Abstract
Aims/hypothesis
Multiple islet autoimmunity increases risk of diabetes, but not all individuals positive for two or more islet autoantibodies progress to disease within a decade. Major islet autoantibodies recognise insulin (IAA), GAD (GADA), islet antigen-2 (IA-2A) and zinc transporter 8 (ZnT8A). Here we describe the baseline characteristics of a unique cohort of ‘slow progressors’ (n = 132) who were positive for multiple islet autoantibodies (IAA, GADA, IA-2A or ZnT8A) but did not progress to diabetes within 10 years.
Methods
Individuals were identified from five studies (BABYDIAB, Germany; Diabetes Autoimmunity Study in the Young [DAISY], USA; All Babies in Southeast Sweden [ABIS], Sweden; Bart’s Oxford Family Study [BOX], UK and the Pittsburgh Family Study, USA). Multiple islet autoantibody characteristics were determined using harmonised assays where possible. HLA class II risk was compared between slow progressors and rapid progressors (n = 348 diagnosed <5 years old from BOX) using the χ2 test.
Results
In the first available samples with detectable multiple antibodies, the most frequent autoantibodies were GADA (92%), followed by ZnT8A (62%), IAA (59%) and IA-2A (41%). High risk HLA class II genotypes were less frequent in slow (28%) than rapid progressors (42%, p = 0.011), but only two slow progressors carried the protective HLA DQ6 allele.
Conclusion
No distinguishing characteristics of slow progressors at first detection of multiple antibodies have yet been identified. Continued investigation of these individuals may provide insights into slow progression that will inform future efforts to slow or prevent progression to clinical diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Immune-mediated destruction of pancreatic beta cells progresses at different speeds in different individuals. Prospective birth cohort studies show that autoantibodies to insulin (IAA), GAD (GADA), islet antigen-2 (IA-2A), and zinc transporter 8 (ZnT8A) can be detected in children at risk of type 1 diabetes from 6 months of age with a peak in seroconversion between 2 and 3 years [1]. Over 90% of individuals diagnosed in childhood have at least one of these autoantibodies [2]. Presence of multiple islet autoantibodies (mAabs) is normally associated with a disease risk of 70% within 10 years [2,3,4,5], but many individuals present with clinical symptoms later in adult life [1].
Conceptually, disease pathogenesis can be divided into three stages: development of autoimmunity as indicated by the presence of mAabs, defined recently as Stage 1; progression from autoimmunity to glucose intolerance (Stage 2); and finally to overt disease (Stage 3) [6]. These stages appear to be controlled by different genetic mechanisms, with different HLA alleles affecting seroconversion and/or progression to disease [7, 8]. Positivity for and/or higher levels of IAA, IA-2A, IA-2βA and ZnT8A have also been associated with more rapid development of hyperglycaemia [9,10,11].
The Slow or Non progressive Autoimmunity to the Islets of Langerhans (SNAIL) study focuses on slow progression to diabetes by studying a large international collection of individuals who develop islet autoimmunity (defined by presence of mAabs) but do not develop disease for at least 10 years. The characteristics of humoral autoimmunity in the earliest mAab-positive sample and the HLA class II profile in this unique ‘slow progressor’ cohort are described here. SNAIL participants derive from BABYDIAB [12], the Diabetes Autoimmunity Study in the Young (DAISY) [13], All Babies in Southeast Sweden (ABIS) [14], the Bart’s Oxford (BOX) Family Study [15] and the Pittsburgh Family Study [5]. These studies all investigated the natural history of diabetes and the contribution of islet autoantibodies to disease risk. Three studies (BABYDIAB, DAISY and ABIS) followed children from birth (with blood samples taken at 9 months or 1 year), while two studies enrolled first degree relatives of individuals with diabetes throughout life. Slow progressors may provide new insights that inform disease prevention strategies in those at risk of type 1 diabetes and help to identify biomarkers associated with slow progression.
Methods
Participants
Slow progressors were characterised by remaining diabetes-free for at least 10 years after mAabs (two or more of IAA, GADA, IA-2A or ZnT8A) were first detected. As a control group, genetic data were available for 2075 individuals diagnosed with type 1 diabetes under the age of 21 years and enrolled in the BOX study. Of these, 348 were ‘rapid progressors’ being diagnosed at less than 5 years of age and 1217 were diagnosed over 10 years of age. All individuals were participants in existing studies approved by local ethical review boards. Written informed consent was obtained from participants and/or their parents/legal guardians.
Participating study cohorts
SNAIL participants were recruited from several natural history studies, which have been described in detail elsewhere (see electronic supplementary material [ESM] Table 1). Brief descriptions of the studies are given below.
BABYDIAB, Germany
BABYDIAB is a longitudinal study examining the natural history of islet autoimmunity and type 1 diabetes in 1650 children born to a mother or father with type 1 diabetes [12]. Recruitment began in 1989 and ended in 2000.
DAISY, USA
DAISY has followed two cohorts of young children at increased risk of type 1 diabetes (total n = 2547); a cohort of relatives of individuals with type 1 diabetes (siblings and offspring) and a newborn high genetic risk general population cohort from the Denver area [13, 16, 17]. Recruitment began in 1993 and ended in 2004, and follow-up is ongoing.
ABIS, Sweden
All mothers that gave birth in southeast Sweden between October 1997 and October 1999 were invited to participate [14]. In total 17,055 children were recruited (78.6% of all births), of whom 7394 provided at least two samples for autoantibody analysis.
The BOX family study, UK
BOX is a longitudinal study examining risk factors for type 1 diabetes in siblings or parents (2774 families) of a proband diagnosed under the age of 21 [15]. Autoantibodies were tested in at least one sample from 5881 relatives, diabetes-free at the time of testing. Of these, 284 have had a diabetes (any type) diagnosis. Recruitment began in 1985 and is ongoing. All participants in SNAIL were recruited before 2001.
The Pittsburgh Family Study, USA
More than 10,000 first-degree relatives of children and adolescents with type 1 diabetes (<19 years old) were recruited from the Children’s Hospital of Pittsburgh registry and followed up from 1979 until 2015 [18]. Relatives were excluded at screening if an OGTT or random glucose level was >7.8 mmol/l [19]. All relatives were screened using islet cell antigen (ICA) testing. Relatives confirmed positive for ICA were tested for IAA, GADA and IA-2A, this included 1484 relatives recruited between 1979 and 1984.
Follow-up
Participants were followed for development of disease through: (1) annual or semi-annual written or telephone contact to ascertain self-reported, clinician diagnosed diabetes; (2) in ABIS, diagnosis was ascertained from the national registration of all individuals with type 1 diabetes [11]; (3) for BABYDIAB and willing DAISY participants, oral glucose tolerance tests were performed at least annually and/or HbA1c monitored [2].
Autoantibody assays
Islet autoantibodies (GADA, IA-2A, IAA and ZnT8A) were tested in each parent study by radiobinding assays as previously described [2, 3, 11, 16, 19,20,21] and an overview of the testing strategy for each study is summarised in ESM Methods. Positivity was defined using a threshold determined by each laboratory and where multiple samples were available, persistent autoimmunity was confirmed. Assays for GADA and IA-2A were calibrated against the WHO standard in international workshops and where samples were available, the presence of mAabs within individuals was confirmed with harmonised assays for GADA and IA-2A [20]. Where harmonised assays failed to confirm mAab, those individuals were excluded.
Genetics
The HLA class II genotype DRB1*03-DQB1*02 (DR3-DQ2)/DRB1*04-DQB1*0302 (DR4-DQ8) (or DQB1*02 [DQ2]/DQB1*0302 [DQ8] if DR typing was not available) was considered high risk, presence of one or two copies of DR3-DQ2 or DR4-DQ8 was considered to confer intermediate risk, while all other genotypes or haplotypes containing DQB1*0602 were considered low risk. HLA typing for BOX and ABIS were carried out at the University of Bristol [22]; other studies provided their own data [5, 13, 23].
Data analysis
Comparisons between antibody prevalence and haplotype frequencies across studies and genotype frequencies between SNAIL participants and rapid progressors were made using χ2 tests.
Results
Overall, 132 participants were identified who remained diabetes-free for more than 10 years after mAabs were first detected (Table 1). After this 10-year period, participants remained under follow-up (median 4 years, IQR 2–9 years). During follow-up in SNAIL, 42 slow progressors were diagnosed with diabetes, but 90 remained diabetes-free based on lack of self-reported disease, absence from a diabetes case registry or lack of metabolic abnormalities.
Islet autoantibody frequency in slow progressors differs between study cohorts
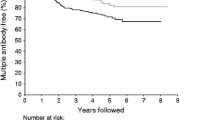
The most frequent autoantibodies in the first mAab-positive samples were GADA (92%), followed by ZnT8A (62%), IAA (59%) and IA-2A (41%) (Fig. 1a–d). Of 117 individuals tested for all four antibodies (Table 2), nine (8%) had four antibodies, with the most common combination of three antibodies being GADA+IA-2A+ZnT8A (in 22 individuals, 19%) and of two antibodies being IAA+GADA (in 31 individuals, 26%). The frequency of IAA, GADA and IA-2A, but not ZnT8A, differed between cohorts (p = 0.001, p = 0.018, p = 0.007 and p = 0.183, respectively, Fig. 1). In ABIS, of ten slow progressors tested at age 11 years for ZnT8A, five were positive.
Proportion of slow progressors positive for (a) IAA, (b) GADA, (c) IA-2A and (d) ZnT8A at the first available mAab-positive sample differed between the cohorts (p=0.001, p=0.018, p=0.007 and p=0.183, for each antibody, respectively). Black bars show overall percentage, white bars show percentage for slow progressors from each study. The ABIS participants were not tested for ZnT8A in their first mAab-positive sample
Slow progressors carry less HLA risk than individuals diagnosed in childhood
High risk HLA class II (DQ2/DQ8) was less frequent (28% vs 42%) while intermediate risk genotypes were more common (55% vs 49%) in the 121 slow progressors with HLA class II data available, than in the 348 children from BOX diagnosed under 5 years of age, who were designated rapid progressors (p = 0.011, Fig. 2a). Genetic risk of the slow progressors was similar to that of the 1217 BOX participants diagnosed over 10 years of age (DQ2/DQ8, 26%). A similar proportion, two of 121 (1.7%) slow progressors, carried the protective DQB1*0602 allele compared with six (1.7%) rapid progressors. HLA class II genetic risk varied between cohorts, but the frequency of DQ2 and DQ8 alleles was not significantly different (Fig. 2b).
HLA risks for (a) proportion of HLA class II high risk (black), intermediate risk (grey) or low risk (white) genotypes in BOX probands according to age at diagnosis (n=2075, including 348 rapid progressors diagnosed under 5 years of age) and slow progressors (SPs, n=121, p=0.011 for HLA class II risk in slow vs rapid progressors); and (b) proportion of participants carrying HLA class II DQ2 (white) or DQ8 (black) haplotypes in BOX (n=36), BABYDIAB (n=22), DAISY (n=30), Pittsburgh study (n=21) and ABIS (n=10)
Discussion
Here we describe a unique group of 132 slow progressors with mAab positivity who remained diabetes-free for at least 10 years while participating in five international natural history studies. Most remained diabetes-free at last contact but could be expected to develop diabetes in the future. Indeed, 32% developed diabetes during 4 years of SNAIL follow-up. Diabetes diagnosis was self-reported for the BOX and Pittsburgh studies. This analysis brought together data from several well-characterised long running studies which will enable further analysis of these rare individuals. Most study participants were first-degree relatives of people with type 1 diabetes but also included individuals selected for HLA risk and from the general population.
All five ‘parent’ studies are longitudinal in nature; serum samples were collected and assayed over decades. Slow progressors were initially identified from historic antibody assay screening results. To allow comparison of data, where possible, the initial antibody-positive sample and/or subsequent samples were retested using standardised assays to confirm autoantibody status and these results are reported here.
The definition of slow progressor was the same for all cohorts included in this analysis, although the parent studies had different structures. The BOX and Pittsburgh family studies recruited relatives of individuals with diabetes at different ages and the age of seroconversion is often unknown. In contrast, BABYDIAB, ABIS and most DAISY participants have been studied from birth and provide valuable evidence that even individuals who develop antibodies early in life can remain diabetes-free for many years. Diabetes was defined by clinical disease, but metabolic abnormalities may appear before overt disease. All participants from BABYDIAB were normoglycaemic, while 1 of 10 DAISY participants tested, and 3 of 11 BOX participants tested recently, have elevated HbA1c. In BABYDIAB the slow progressors described here represent 25% of the children who became mAab-positive in the study [24]. Together these cohorts cover all stages in the natural history of islet autoimmunity; however, individuals with a family history of disease are better represented. The only cohorts included that are representative of the general population are the ABIS study where individuals with no family history were recruited and DAISY where some were recruited from the general population after genetic screening.
All SNAIL participants had mAabs, but the proportion of individuals with each autoantibody varied between study cohorts. The first antibodies to be detected in about two-thirds of young children in BABYDIAB were IAA and their loss was associated with delayed progression [25]. In the SNAIL cohorts, IAA were more common in the first mAab-positive samples from BABYDIAB and ABIS children, but half of BOX and Pittsburgh family study participants were also IAA-positive in their first sample, despite most being tested first as adults. Autoantibodies to GAD are the first islet autoantibody detected in about a third of children who develop diabetes but are also prevalent in adult onset disease [21, 26]. In SNAIL participants, GADA were common in all cohorts but more frequent in older individuals. Antibodies that recognise IA-2 and ZnT8 develop later in disease pathogenesis and are associated with progression [11]; however, ZnT8A were the second most frequent antibodies found in slow progressors. In contrast, IA-2A were less common and BABYDIAB participants had a particularly low prevalence of IA-2A (14%). During follow-up, however, 18 of 22 (82%) BABYDIAB SNAIL participants seroconverted to IA-2A positivity, indicating that antigen spreading continued despite slower progression (data not shown).
The slow progressors in SNAIL have a lower prevalence of DQ2/DQ8 compared with well-characterised rapid progressors diagnosed under the age of five from BOX but a similar genetic risk profile to individuals diagnosed in adolescence. In contrast, BABYDIAB showed that children who develop islet autoantibodies early in life have similar high HLA class II risk, independent of whether they progress to diabetes [24]. One reason for this discrepancy may be that the average age of detection of islet autoantibodies in SNAIL participants was during adolescence. Later development of autoantibodies is associated with lower HLA class II risk as these genes are known to influence antibody development, whereas HLA class I genes have a more dominant role in progression from autoimmunity to disease [7, 8]. The frequency of DQ2 and DQ8 did not differ between cohorts despite differences in participant age between the studies, but this may have been due to a lack of statistical power for comparisons between cohorts. The different structures, ages or ethnicities of the SNAIL cohorts should be considered in interpretation of our analyses. HLA class II risk in children diagnosed under 5 years old is likely to be similar in Western populations, i.e. 36–42% have the highest HLA risk in the type 1 diabetes genetics consortium, DAISY and BOX studies [27]. In addition, over 95% of participants in BABYDIAB, ABIS, BOX and Pittsburgh were of white European origin. For older participants, the age of multiple antibody seroconversion is not known, limiting the interpretation of HLA class II data. Those who seroconverted at a younger age however appear to have similar HLA class II regardless of future progression rate.
On the basis of their mAab positivity, slow progressors are at high risk of developing type 1 diabetes. Despite having a lower genetic risk of disease than rapid progressors, almost 30% of slow progressors carry the highest HLA class II risk haplotype, highlighting the importance of other factors in influencing progression to clinical disease. Continued study of SNAIL participants will focus on characteristics of the immune response, including antibody characteristics, and investigate whether these individuals develop the disease slowly or not at all because of immune regulation.
Data availability
Requests for data sharing would need to be referred to the studies in question. More information can be provided by the corresponding author if required.
Abbreviations
- ABIS:
-
All Babies in Southeast Sweden
- BOX:
-
Bart’s Oxford Family Study
- DAISY:
-
Diabetes Autoimmunity Study in the Young
- GADA:
-
Autoantibodies to GAD
- IA-2A:
-
Autoantibodies to islet antigen-2
- IAA:
-
Autoantibodies to insulin
- ICA:
-
Islet cell antigen
- mAab:
-
Multiple islet autoantibody
- SNAIL:
-
Slow or Non progressive Autoimmunity to the Islets of Langerhans
- ZnT8A:
-
Autoantibodies to zinc transporter 8
References
Ziegler AG, Bonifacio E, BABYDIAB-BABYDIET Study Group (2012) Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 55:1937–1943
Ziegler AG, Rewers M, Simell O et al (2013) Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309:2473–2479
Long AE, Gooneratne AT, Rokni S, Williams AJK, Bingley PJ (2012) The role of autoantibodies to zinc transporter 8 in prediction of type 1 diabetes in relatives: lessons from the European Nicotinamide Diabetes Intervention Trial (ENDIT) cohort. J Clin Endocrinol Metab 97:632–627
Steck AK, Johnson K, Barriga KJ et al (2011) Age of islet autoantibody appearance and mean levels of insulin, but not GAD or ,autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care 34:1397–1399
Pietropaolo M, Becker DJ, LaPorte RE et al (2002) Progression to insulin-requiring diabetes in seronegative prediabetic subjects: the role of two HLA-DQ high-risk haplotypes. Diabetologia 45:66–76
Insel RA, Dunne JL, Atkinson MA et al (2015) Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38:1964–1974
Lipponen K, Gombos Z, Kiviniemi M et al (2010) Effect of HLA class I and class II alleles on progression from autoantibody positivity to overt type 1 diabetes in children with risk-associated class II genotypes. Diabetes 59:3253–3256
Tait BD, Colman PG, Morahan G et al (2003) HLA genes associated with autoimmunity and progression to disease in type 1 diabetes. Tissue Antigens 61:146–153
Achenbach P, Warncke K, Reiter J et al (2004) Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 53:384–392
Steck AK, Vehik K, Bonifacio E et al (2015) Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 38:808–813
Akerman L, Ludvigsson J, Swartling U, Casas R (2017) Characteristics of the pre-diabetic period in children with high risk of type 1 diabetes recruited from the general Swedish population–the ABIS study. Diabetes Metab Res Rev. https://doi.org/10.1002/dmrr.2900
Ziegler AG, Hummel M, Schenker M, Bonifacio E (1999) Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes. The 2-year analysis of the German BABYDIAB study. Diabetes 48:460–468
Rewers M, Bugawan TL, Norris JM et al (1996) Newborn screening for HLA markers associated with IDDM: Diabetes autoimmunity study in the young (DAISY). Diabetologia 39:807–812
Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A (2015) Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia 58:1188–1197
Bingley PJ, Gale EAM (1989) The incidence of insulin-dependent diabetes in England: a study in the Oxford region 1985-6. Br Med J 298:558–560
Wenzlau JM, Liu Y, Yu L et al (2008) A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 57:2693–2697
Norris JM, Beaty B, Klingensmith G et al (1996) Lack of association between early exposure to cow’s milk protein and beta-cell autoimmunity. Diabetes autoimmunity study in the young (DAISY). JAMA 276:609–614
Morran MP, Casu A, Arena VC et al (2010) Humoral autoimmunity against the extracellular domain of the neuroendocrine autoantigen IA-2 heightens the risk of type 1 diabetes. Endocrinology 151:2528–2537
Pietropaolo M, Yu S, Libman IM et al (2005) Cytoplasmic islet cell antibodies remain valuable in defining risk of progression to type 1 diabetes in subjects with other islet autoantibodies. Pediatr Diabetes 6:184–192
Bonifacio E, Yu L, Williams AK et al (2010) Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 95:3360–3367
Yu L, Rewers M, Gianani R et al (1996) Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 81:4264–4267
Gillespie KM, Valovin SJ, Saunby J et al (2000) HLA class II typing of whole genome amplified mouth swab DNA. Tissue Antigens 56:530–538
Walter M, Albert E, Conrad M et al (2003) IDDM2/insulin VNTR modifies risk conferred by IDDM1/HLA for development of type 1 diabetes and associated autoimmunity. Diabetologia 46:712–720
Achenbach P, Hummel M, Thumer L, Boerschmann H, Hofelmann D, Ziegler AG (2013) Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children. Diabetologia 56:1615–1622
Endesfelder D, Hagen M, Winkler C et al (2016) A novel approach for the analysis of longitudinal profiles reveals delayed progression to type 1 diabetes in a subgroup of multiple-islet-autoantibody-positive children. Diabetologia 59:2172–2180
Gorus FK, Goubert P, Semakula C et al (1997) IA-2-autoantibodies complement GAD65-autoantiobdies in new-onset IDDM patients and help predict impending diabetes in their siblings. The Belgian diabetes registry. Diabetologia 40:95–99
Steck AK, Armstrong TK, Babu SR, Eisenbarth GS, Type 1 Diabetes Genetics Consortium (2011) Stepwise or linear decrease in penetrance of type 1 diabetes with lower-risk HLA genotypes over the past 40 years. Diabetes 60:1045–1049
Acknowledgements
The authors would like to thank all individuals who have participated in the BABYDIAB, DAISY, ABIS, BOX and Pittsburgh studies; without their dedication this research would not be possible.
Contribution statement
AEL collated and analysed the data and wrote the manuscript. All authors supported data acquisition/interpretation, reviewed/edited the manuscript, contributed to the discussion and approved the final version. KMG devised the SNAIL study. KMG is the guarantor of this work and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
AEL was funded by a Diabetes UK–Fulbright Award and a Diabetes UK Moffat Travelling Fellowship (13/0004636). The BOX study is funded by Diabetes UK (14/0004869) and the SNAIL study is funded by a Strategic Research Agreement from the JDRF (17-2013-529). The BABYDIAB study was funded by Deutsche Forschungsgemeinschaft (DFG ZI-310/14-1 to 14-4). DAISY was supported by JDRF grant 17-2013-535 and the National Institutes of Health (NIH) grants R01 DK32083 and DK32493. The ABIS study received funds from Vetenskapsrådet (VR 2009-2010); Novo Nordisk; Barndiabetesfonden; ALF grants, Region Östergötland and Medical Research Council of southeast Sweden (Forskningsrådet i sydöstrasverige, FORSS), grant/award number: 12594. The Pittsburgh registry was supported by NIH grants R01 DK53456, DK56200 and NIDDK PA-04-081; the Brehm Coalition by the University of Michigan Center for Computational Medicine and Biology Pilot Research Program NIH Grant R01 DK46864; NIH Grant R01 DK24021; General Clinical Research Center of the Children’s Hospital of Pittsburgh Grant MO1 RR 00084 and the Renziehausen fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Electronic supplementary material
ESM
(PDF 96.5 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Long, A.E., Wilson, I.V., Becker, D.J. et al. Characteristics of slow progression to diabetes in multiple islet autoantibody-positive individuals from five longitudinal cohorts: the SNAIL study. Diabetologia 61, 1484–1490 (2018). https://doi.org/10.1007/s00125-018-4591-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4591-5