Abstract
Aims/hypothesis
Wingless-type (Wnt) inducible signalling pathway protein-1 (WISP1) has been recently identified as a proinflammatory adipokine. We examined whether WISP1 expression and circulating levels are altered in type 2 diabetes and whether WISP1 affects insulin signalling in muscle cells and hepatocytes.
Methods
Serum and visceral adipose tissue (VAT) biopsies, for analysis of circulating WISP1 levels by ELISA and WISP1 mRNA expression by real-time quantitative RT-PCR, were collected from normal-weight men (control group, n = 33) and obese men with (n = 46) and without type 2 diabetes (n = 56) undergoing surgery. Following incubation of primary human skeletal muscle cells (hSkMCs) and murine AML12 hepatocytes with WISP1 and insulin, insulin signalling was analysed by western blotting. The effect of WISP1 on insulin-stimulated glycogen synthesis and gluconeogenesis was investigated in hSkMCs and murine hepatocytes, respectively.
Results
Circulating WISP1 levels were higher in obese men (independent of diabetes status) than in normal-weight men (mean [95% CI]: 70.8 [55.2, 86.4] ng/l vs 42.6 [28.5, 56.6] ng/l, respectively; p < 0.05). VAT WISP1 expression was 1.9-fold higher in obese men vs normal-weight men (p < 0.05). Circulating WISP1 levels were positively associated with blood glucose in the OGTT and circulating haem oxygenase-1 and negatively associated with adiponectin levels. In hSkMCs and AML12 hepatocytes, recombinant WISP1 impaired insulin action by inhibiting phosphorylation of insulin receptor, Akt and its substrates glycogen synthase kinase 3β, FOXO1 and p70S6 kinase, and inhibiting insulin-stimulated glycogen synthesis and suppression of gluconeogenic genes.
Conclusions/interpretation
Circulating WISP1 levels and WISP1 expression in VAT are increased in obesity independent of glycaemic status. Furthermore, WISP1 impaired insulin signalling in muscle and liver cells.
Similar content being viewed by others

Introduction
The wingless-type (Wnt) signalling pathway is closely associated with the pathophysiology of cancers and has been linked to adipogenesis and hypertrophic obesity [1, 2]. Regulation of the Wnt signalling system is complex and involves multiple components, including 19 different Wnt ligands that can bind to a family of ten distinct frizzled receptors and seven families of co-receptors, the most well-known being the LDL receptor-related proteins (LRPs) 5 and 6 [3]. Numerous secreted proteins that antagonise Wnt signalling by preventing ligand–receptor interactions have been identified, including members of the secreted frizzled-related protein (sFRP) and Dickkopf families [4]. Depending on the ligand–receptor combination, activation of Wnt signalling pathways results in the activation of either the canonical or the less-well-characterised non-canonical Wnt pathway [5, 6]. In the canonical pathway, activation of the frizzled receptors and LRP5/6 leads to inhibition of the activity of cytosolic glycogen synthase kinase 3β (GSK3β) [7, 8]. This results in stabilisation of the transcriptional regulator β-catenin and induction of the expression of Wnt target genes [5, 6]. Importantly, in vitro studies on pre-adipocytes report an association between alterations in Wnt and LRP5 activity and the regulation of the insulin-mediated phosphorylation of Akt and its substrate GSK3β, suggesting that changes in the activity of Wnt signalling may participate in the pathophysiology of insulin resistance and type 2 diabetes [9].
Adipocytes have been found to express and/or release many targets and regulators of the Wnt signalling pathway, including Wnt-inducible signalling protein (WISP) 2, sFRP1, sFRP2, sFRP4 and sFRP5 [10,11,12]. Moreover, expression and release of these proteins is altered in obesity [10,11,12]. Recently, we identified WISP1 (also known as CCN4) as an adipokine in human and murine adipose tissue [13]. Cross-sectional observations in glucose-tolerant humans have demonstrated that WISP1 mRNA levels in adipose tissue are positively associated with fasting insulin levels and macrophage infiltration in adipose tissue and are associated negatively with insulin sensitivity, as measured by hyperinsulinaemic–euglycaemic clamp [13]. Furthermore, WISP1 expression is affected by diet-induced changes in body weight [13]. In humans, weight loss led to a reduction in WISP1 expression, whereas feeding mice a high-fat diet increased the expression of WISP1 in adipose tissue [13]. A study in women with gestational diabetes and insulin resistance showed increased circulating levels of WISP1 and identified BMI and insulin resistance as determinants of WISP1 levels [14]. Another very recently published study observed a direct relationship between circulating WISP1 levels and visceral adiposity and signs of insulin resistance, independently of glucose tolerance [15].
Despite these observations, data on the potential relevance of WISP1 in the pathophysiology of insulin resistance and type 2 diabetes are limited. This study examined whether circulating levels of WISP1 and its expression in visceral adipose tissue (VAT) are altered in obese men with or without type 2 diabetes vs normal-weight men. Furthermore, we investigated in vitro whether WISP1 impairs insulin action in primary human skeletal muscle cells (hSkMCs) and murine AML12 hepatocytes.
Methods
Participant characteristics
We recruited 33 normal-weight men and 102 obese men, of whom 46 had type 2 diabetes according to the American Diabetes Association criteria [16], from the Obster [17] and HepObster [18] cohort. Obese men were scheduled for bariatric surgery, whereas normal-weight men were scheduled for elective surgery in the abdominal region. On the day of surgery, a blood sample was collected after overnight fasting. During surgery, VAT and subcutaneous adipose tissue (SAT) biopsies were collected. In a subcohort of obese men (n = 42), an OGTT was performed using 75 g glucose. The study protocol was approved by the Ethics Committee of Ghent University Hospital and conducted according to the principles of the Declaration of Helsinki. All participants gave their written informed consent. See electronic supplementary material (ESM) Methods for more details. Samples collected from the participants were not randomised and no blinding regarding group assignment was applied.
Serum analysis
Serum WISP1 levels were determined using a Duoset human WISP-1/CCN4 ELISA assay (R&D systems, Wiesbaden, Germany). C-reactive protein (CRP), insulin, adiponectin, chemerin, monocyte chemotactic protein 1 (MCP-1), leptin, omentin and haem oxygenase-1 (HO-1) serum levels were quantified using commercial assays as described in ESM Methods.
Determination of adipocyte cell size
VAT and SAT biopsies were used to assess adipocyte cell size by hematoxylin–eosin staining of VAT and SAT paraffin slides. See ESM Methods for details.
Culture of adipose tissue explants
Adipose tissue explants were obtained from obese individuals and cultured in DMEM/F12 (Gibco, Darmstadt, Germany). See ESM Methods for details.
Cell culture
hSkMCs were differentiated from proliferating satellite cells and were cultured as previously described [19]. The mouse AML12 (alpha mouse liver 12) hepatocyte cell line was maintained in DMEM/F12 (Thermo Fisher Scientific, Waltham, MA, USA) for 48 h. Primary hepatocytes were isolated from male C57BL/6 mice as described [20, 21]. 3T3-L1 fibroblasts were differentiated to adipocytes in Iscove’s Modified Dulbecco’s Medium IMDM, PAN Biotech, Aidenbach, Germany). See ESM Methods for details.
Cell treatment with recombinant WISP1
Myotubes and primary hepatocytes were serum-starved and AML12 cells were cultured in medium without insulin and serum for 4 h followed by 24 h exposure to recombinant WISP1 prior to insulin stimulation. See ESM Methods for details.
Knockdown of WISP1 in 3T3-L1 adipocytes
Before initiating differentiation into adipocytes, 3T3-L1 cells were transfected with siRNA for WISP1 or scrambled siRNA (used as a control). See ESM Methods for details.
Gene expression analysis
Real-time quantitative RT-PCR using specific primers (ESM Table 1) was performed to determine expression levels of mRNAs for WISP1, CCL2 (encoding MCP-1), RARRES2 (encoding chemerin), ITLN1 (encoding omentin), HMOX1 (encoding HO-1), RPS18, UBE2D2, YWHAZ and RPLP0 in human adipose tissue and Wisp1, Il6, Tnf, Ccl2, G6pc Pck, Rplp0 and 18s in mouse 3T3-L1 adipocytes or primary hepatocytes. See ESM Methods and ESM Table 1 for details.
Analysis of protein phosphorylation and abundance
Analysis of insulin signalling was performed in hSkMCs and AML12 lysates by western blotting with antibodies recognising phosphorylated and/or non-phosphorylated Akt, glycogen synthase kinase, forkhead box O1 (FOXO1), p70S6kinase (p70S6K), insulin receptor β (IRβ), IRS1 and IRS2, α-tubulin and GAPDH all used as per manufacturer’s instructions and as described in ESM Methods.
Analysis of glycogen synthesis in primary hSkMCs
Glycogen synthesis in hSkMCs was assessed by the incorporation of d-[U-14C]glucose into glycogen in the absence or presence of insulin (100 nmol/l). See ESM Methods for details.
Statistical analysis
Statistical analysis of the clinical data and cell culture experiments was performed using SPSS Statistics (version 24.0; IBM, Armonk, NY, USA) and GraphPad Prism (version 7.0; GraphPad Software, La Jolla, CA, USA), respectively. The data are expressed as mean (95% CI) unless stated otherwise. Differences between groups were calculated by ANOVA followed by a Bonferroni correction for multiple comparisons. Gutt index of insulin sensitivity was calculated as described [22]. Linear regression analysis with adjustments for age and/or BMI was applied to identify correlates of WISP1 mRNA expression in VAT as well as circulating WISP1 levels and determinants of adipose tissue and glucose metabolism. In the correlation and regression analysis of glucose in OGTT, HOMA-IR and Gutt index, individuals treated with insulin (inclusive of all individuals with diabetes) were excluded. Variables showing a skewed distribution were log-transformed prior to analysis. In all analyses, a p value of <0.05 was considered as statistically significant.
Results
Participant cohort
The characteristics of the 33 normal-weight men and 102 obese men, of whom 46 had type 2 diabetes, are listed in Table 1. The obese men without type 2 diabetes had a higher BMI, fat mass, and larger adipocytes than normal-weight men (all p < 0.001), increased fasting insulin levels (p < 0.01), a reduced insulin sensitivity (p < 0.05) and increased beta cell function (p < 0.001), both estimated by HOMA modelling (p < 0.05). The obese participants with type 2 diabetes were older than the obese men without type 2 diabetes (p < 0.001). In addition, fat mass (p < 0.05) and fasting glucose levels (p < 0.001) were higher, and beta cell function (p < 0.05) was reduced in the obese men with type 2 diabetes vs those without type 2 diabetes. A subgroup of 42 obese men, of whom eight had type 2 diabetes, underwent an OGTT. As expected, the post-load glucose levels were higher in individuals with type 2 diabetes (ESM Fig. 1a, b), while post-load insulin levels were lower (ESM Fig. 1c, d), when compared with participants without type 2 diabetes.
WISP1 serum levels and gene expression in VAT in obesity and type 2 diabetes
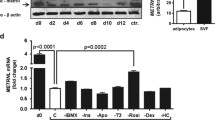
WISP1 circulating levels were obtained in 123 out of 135 participants. In samples from two participants (one normal weight and one obese), WISP1 levels were below the limit of detection; in the other participants without data, no serum sample was available for analysis. The circulating WISP1 level was 42.6 (28.5, 56.6) ng/l in normal-weight men (n = 30) and 70.8 (55.2, 86.4) ng/l in obese men (n = 93) (p < 0.05, Fig. 1a). There was no statistically significant difference in circulating WISP1 level between obese men with (69.4 [48.5, 90.3] ng/l; n = 44) and without type 2 diabetes (72.2 [48.4, 95.6] ng/l; n = 49) (Fig. 1a).
WISP1 serum levels and gene expression in VAT. (a, b) Quantification of circulating WISP1 levels (a) and gene expression in VAT (b) from normal-weight (control, n = 30 [a], n = 16 [b]) and obese men with (n = 44 [a], n = 31 [b]) and without (n = 49 [a], n = 45 [b]) type 2 diabetes. (c) Expression of WISP1 in paired samples of VAT and SAT (c, n = 6). Data (a–c) are presented as dot plot in which the mean and 95% CI for each group are shown. Differences among normal-weight men and obese men with and without T2D (brackets) were analysed by Mann–Whitney U test (a, b). Brackets link data for obese men without and with T2D for analysis. Differences among all groups were analysed by ANOVA and Bonferroni correction for multiple comparisons (b). Data were analysed by paired Student’s t test (c). *p < 0.05, obese vs normal-weight men (a, b); *p < 0.05, VAT vs SAT (c). T2D, type 2 diabetes
VAT biopsies to analyse WISP1 gene expression levels were available for 92 participants from the ‘HepObster’ cohort. The expression of WISP1 was 1.9-fold higher in VAT from obese men (n = 76) than in VAT from normal-weight men (n = 16) (p < 0.05). As with circulating WISP1 levels, we observed no statistically significant differences in WISP1 expression levels between obese men with (n = 31) and without type 2 diabetes (n = 45) (Fig. 1b). In paired SAT samples, WISP1 mRNA levels were very low; reliable detection of WISP1 (Ct < 35 and single-peak melting curve) was achieved in only nine of the 33 samples analysed (data not shown). In addition, we compared WISP1 mRNA expression levels in the explants of paired SAT and VAT samples collected from study participants with obesity (four men, two women, BMI 47.94 ± 3.96 kg/m2, age 43.73 ± 4.59 years). In accordance with our previous report [13], WISP1 expression was ninefold higher in VAT than in SAT (p < 0.05) (Fig. 1c). Therefore, only values of the WISP1 expression in VAT were used for further analysis.
Correlation of WISP1 serum levels and WISP1 VAT gene expression with determinants of body composition and glucose metabolism
WISP1 VAT expression was positively associated with HOMA-IR (r = 0.272, p = 0.034) and with fasting glucose levels (r = 0.262, p = 0.042) (ESM Table 2). Furthermore, a positive association with fasting insulin levels was observed (r = 0.231), although this did not reach statistical significance (p = 0.073). Adjusting for age had no major impact on the relationship between WISP1 gene expression and HOMA-IR (β = 0.261, p = 0.041) but the associations with fasting glucose (β = 0.232, p = 0.085) and fasting insulin (β = 0.233, p = 0.069) lost their statistical significance.
Circulating WISP1 levels displayed a negative association with age (r = −0.193, p = 0.034) and positive associations with determinants of body composition (e.g. BMI [r = 0.207, p = 0.022]) as well as with subcutaneous adipocyte cell size (r = 0.368, p = 0.015) (ESM Table 2). There was also an association between circulating WISP1 level and visceral adipocyte cell size (r = 0.292, p = 0.058), although this did not reach statistical significance. Several determinants of glucose metabolism were positively associated with serum WISP1 levels: fasting insulin (r = 0.255, p = 0.022) and glucose (r = 0.188, p = 0.094); HOMA-IR (r = 0.275, p = 0.014); post-load glucose levels at multiple time points (30 min [r = 0.312, p = 0.072] 60 min [r = 0.443, p = 0.009], 120 min [r = 0.335, p = 0.052]) and AUC for glucose between 0 and 120 min (r = 0.413, p = 0.015) (ESM Table 2). The associations with BMI (β = 0.192, p = 0.033), subcutaneous adipocyte size (β = 0.322, p = 0.039), post-load glucose at 60 min (β = 0.442, p = 0.013) and AUC glucose0–120min (β = 0.428, p = 0.021) remained significant after adjusting for age (Table 2). After adjusting for age and BMI, the associations between circulating WISP1 and post-load glucose at 60 min (β = 0.434, p = 0.018) and AUC glucose 0-120min (β = 0.420, p = 0.028) were still statistically significant (Table 2). Moreover, circulating WISP1 levels were negatively associated with Gutt’s index of insulin sensitivity (r = −0.401, p = 0.035; n = 28; ESM Table 2) and this association remained significant after adjusting for age and BMI (β = −0.438, p = 0.037; Table 2).
As shown in ESM Table 3, the association between HOMA-IR and WISP1 gene expression lost statistical significance upon adjustment for age and BMI (β = 0.240, p = 0.101).
Correlation of WISP1 serum and gene expression levels with markers of adipose tissue and systemic inflammation
Because WISP1 has been shown to induce an inflammatory response in human macrophages [13], we additionally investigated the association of WISP1 expression and serum levels with markers of adipose tissue inflammation (mRNA levels of ADIPOQ, RARRES2, CCL2, ITLN1) and systemic inflammation (adiponectin, CRP, chemerin, leptin, MCP-1, omentin). Moreover, we investigated the association of WISP1 with serum HO-1 which is associated with insulin resistance and adipose tissue inflammation and activates Wnt signalling [23, 24]. Compared with normal-weight participants, circulating levels of adiponectin, leptin and CRP were increased and VAT ADIPOQ mRNA levels were decreased in obese individuals (p < 0.001, p < 0.001, p = 0.014 and p = 0.002, respectively) (Table 1). Serum levels of chemerin and VAT levels of RARRES2 mRNA were higher in obese individuals with vs without diabetes (p = 0.014 and p = 0.010, respectively).
Circulating WISP1 levels showed a negative association with serum adiponectin (r = −0.190, p = 0.031) and a highly significant positive association with serum HO-1 levels (r = 0.437, p = 1.3 × 10−5) (ESM Table 4). The association with serum adiponectin remained significant after adjusting for age (β = − 0.176, p = 0.041); the association with HO-1 remained significant after adjusting for age (β = 0.319, p = 0.002) and for age and BMI (β = 0.303, p = 0.004). WISP1 mRNA expression in VAT showed a positive correlation with CCL2 expression (r = 0.253, p = 0.021) (ESM Table 4) and this association remained statistically significant after adjusting for age and BMI (β = 0.266, p = 0.015).
WISP1 silencing with siRNA in 3T3-L1 adipocytes had no effect on mRNA expression of Il6, Tnf and Ccl2 (ESM Fig. 2).
Effects of WISP1 on insulin signalling
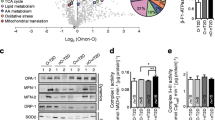
The observed relationship between circulating WISP1 and HOMA-IR as well as post-load glucose levels, together with our previous observation of the negative association between WISP1 VAT expression and hyperinsulinaemic–euglycaemic clamp-derived insulin sensitivity [13], led us to test the hypothesis that WISP1 may interfere with insulin signalling. To examine this in more detail, we exposed primary hSkMCs to recombinant WISP1 before analysis of insulin action. As shown in Fig. 2 a, b, the insulin-mediated induction of Akt on Thr308 and Ser473 phosphorylation in primary hSkMCs was reduced by 40% in cells that were exposed to 1 μg/l WISP1 before the addition of insulin. The reductions in insulin-mediated Akt phosphorylation induced by WISP1 were accompanied by decreases in insulin-mediated GSK3β-Ser9, p70S6K-Thr389 and IRβ-Tyr-1150/1151 phosphorylation (Fig. 2c–e). This inhibition of the insulin-mediated phosphorylation was achieved already in the presence of 0.1 μg/l WISP1. Of further note, the reductions in phosphorylation levels could not be ascribed to changes in the protein abundances of Akt, IRβ, GSK3β or p70S6K (ESM Fig. 3). In contrast, the abundance of IRS1 protein was reduced by ~50% in cells that were exposed to WISP1 (Fig. 2f).
Effect of WISP1 on insulin signalling in primary hSkMCs. Representative western blots and bar graphs show the effects of WISP1 on insulin-stimulated phosphorylation of Akt-Thr308 (a), Akt-Ser473 (b) GSK3β-Ser9 (c), p70S6 kinase (K)-Thr389 (d) and IRβ-Tyr1150/1151 (e) and on protein abundance of IRS1 (f). The dividing lines in the blots in (f) indicate places where the blot has been cut. The scattered bar graphs indicate the mean ± SD for the phosphorylation levels obtained in three to five independent experiments in primary hSkMCs obtained from different donors. The phosphorylation levels were normalised for the protein abundances of the non-phosphorylated proteins, GAPDH or α-tubulin, as indicated. The values obtained in cells incubated with 100 nmol/l insulin only were considered as the control and set at 100%. The effects of WISP1 and insulin were analysed by two-way ANOVA with Bonferroni correction for multiple comparisons. *p < 0.05, **p < 0.01 and ***p < 0.001, with vs without WISP1 incubation; †p < 0.05, ††p < 0.01 and †††p < 0.001, with vs without insulin stimulation
To demonstrate the functional relevance of the reduced insulin signalling, we further investigated effects of the WISP1 treatment on insulin-stimulated glycogen synthesis. Insulin treatment (100 nmol/l) for 3 h significantly increased glycogen synthesis in primary human myotubes compared with basal conditions, whereas in cells pre-incubated with 0.1 or 1 μg/l WISP1 for 24 h, this effect was completely abrogated (Fig. 3).
Effect of WISP1 on insulin-stimulated glycogen synthesis in primary hSkMCs. Myotubes were exposed to WISP1 for 24 h before glycogen synthesis was determined as incorporation of d-[U-14C]glucose into glycogen in the absence or presence of insulin (100 nmol/l) for 3 h. Data are normalised to amount of protein for three independent experiments (n = 3) and are shown as mean ± SD. The effects of WISP1 and insulin were analysed by two-way ANOVA and Bonferroni correction for multiple comparisons. ††p < 0.01, with vs without insulin
Largely comparable data were obtained for the murine hepatocyte cell line AML12. Exposing AML12 cells to WISP1 caused a dose-dependent reduction in insulin-induced Akt-Thr308 and Akt-Ser473 phosphorylation (Fig. 4a, b). The induction of GSK3β-Ser9 phosphorylation by insulin in hepatocytes was not affected by WISP1 (Fig. 4c) whereas the inhibition of insulin-induced Akt phosphorylation was accompanied by a reduction in p70S6K-Thr389 and IRβ-Tyr-1150/1151 phosphorylation (Fig. 4d, e). Therefore, to corroborate the effects of WISP1 on distal Akt signalling, we analysed another Akt substrate, namely phosphorylation of FOXO1 at Ser256. Figure 4f shows that incubating AML12 cells with 0.1 and 1 μg/l WISP1 impaired the induction of FOXO1-Ser256 phosphorylation by insulin. In contrast to the findings in myotubes, the abundance of IRS1 protein was doubled in AML12 cells exposed to WISP1 (Fig. 4g), whereas the abundances of Akt, GSK3β, P70S6K, IRβ and FOXO1 were not affected (ESM Fig. 4). In addition, WISP1 incubation had no effect on the abundance of IRS2, the preferred insulin receptor substrate in the liver [25]. Further, WISP1 treatment abrogated the insulin-mediated suppression of the gluconeogenic genes Pck1 and G6pc in mouse primary hepatocytes (Fig. 5).
Effect of WISP1 on insulin signalling in AML12 hepatocytes. Representative western blots and bar graphs show the effects of WISP1 on insulin-stimulated phosphorylation of Akt-Thr308 (a), Akt-Ser473 (b), GSK3β-Ser9 (c), p70S6 kinase (K)-Thr389 (d), IRβ-Tyr1150/1151 (e) and FOXO1-Ser256 (f) and on protein abundance of IRS1 (g). The dividing lines in the blots in (g) indicate places where the blot has been cut. The scattered bar graphs indicate the mean ± SD for the phosphorylation levels obtained in three or four independent experiments. The phosphorylation levels were normalised for the protein abundances of the non-phosphorylated protein, GAPDH or α-tubulin, as indicated. The values obtained in cells incubated with 100 nmol/l insulin only were considered as the control and set at 100%. The effects of WISP1 and insulin were analysed by two-way ANOVA with Bonferroni correction for multiple comparisons. *p < 0.05 and **p < 0.01, with vs without WISP1 incubation; †p < 0.05, ††p < 0.01 and †††p < 0.001, with vs without insulin stimulation
WISP1 impairs the insulin-mediated suppression of gluconeogenic gene expression in primary hepatocytes. Hepatocytes were exposed to WISP1 for 24 h. The last 60 min of the WISP1 incubation was carried out in the absence or presence of insulin (100 nmol/l). Pck1 (a) and G6pc (b) expression levels were assessed by quantitative RT-PCR and normalised to 18S. Data represent mean ± SD of the expression levels of five independent experiments using cells from different mice. The values obtained for untreated cells were considered as controls and set as 1. Differences among conditions were calculated by two-way ANOVA and Bonferroni correction for multiple comparisons. *p < 0.05, with vs without WISP1 incubation; ††p < 0.01, with vs without insulin stimulation
Discussion
Our study showed that both circulating levels of WISP1 and expression of WISP1 mRNA in VAT are increased in obese men, irrespective of type 2 diabetes status. Regression analysis showed that WISP1 expression and circulating levels of WISP1 were associated with variables reflecting insulin resistance and adipose tissue inflammation, even after adjusting for age and BMI. In vitro studies on primary hSkMCs as well as the mouse hepatocyte cell line AML12 showed that recombinant WISP1 directly impaired insulin action by inhibiting the Akt signalling pathway.
The increased WISP1 expression in VAT of obese men is in line with previous reports from our group and others [13,14,15, 26] suggesting that WISP1 expression is regulated by body weight. High-fat feeding in mice increased the expression of WISP1 in adipose tissue [13, 27] while diet-induced weight loss in humans lowered WISP1 expression in adipose tissue [13]. The present study also detailed the relationship between WISP1 and insulin sensitivity. In individuals with normal glucose tolerance, WISP1 expression in adipose tissue is related to determinants of insulin sensitivity [13, 26]. Here, we additionally showed that circulating levels of WISP1 are negatively associated with insulin sensitivity. Although the associations with fasting glucose and insulin levels as well as HOMA-IR in this cohort of obese men were confounded by BMI, this was not the case when considering glucose clearance after an OGTT. The association between circulating WISP1 and post-load glucose levels was not affected by adjustments for age and BMI, strongly suggesting that WISP1 may interfere with insulin signalling in target tissues for insulin action.
Another interesting finding is that circulating WISP1 level is positively associated with plasma glucose in the OGTT (independent of BMI) and negatively with the Gutt index of insulin sensitivity, confirming the association of WISP1 with insulin resistance. However, circulating WISP1 is not further increased in obese individuals with diabetes and shows no association with HOMA-IR after adjustment for BMI. It should be noted that in the analysis of glucose in the OGTT, HOMA-IR and Gutt index, individuals treated with insulin were excluded from the analysis. In our cohort, all individuals with diabetes were treated with insulin, so that all those with diabetes were completely excluded from the analysis. For the correct analysis of WISP1 association with plasma glucose in future studies, drug-naive individuals with diabetes should be investigated. Nevertheless, our results confirm that WISP1 level is directly related to adiposity independent of glycaemic status. This is in agreement with previously published data of Barchetta et al [15] and Tacke et al [26], who also found no difference in plasma WISP1 levels between diabetic and non-diabetic individuals.
The in vitro data reported in this study corroborate the suggestion that WISP1 may interfere with insulin signalling in insulin target tissues (Fig. 6). Recombinant WISP1 was found to impair insulin signalling in two different cell types, namely primary hSkMCs and murine AML12 hepatocytes. In both cell types, WISP1 inhibited the insulin-mediated phosphorylation of Akt, an important regulator of multiple aspects of glucose metabolism, such as glucose uptake, glycogen synthesis and suppression of hepatic glucose production by insulin. Moreover, we were able to demonstrate the functional relevance of the reduced insulin signalling in both cell types. Human myotubes pre-incubated with WISP1 displayed impaired insulin-stimulated glycogen synthesis and exposure to WISP1 abrogated the insulin-mediated suppression of the gluconeogenic genes Pck1 and G6pc in mouse primary hepatocytes. These in vitro observations should be validated in further animal studies in vivo.
Schematic diagram showing how WISP1 impairs insulin action in myotubes and hepatocytes. During obesity, WISP1 VAT expression and WISP1 serum levels increase. In vitro, physiological concentrations of WISP1 impair insulin action on phosphorylation of IRβ, Akt and GSK3β in myotubes and reduce insulin-mediated phosphorylation of IRβ, Akt, GSK3β and FOXO1 in hepatocytes (purple arrow downwards). WISP1 abrogates insulin-mediated induction of glycogen synthesis in myotubes (purple arrow downwards) and insulin-induced suppression of gluconeogenic gene expression (purple arrow upwards). Examined effects (solid arrow), potential effects (dotted arrow). Images ©Les Laboratoires Servier, courtesy of Servier Medical Art (https://smart.servier.com/), reproduced under the Creative Commons Attribution 3.0 France (CC BY 3.0 FR) license (https://creativecommons.org/licenses/by/3.0/fr/)
WISP1 deficiency in mice leads to a slightly lower body weight than found in wild-type animals but unfortunately no data on glucose traits or insulin sensitivity are available yet [28]. In contrast, WISP1 did not interfere with insulin-mediated Akt phosphorylation in our previous study on 3T3L1 adipocytes [13]. Apart from potential tissue-specific effects, other possible reasons for this difference may include the duration of WISP1 exposure (24 h in the present study vs 30 min in the 3T3-L1 experiment) as well as the concentration of WISP1 applied. Here, we used WISP1 concentrations representative of the levels found in the circulation whereas the 3T3-L1 adipocytes were exposed to supraphysiological concentrations of WISP1. Moreover, different signalling pathways might be involved in the actions of WISP1 in various cell types [27]. Importantly, the impaired insulin-mediated Akt phosphorylation in primary hSkMCs and AML12 hepatocytes was accompanied by inhibition of the phosphorylation of several of its well-characterised substrates. In myotubes, reductions in the insulin-mediated phosphorylation of GSK3β and p70S6K paralleled the inhibition of Akt phosphorylation. Strikingly, in AML12 hepatocytes, the insulin-mediated phosphorylation of GSK3β was not impaired by recombinant WISP1, whereas the phosphorylation of p70S6K and FOXO1 were markedly reduced or even abrogated. Tissue-specific differences in the phosphorylation of Akt substrates have also been reported by others [29, 30]. Gene silencing experiments in cultured cells as well as studies on mouse models suggest that tissue-specific changes in the abundance of Akt isoforms as well as the insulin receptor substrates IRS1 and IRS2 contribute to Akt substrate selection [29, 30].
As previously mentioned, WISP1 is a proinflammatory adipokine [13] and circulating WISP1 showed an association with systemic levels of IL-8 [15]. In our study, we further reveal the association between circulating WISP1 and markers of adipose tissue and systemic inflammation. Interestingly, the serum HO-1 level was the strongest predictor of circulating WISP1, even after adjustment for age and BMI. HO-1, encoded by HMOX1, is a stress-induced protein that is critical for stem cell differentiation [31]. Induction of the Hmox1 gene in mice is protective against deleterious obesity phenotype as characterised by a reduced number of enlarged adipocytes, an increased number of small adipocytes and a higher adiponectin concentration [24]. Moreover, increased HO-1 expression in human mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation of adipocytes via upregulation of the Wnt signalling cascade [23]. These data allow us to speculate that upregulation of Wnt signalling by HO-1 leads to higher expression levels and possibly production of WISP1 from younger adipocytes. This, in turn, might result in the redistribution of in-tissue and in-body insulin sensitivity and an increased insulin resistance in older cells (i.e. redistribution of glucose and other substrates with better supply of young and proliferating cells).
A limitation of the present study is that we could not detail the mechanism by which WISP1 inhibits insulin action especially because of the high complexity of the Wnt signalling pathway [3]. Based on the modular structure of WISP1, several functional receptors have been identified. Previous studies showed that WISP1 interacted with integrin α(5)β in human bone marrow stromal cells [32]. Furthermore, WISP1 has been shown to bind decorin and biglycan in the extracellular matrix of fibroblasts [33]. The presence of an IGF binding protein domain in CCN proteins including WISP1 suggests an interference with the IGF and insulin signalling pathway [34,35,36]. Our observations showed a decrease in the insulin-mediated tyrosine phosphorylation of insulin receptor-β/IGF-1 receptor by WISP1, indicating that WISP1 might act by interfering with these receptors in myotubes and hepatocytes. The inhibition of insulin action by adipo(cyto)kines is frequently associated with a decreased protein abundance of IRS1 [19, 25]. Although WISP1 was found to decrease the abundance of IRS1 in myotubes, this was not the case in AML12 hepatocytes. The latter cell line even displayed an increase in IRS1 protein levels following WISP1 exposure, whereas the abundance of IRS2, which is considered to be the major insulin receptor substrate in the liver [25], was unaltered by WISP1 treatment. This raises the possibility that WISP1 may use alternative mechanisms to impair insulin action in myotubes and hepatocytes.
The second limitation of our study is that it was conducted in men (but not in women), and that the OGTT data were collected in a subgroup of the obese men. Without additional studies, caution should be taken when generalising these findings to the entire population.
Taken together, our study identifies WISP1 as a determinant of obesity in men. The increases in circulating WISP1 levels associate with variables reflecting insulin resistance in vivo in individuals with obesity. In vitro studies corroborate the associations observed in the clinical study by showing that recombinant WISP1 impairs insulin signalling in myotubes and hepatocytes with consequent impaired gluconeogenesis and glycogen synthesis (Fig. 6). Thus, WISP1 may regulate whole-body insulin sensitivity and glucose uptake due to its effects on insulin signalling in liver and muscle and is an attractive target for the prevention and treatment of obesity and diabetes.
Data availability
The datasets analysed in this study are available from the corresponding author upon reasonable request.
Abbreviations
- CRP:
-
C-reactive protein
- FOXO1:
-
Forkhead box O1
- GSK3β:
-
Glycogen synthase kinase 3β
- HO-1:
-
Haem oxygenase-1
- hSkMC:
-
Human skeletal muscle cell
- IRβ:
-
Insulin receptor β
- LRP:
-
LDL receptor-related protein
- MCP-1:
-
Monocyte chemotactic protein 1
- p70S6K:
-
p70 S6 kinase
- SAT:
-
Subcutaneous adipose tissue
- sFRP:
-
Secreted frizzled-related protein
- VAT:
-
Visceral adipose tissue
- Wnt:
-
Wingless-type
- WISP1:
-
Wnt-inducible signalling protein-1
References
Gustafson B, Hammarstedt A, Hedjazifar S, Smith U (2013) Restricted adipogenesis in hypertrophic obesity: the role of WISP2, WNT, and BMP4. Diabetes 62:2997–3004
Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U (2015) Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab 26:193–200
Niehrs C (2012) The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13:767–779
Cruciat CM, Niehrs C (2013) Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol 5:a015081
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–1205
Nusse R, Clevers H (2017) Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169:985–999
Zeng X, Huang H, Tamai K et al (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135:367–375
Zeng X, Tamai K, Doble B et al (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438:873–877
Palsgaard J, Emanuelli B, Winnay JN, Sumara G, Karsenty G, Kahn CR (2012) Cross-talk between insulin and Wnt signaling in preadipocytes: role of Wnt co-receptor low density lipoprotein receptor-related protein-5 (LRP5). J Biol Chem 287:12016–12026
Dahlman I, Elsen M, Tennagels N et al (2012) Functional annotation of the human fat cell secretome. Arch Physiol Biochem 118:84–91
Ehrlund A, Mejhert N, Lorente-Cebrian S et al (2013) Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab 98:E503–E508
Ouchi N, Higuchi A, Ohashi K et al (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329:454–457
Murahovschi V, Pivovarova O, Ilkavets I et al (2015) WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes 64:856–866
Sahin Ersoy G, Altun Ensari T, Subas S, Giray B, Simsek EE, Cevik O (2016) WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med 1–5
Barchetta I, Cimini FA, Capoccia D et al (2017) WISP1 is a marker of systemic and adipose tissue inflammation in dysmetabolic subjects with or without type 2 diabetes. J Endocr Soc 1:660–670
Diabetes AATFoI (2006) American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control. Diabetes Care 29:1955–1962
Ruige JB, Bekaert M, Lapauw B et al (2012) Sex steroid-induced changes in circulating monocyte chemoattractant protein-1 levels may contribute to metabolic dysfunction in obese men. J Clin Endocrinol Metab 97:E1187–E1191
Bekaert M, Ouwens DM, Horbelt T et al (2016) Reduced expression of chemerin in visceral adipose tissue associates with hepatic steatosis in patients with obesity. Obesity (Silver Spring) 24:2544–2552
Wiza C, Herzfeld de Wiza D, Nascimento EB, Lehr S, Al-Hasani H, Ouwens DM (2013) Knockdown of PRAS40 inhibits insulin action via proteasome-mediated degradation of IRS1 in primary human skeletal muscle cells. Diabetologia 56:1118–1128
Jelenik T, Kaul K, Sequaris G et al (2017) Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes 66:2241–2253
Akie TE, Cooper MP (2015) Determination of fatty acid oxidation and lipogenesis in mouse primary hepatocytes. J Vis Exp e52982
Gutt M, Davis CL, Spitzer SB et al (2000) Validation of the insulin sensitivity index (ISI0,120): comparison with other measures. Diabetes Res Clin Pract 47:177–184
Vanella L, Sodhi K, Kim DH et al (2013) Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther 4:28
Li M, Kim DH, Tsenovoy PL et al (2008) Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 57:1526–1535
Eckstein SS, Weigert C, Lehmann R (2017) Divergent roles of IRS (insulin receptor substrate) 1 and 2 in liver and skeletal muscle. Curr Med Chem 24:1827–1852
Tacke C, Aleksandrova K, Rehfeldt M et al (2017) Assessment of circulating Wnt1 inducible signalling pathway protein 1 (WISP-1)/CCN4 as a novel biomarker of obesity. J Cell Commun Signal. https://doi.org/10.1007/s12079-017-0427-1
Ferrand N, Bereziat V, Moldes M, Zaoui M, Larsen AK, Sabbah M (2017) WISP1/CCN4 inhibits adipocyte differentiation through repression of PPARγ activity. Sci Rep 7:1749
Maeda A, Ono M, Holmbeck K et al (2015) WNT1-induced secreted protein-1 (WISP1), a novel regulator of bone turnover and WNT signaling. J Biol Chem 290:14004–14018
Gonzalez E, McGraw TE (2009) The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle (Georgetown, Tex) 8:2502–2508
Bouzakri K, Zachrisson A, Al-Khalili L et al (2006) siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 4:89–96
Kozakowska M, Szade K, Dulak J, Jozkowicz A (2014) Role of heme oxygenase-1 in postnatal differentiation of stem cells: a possible cross-talk with microRNAs. Antioxid Redox Signal 20:1827–1850
Ono M, Inkson CA, Kilts TM, Young MF (2011) WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res 26:193–208
Desnoyers L, Arnott D, Pennica D (2001) WISP-1 binds to decorin and biglycan. J Biol Chem 276:47599–47607
Lorenzatti G, Huang W, Pal A, Cabanillas AM, Kleer CG (2011) CCN6 (WISP3) decreases ZEB1-mediated EMT and invasion by attenuation of IGF-1 receptor signaling in breast cancer. J Cell Sci 124:1752–1758
Lopez-Bermejo A, Khosravi J, Fernandez-Real JM et al (2006) Insulin resistance is associated with increased serum concentration of IGF-binding protein-related protein 1 (IGFBP-rP1/MAC25). Diabetes 55:2333–2339
Yamanaka Y, Wilson EM, Rosenfeld RG, Oh Y (1997) Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J Biol Chem 272:30729–30734
Acknowledgements
Part of this work was presented as an abstract at the 52nd European Association for the Study of Diabetes (EASD) Annual Meeting 2016.
Funding
This work was financially supported by a grant to NR and DMO from the German Center for Diabetes Research (‘WISP1 is a novel target for regulation of glucose metabolism’), by a grant to NR and DMO from European Foundation for Study of Diabetes (EFSD/AZ Cellular Plasticity, ‘Unravelling the role of WISP1 on metabolic and cellular plasticity in white adipose tissue’) and by an internal grant of the German Institute of Human Nutrition to MM (2017, ‘Effects of dietary protein intake on lipid metabolism and inflammatory markers in human adipose tissue’).
Author information
Authors and Affiliations
Contributions
TH, NR, OP and DMO designed the study, conducted experiments, performed data analysis and drafted the manuscript. CT, MM, WJ, AFHP, SH, AR, VL, GHT and AS conducted experiments, performed data analysis and drafted the manuscript. DHdW, MR, NS and EF conducted experiments, performed data analysis and reviewed the manuscript. FVdV and MB collected clinical samples, analysed clinical data, maintained participants records, supervised clinical chemistry and reviewed the manuscript. YVN and BL designed and supervised the clinical study and reviewed the manuscript. OK performed statistical analysis and reviewed the manuscript. DMO and OP are the guarantors of this work, had full access to all the data and take full responsibility for the integrity of the data and the accuracy of the data analysis. All authors have seen and approved the final version of the manuscript.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Electronic supplementary material
ESM
(PDF 1345 kb)
Rights and permissions
About this article
Cite this article
Hörbelt, T., Tacke, C., Markova, M. et al. The novel adipokine WISP1 associates with insulin resistance and impairs insulin action in human myotubes and mouse hepatocytes. Diabetologia 61, 2054–2065 (2018). https://doi.org/10.1007/s00125-018-4636-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4636-9