologists. Fasting plasma glucose was measured using the hexokinase method on an autoanalyser (AU 680, Beckman Coulter, Brea, USA). HbA1c was measured by HPLC (MQ-2000PT, Medconn, China). Serum total cholesterol and triacylglycerols were measured with a Beckman Coulter AU 680 Analyzer (Beckman Coulter, Brea, USA).
Assessment of famine exposure
According to the date of birth reported at enrolment, we categorised participants into four famine exposure subgroups: nonexposed (born between 1 January 1963 and 31 December 1974), fetal-exposed (born between 1 January 1959 and 31 December 1962), childhood-exposed (born between 1 January 1949 and 31 December 1958), and adolescence-exposed (born between 1 January 1941 and 31 December 1948), as described in previous studies [9, 19, 20].
Genotyping
Details of the genotyping process and arrays have been described elsewhere [21]. Briefly, DNA was extracted from white blood cells using a commercial blood genomic DNA extraction kit (DP603, TIANGEN BIOTECH Beijing, China) on an automated nucleic acid extraction instrument (YOSE-S32, TIANGEN BIOTECH, Beijing, China) according to the manufacturer’s standard protocol. Specific assays were designed using the Geneious Pro v4.8.3 software (https://www.geneious.com/). SNP genotyping was carried out with the Juno 96.96 Dynamic Array integrated fluidic circuits and the BioMark HD system (Fluidigm Corporation, South San Francisco, CA). Data were analysed using Fluidigm SNP Genotyping Analysis v4.1.3 software. The call rates of all SNPs were >98%.
Construction of genetic risk score
We selected SNPs associated with type 2 diabetes that have been identified from large-scale GWAS and meta-analysis in East Asians [22, 23] and then successfully used to construct diabetes genetic risk scores in Chinese adults [24]. Because of limited funds, we gave preference to those SNPs without pleiotropic effects as previously reported [24] to facilitate mendelian randomisation analysis in our population. Therefore, 21 SNPs were selected for genotyping. They all reached a genome-wide significance level (p < 5× 10−8) and were not in linkage disequilibrium (r2 = 0). The full list of the SNPs is presented in Electronic supplementary material (ESM) Table 1.
Genetic risk scores were created following an additive model, defined as the sum of the number of risk alleles (0, 1 or 2) at each SNP weighted by the respective effect size (log of the OR) on the risk of diabetes based on previous GWAS [22]. We excluded participants with more than two SNPs missing. For those who were missing one or two SNPs, the mean genotype of that variant was assigned. The weighted genetic risk score ranges from 11.0 to 35.4, with higher scores indicating a higher genetic predisposition to type 2 diabetes.
Ascertainment of type 2 diabetes
In accordance with the 2010 ADA criteria [25], type 2 diabetes was defined as fasting plasma glucose ≥7.0 mmol/l and/or HbA1c ≥ 48 mmol/mol (6.5%) and/or a self-reported previous diagnosis by healthcare professionals. The validity of reported diabetes diagnosis was affirmed by reviewing medical records.
Statistical analyses
Baseline characteristics of participants across famine exposure subgroups were summarised as mean ± SD or median (IQR) for continuous variables and number (percentages) for categorical variables. Hierarchical logistic regression models were used to examine the association of famine exposure and genetic risk with type 2 diabetes while accounting for the clustering of participants within each sampling site and for fixed regional-level economic data. Genetic risk was classified as low risk, intermediate risk and high risk based on genetic risk score in tertiles. For multivariable-adjusted analyses, model 1 was adjusted for age (years) and sex; model 2 was further adjusted for education (less than high school or high school or above), smoking status (never, former or current), drinking status (never, former or current), family history of diabetes (yes or no), physical activity (none, mild or moderate to vigorous) and economic status (low or high); model 3 included variables in model 2 plus BMI (kg/m2), systolic BP (mmHg) and total cholesterol (mmol/l). In the analysis of famine exposure, we additionally adjusted for genetic risk score in model 4. Similarly, famine exposure was additionally adjusted for the analysis of genetic risk.
We used hierarchical logistic regression to estimate prevalence rate of diabetes according to famine exposure and genetic risk, which were standardised to the mean of all predictor variables. The association between famine exposure and diabetes was evaluated across genetic risk strata. A combined analysis of famine exposure and genetic risk in relation to diabetes was further performed, with nonexposed participants at low genetic risk as the reference. We tested interactions of famine exposure and genetic risk using a likelihood ratio test comparing models with and without the cross-product term. Meanwhile, interaction with a single SNP was assessed, and the Benjamini–Hochberg false discovery rate (FDR) method was used to correct for multiple testing. To minimise the bias derived from age differences between famine-exposed and nonexposed groups, we adopted an ‘age-balanced’ method by combining postfamine and prefamine births into the control group [26].
Since the exact beginning and end of the Chinese famine is unknown, we conducted a sensitivity analysis by excluding participants born in 1959 and 1962 to reduce potential misclassification of the exposure periods. Furthermore, we repeated the analyses by creating an extensive genetic risk score based on 35 SNPs available in a subsample of participants. A two-sided p value <0.05 was considered statistically significant. All statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC).
Results
Participant characteristics
Of the 8350 participants with complete data, 3306 (39.6%) were men, and the mean ± SD age was 57.2 ± 8.9 years. Baseline characteristics of participants are shown in Table 1. In the study population, 1113 (13.3%) had been exposed to the Chinese famine during fetal life, 3131 (37.5%) had been exposed during childhood, and 1472 (17.6%) had been exposed during adolescence. As expected, famine-exposed participants were significantly older than nonexposed participants. Compared with nonexposed participants, fetal-exposed participants were more likely to smoke and to have a family history of diabetes, while childhood-exposed and adolescence-exposed participants were less educated and more likely to be men. In general, famine-exposed participants had poorer metabolic measures, including higher BMI, BP, fasting glucose, HbA1c, total cholesterol and triacylglycerols. There was no significant difference in genetic risk score between the nonexposed group and famine-exposed subgroups.
Early-life famine exposure and type 2 diabetes
The overall prevalence of type 2 diabetes was 16.4%. Of the cases, 55.6% were self-reported. Participants exposed to famine in the fetal stage had a higher risk of diabetes in adulthood than those not exposed to famine (Table 2). In model 1, adjusting for age and sex, the ORs (95% CIs) of diabetes were 1.50 (1.15, 1.94), 1.21 (0.84, 1.73) and 1.03 (0.60, 1.75) among fetal-exposed, childhood-exposed and adolescence-exposed participants, respectively, compared with nonexposed participants. Additional adjustment for other covariates including socioeconomic status, lifestyles, BMI, BP and total cholesterol did not appreciably change the results. The multivariable-adjusted OR (95% CI) was 1.47 (1.13, 1.93) in fetal-exposed participants vs nonexposed participants, which was unchanged after further adjustment for genetic risk score. When compared with prefamine and postfamine births combined in the age-balanced analysis, fetal-exposed participants still showed an increased risk of diabetes (ESM Table 2).
Genetic risk score and type 2 diabetes
Genetic risk scores approximated a normal distribution (ESM Fig. 1). The OR (95% CI) of diabetes associated with per SD (3.19 points) increase in diabetes genotype score was 1.22 (1.15, 1.30) after adjustment for age and sex (Table 2). Additional adjustment for covariates had little effect on this association. A graded risk of diabetes was observed across higher categories of genetic risk score. The adjusted ORs (95% CIs) comparing participants at intermediate and high genetic risk with those at low genetic risk were 1.24 (1.06, 1.45) and 1.56 (1.34, 1.81), respectively (p for linear trend <0.001). Likewise, the estimates remained essentially unchanged after adjustment for famine exposure.
Early-life famine exposure, genetic risk score and type 2 diabetes
We then examined the association of famine exposure and diabetes by genetic risk category (ESM Table 3). Among participants at high genetic risk, fetal exposure to famine was associated with a significantly increased risk of diabetes (OR 1.74; 95% CI 1.12, 2.70). There were no significant interactions between famine exposure at different stages and genetic risk category (all p for interaction >0.05). We also did not find interactions between any single SNP and famine exposure on diabetes risk (FDR >0.05). The standardised prevalence of diabetes according to famine exposure and genetic risk category is depicted in Fig. 2. Among participants at low genetic risk, the standardised prevalence of diabetes was 9.6% in nonexposed individuals, 14.2% in fetal-exposed individuals, 11.7% in childhood-exposed individuals, and 10.3% in adolescence-exposed individuals, which increased to 14.5%, 21.2%, 17.4% and 14.9%, respectively, among those at high genetic risk. When considered jointly, the highest odds of diabetes was observed in fetal-exposed participants at high genetic risk (Fig. 3). Compared with no exposure among participants at low genetic risk, fetal exposure to famine was associated with adjusted ORs (95% CIs) of 1.56 (1.01, 2.40) among those at low genetic risk, 1.67 (1.09, 2.54) among those at intermediate genetic risk, and 2.60 (1.71, 3.93) among those at high genetic risk. Participants at high genetic risk with childhood famine exposure also showed an increased risk of diabetes (OR 1.95; 95% CI 1.24, 3.05). The results did not markedly differ by sex (p for interaction >0.05), even though the risk estimates in childhood- and adolescence-exposed subgroups were greater in women (ESM Fig. 2).
Multivariable-adjusted ORs (95% CIs) for type 2 diabetes according to famine exposure and genetic risk. Participants at low genetic risk without famine exposure served as the reference group. The models were adjusted for age, sex, education level, smoking status, drinking status, family history of diabetes, physical activity, economic status, BMI, systolic BP and total cholesterol
Sensitivity analyses excluding individuals born in 1959 and 1962 yielded consistent results (data not shown). Furthermore, in a subset of 3768 participants using an extensive genetic risk score of 35 SNPs, we observed stronger genetic associations and joint effects of genetic risk and famine exposure on diabetes risk (ESM Table 4).
Discussion
In this population-based study of Chinese adults, prenatal exposure to famine and higher genotype scores, based on 21 variants common in the East Asian population, were independently associated with an increased risk of type 2 diabetes in adulthood. The relative effects of famine exposure on diabetes were comparable across genetic risk categories. Coexistence of prenatal famine exposure and high genetic risk were associated with a more than 2.5-fold increased risk of type 2 diabetes. Our findings extend the current knowledge by generating quantitative data about the potential interplay between famine exposure and genetic susceptibility on type 2 diabetes development.
The association of famine exposure in early life and risk of type 2 diabetes has been reported previously [6, 8,9,10]. Findings from the Ukraine famine study showed a 1.5-times increase in odds of developing type 2 diabetes in adulthood among individuals with famine exposure during fetal life [6]. The China Kadoorie Biobank study demonstrated that fetal exposure to the Chinese Great Famine was associated with an increased risk of type 2 diabetes [8]. Recently, results from a nationwide cohort of Chinese adults confirmed the adverse effect of fetal famine exposure on the incidence of type 2 diabetes [9]. Our previous study reported a relationship between early famine exposure and diabetes development using births from three areas in East China [27]. In this study, we added two areas (Jiangsu and Anhui Province) to provide diversity in exposure and disease patterns. Consistently, we found an increased adulthood risk of type 2 diabetes in fetal-exposed individuals than in nonexposed individuals. To tackle the bias from age differences between famine births and control births, we applied an age-balanced analysis by combining pre- and postfamine births and still observed significantly increased risk of diabetes associated with fetal famine exposure. Adjustment for BMI, BP and total cholesterol did not alter the results; thus, the effect of famine exposure might not be mediated by these cardiometabolic indicators. Our study, together with other studies, suggests the fetal period is a critical timing window for determining the risk of type 2 diabetes in adult life.
Both genetic and environmental factors contribute to the progression to type 2 diabetes [11]. However, evidence regarding the influence of genetic susceptibility on the association between famine exposure and type 2 diabetes is limited. Several studies based on the Dutch Famine Birth Cohort showed that variants in PPARG2 and IGF2BP2 gene may interact with prenatal exposure to famine to affect glucose metabolism [14, 15]. Similarly, another study conducted in the same population suggested an interaction between SIRT1 variants and prenatal famine exposure in the risk of type 2 diabetes [16]. In the current study, we quantified genetic susceptibility using a genetic risk score comprising 21 diabetes variants that are common in East Asians. The observed robust genetic association with a higher risk of type 2 diabetes was well aligned with previous studies of Chinese populations [24, 28]. We then performed stratified analysis by genetic risk categories and detected no meaningful interactions between famine exposure and genetic susceptibility to diabetes risk, although the estimates appeared to be a little more pronounced in individuals at high genetic risk. However, because we did not include the relevant loci (i.e. IGF2BP2, SIRT1) with evidence of effect modification, such findings are not comparable.
It is noteworthy that genetic susceptibility and famine exposure might exert an additive effect on the development of type 2 diabetes. Individuals with both a high genetic risk and fetal famine exposure had the highest risk of type 2 diabetes. These findings support the notion that undernutrition during fetal development may increase predisposition to type 2 diabetes in adulthood regardless of genetic risk, which implies the importance of active interventions (i.e. lifestyle modification) for preventing type 2 diabetes across genetic risk gradients in populations who experienced fetal undernutrition. An alternative approach is to target intensive measures to those at high genetic risk.
Several potential mechanisms underlying the association of early famine exposure and type 2 diabetes in later life have been proposed. The developmental origins hypothesis postulates that adaptations in response to fetal malnutrition cause long-term metabolic changes [3, 29]. Imbalanced maternal nutrition during gestation in animal models could induce pancreatic beta cell dysfunction in offspring through epigenetic modification [30]. In individuals prenatally exposed to the Dutch famine, differential DNA methylation of the imprinted IGF2 gene and the candidate genes involved in growth and metabolism were reported, suggesting an important role of epigenetic modulation in promoting metabolic disorders associated with prenatal malnutrition [31,32,33]. The epigenetic changes resulting from famine exposure may theoretically overlap with pathways linking genetic variations to type 2 diabetes [29]. Because of the limited variants in our analysis, it might not be possible to uncover potential interactions that are driven by specific modulations of biological pathways or mechanisms. The interplay between famine exposure and genetic susceptibility to type 2 diabetes continues to be elucidated by the inclusion of more relevant loci.
To our knowledge, this study is the first to investigate the joint effects of early famine exposure and genetic susceptibility on the risk of type 2 diabetes in adulthood. The strengths of this study include high-quality control across centres and comprehensive adjustments for covariates. Questionnaire surveys, anthropometric measurements and genotyping were performed by the same trained staff and laboratory technicians. Besides, inclusion of a geographically diverse population from urban and rural areas makes our sample representative. Since the participants are mostly (>99%) Han people, bias from population stratification is minimal.
Our study has several limitations. Because the exact beginning and end of the Chinese famine is unknown, misclassification of famine exposure was inevitable. However, famine exposure is commonly defined using birth dates. Our results were also robust after excluding participants born in 1 year between three famine exposure subgroups. Furthermore, the lack of postprandial glucose measures resulted in some underestimation of diabetes cases. Such underestimation is likely to be nondifferential and would attenuate effect estimates. We constructed the genetic risk score based on only 21 common variants, which may not reflect the full diabetes genetic profile. However, a similar genetic association was observed in the sensitivity analysis by including 35 SNPs in a subsample. As the incidence of diabetes is highly correlated with ageing, it is challenging to disentangle the effect of famine exposure from the ageing effect. Although age-balanced analysis as recommended by previous studies provided more convincing results, there might be limitations of combining the age groups. In addition, the relatively small sample size within strata according to genetic risk and famine exposure may reduce statistical power to detect significant interactions, especially when the effects are modest. Finally, we did not collect information on several factors potentially affecting early exposure and diabetes risk such as maternal health and adult dietary patterns. Therefore, the possibility of residual confounding cannot be ruled out. The present analyses were performed in Chinese individuals using SNPs identified in East Asians; hence, our results might not be directly generalisable to other ethnic populations.
In conclusion, we found that prenatal exposure to famine was associated with an increased risk of type 2 diabetes in Chinese adults. Early famine exposure and genetic susceptibility, measured by a genetic risk score using 21 variants common in the East Asian population, exhibited an additive effect on diabetes development without evidence of potential interactions. Our findings support public health efforts that emphasise active interventions to reduce diabetes risk for all who experienced fetal malnutrition, although intensive measures may be targeted to those with high-risk genetic profiles.
Data availability
Data are available on request from the corresponding author.
Abbreviations
- FDR:
-
False discovery rate
- GWAS:
-
Genome-wide association studies
References
International Diabetes Federation (2019) IDF diabetes atlas, 9th edition. Brussels. http://www.diabetesatlas.org/. Accessed 12 Sept 2020
Ley SH, Hamdy O, Mohan V, Hu FB (2014) Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383:1999–2007. https://doi.org/10.1016/S0140-6736(14)60613-9
Gluckman PD, Hanson MA, Bateson P et al (2009) Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet 373:1654–1657. https://doi.org/10.1016/S0140-6736(09)60234-8
Berends LM, Ozanne SE (2012) Early determinants of type-2 diabetes. Best Pract Res Clin Endocrinol Metab 26:569–580. https://doi.org/10.1016/j.beem.2012.03.002
Ravelli AC, van der Meulen JH, Michels RP et al (1998) Glucose tolerance in adults after prenatal exposure to famine. Lancet 351:173–177. https://doi.org/10.1016/S0140-6736(97)07244-9
Lumey LH, Khalangot MD, Vaiserman AM (2015) Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes Endocrinol 3:787–794. https://doi.org/10.1016/S2213-8587(15)00279-X
Smil V (1999) China’s great famine: 40 years later. BMJ 319:1619–1621. https://doi.org/10.1136/bmj.319.7225.1619
Meng R, Lv J, Yu C et al (2018) China Kadoorie Biobank Collaborative Group. Prenatal famine exposure, adulthood obesity patterns and risk of type 2 diabetes. Int J Epidemiol 47:399–408. https://doi.org/10.1093/ije/dyx228
Lu J, Li M, Xu Y et al (2020) Early life famine exposure, ideal cardiovascular health metrics, and risk of incident diabetes: findings from the 4C study. Diabetes Care 43:1902–1909. https://doi.org/10.2337/dc19-2325
Wang J, Li Y, Han X et al (2016) Exposure to the Chinese famine in childhood increases type 2 diabetes risk in adults. J Nutr 146:2289–2295. https://doi.org/10.3945/jn.116.234575
Franks PW, McCarthy MI (2016) Exposing the exposures responsible for type 2 diabetes and obesity. Science 354:69–73. https://doi.org/10.1126/science.aaf5094
Pulizzi N, Lyssenko V, Jonsson A et al (2009) Interaction between prenatal growth and high-risk genotypes in the development of type 2 diabetes. Diabetologia 52:825–829. https://doi.org/10.1007/s00125-009-1291-1
Li Y, Qi Q, Workalemahu T, Hu FB, Qi L (2012) Birth weight, genetic susceptibility, and adulthood risk of type 2 diabetes. Diabetes Care 35:2479–2484. https://doi.org/10.2337/dc12-0168
de Rooij SR, Painter RC, Phillips DI et al (2006) The effects of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma2 gene on glucose/insulin metabolism interact with prenatal exposure to famine. Diabetes Care 29:1052–1057. https://doi.org/10.2337/dc05-1993
van Hoek M, Langendonk JG, de Rooij SR, Sijbrands EJ, Roseboom TJ (2009) Genetic variant in the IGF2BP2 gene may interact with fetal malnutrition to affect glucose metabolism. Diabetes 58:1440–1444. https://doi.org/10.2337/db08-1173
Botden IP, Zillikens MC, de Rooij SR et al (2012) Variants in the SIRT1 gene may affect diabetes risk in interaction with prenatal exposure to famine. Diabetes Care 35:424–426. https://doi.org/10.2337/dc11-1203
Dietrich S, Jacobs S, Zheng JS, Meidtner K, Schwingshackl L, Schulze MB (2019) Gene-lifestyle interaction on risk of type 2 diabetes: a systematic review. Obes Rev 20:1557–1571. https://doi.org/10.1111/obr.12921
Wang N, Cheng J, Ning Z et al (2018) Type 2 diabetes and adiposity induce different lipid profile disorders: a Mendelian randomization analysis. J Clin Endocrinol Metab 103:2016–2025. https://doi.org/10.1210/jc.2017-02789
Wang N, Wang X, Li Q et al (2017) The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr 36:253–259. https://doi.org/10.1016/j.clnu.2015.11.010
Du R, Zheng R, Xu Y et al (2020) Early-life famine exposure and risk of cardiovascular diseases in later life: findings from the REACTION study. J Am Heart Assoc 9:e014175. https://doi.org/10.1161/JAHA.119.014175
Chen C, Zhai H, Cheng J et al (2019) Causal link between vitamin D and total testosterone in men: a Mendelian randomization analysis. J Clin Endocrinol Metab 104:3148–3156. https://doi.org/10.1210/jc.2018-01874
Cho YS, Lee JY, Park KS, Nho CW (2012) Genetics of type 2 diabetes in East Asian populations. Curr Diab Rep 12:686–696. https://doi.org/10.1007/s11892-012-0326-z
Cho YS, Chen CH, Hu C et al (2011) Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet 44:67–72. https://doi.org/10.1038/ng.1019
Xu M, Huang Y, Xie L et al (2016) Diabetes and risk of arterial stiffness: a Mendelian randomization analysis. Diabetes 65:1731–1740. https://doi.org/10.2337/db15-1533
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33:S62–S69
Li C, Tobi EW, Heijmans BT, Lumey LH (2019) The effect of the Chinese Famine on type 2 diabetes mellitus epidemics. Nat Rev Endocrinol 15:313–314. https://doi.org/10.1038/s41574-019-0195-5
Wang N, Wang X, Han B et al (2015) Is exposure to famine in childhood and economic development in adulthood associated with diabetes? J Clin Endocrinol Metab 100:4514–4523. https://doi.org/10.1210/jc.2015-2750
Li H, Khor CC, Fan J et al (2020) Genetic risk, adherence to a healthy lifestyle, and type 2 diabetes risk among 550,000 Chinese adults: results from 2 independent Asian cohorts. Am J Clin Nutr 111:698–707. https://doi.org/10.1093/ajcn/nqz310
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73. https://doi.org/10.1056/NEJMra0708473
Sandovici I, Hammerle CM, Ozanne SE, Constancia M (2013) Developmental and environmental epigenetic programming of the endocrine pancreas: consequences for type 2 diabetes. Cell Mol Life Sci 70:1575–1595. https://doi.org/10.1007/s00018-013-1297-1
Tobi EW, Slieker RC, Stein AD et al (2015) Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. Int J Epidemiol 44:1211–1223. https://doi.org/10.1093/ije/dyv043
Heijmans BT, Tobi EW, Stein AD et al (2008) Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 105:17046–17049
Tobi EW, Goeman JJ, Monajemi R et al (2014) DNA methylation signatures link prenatal famine exposure to growth and metabolism [published correction in Nat Commun 2015;6:7740]. Nat Commun 5:5592
Acknowledgements
The investigators are grateful to all participants for their cooperation in the study.
Authors' relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This study was supported by the National Natural Science Foundation of China (91857117, 81600614); Science and Technology Commission of Shanghai Municipality (19140902400, 18410722300); the Major Science and Technology Innovation Program of Shanghai Municipal Education Commission (2019-01-07-00-01-E00059); Commission of Health and Family Planning of Pudong District (PWZxq2017-17); Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ053); and the Shanghai JiaoTong University School of Medicine (19XJ11007).
Author information
Authors and Affiliations
Contributions
BW, NW and YL conceived and designed the study. BW and JC analysed the data and drafted the manuscript. All authors contributed to data interpretation and editing of the manuscript. NW and YL critically revised the manuscript for important intellectual content. All authors have approved the final version to be published. YL is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 424 kb)
Rights and permissions
About this article
Cite this article
Wang, B., Cheng, J., Wan, H. et al. Early-life exposure to the Chinese famine, genetic susceptibility and the risk of type 2 diabetes in adulthood. Diabetologia 64, 1766–1774 (2021). https://doi.org/10.1007/s00125-021-05455-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05455-x