Abstract
Aims/hypothesis
Previous studies have suggested that the haemoglobin glycation index (HGI) can be used as a predictor of diabetes-related complications in individuals with type 1 and type 2 diabetes. We investigated whether HGI was a predictor of adverse outcomes of intensive glucose lowering and of diabetes-related complications in general, using data from the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial.
Methods
We studied participants in the ADVANCE trial with data available for baseline HbA1c and fasting plasma glucose (FPG) (n = 11,083). HGI is the difference between observed HbA1c and HbA1c predicted from a simple linear regression of HbA1c on FPG. Using Cox regression, we investigated the association between HGI, both categorised and continuous, and adverse outcomes, considering treatment allocation (intensive or standard glucose control) and compared prediction of HGI and HbA1c.
Results
Intensive glucose control lowered mortality risk in individuals with high HGI only (HR 0.74 [95% CI 0.61, 0.91]; p = 0.003), while there was no difference in the effect of intensive treatment on mortality in those with high HbA1c. Irrespective of treatment allocation, every SD increase in HGI was associated with a significant risk increase of 14–17% for macrovascular and microvascular disease and mortality. However, when adjusted for identical covariates, HbA1c was a stronger predictor of these outcomes than HGI.
Conclusions/interpretation
HGI predicts risk for complications in ADVANCE participants, irrespective of treatment allocation, but no better than HbA1c. Individuals with high HGI have a lower risk for mortality when on intensive treatment. Given the discordant results and uncertain relevance beyond HbA1c, clinical use of HGI in type 2 diabetes cannot currently be recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
HbA1c is an established means of monitoring average blood glucose levels and a surrogate marker of the effect of glucose-lowering interventions [1]. It is highly associated with the risk for diabetes-related complications, in particular those of microvascular origin [2,3,4,5]. Although HbA1c is almost universally accepted to guide and monitor diabetes treatment, its use in clinical practice has arguable limitations. There is a proposed inter-individual variation in the propensity for glycation, in both healthy individuals and those with diabetes [6,7,8,9,10,11,12,13], limiting the use of HbA1c as a one-size-fits-all measurement. Moreover, the value of HbA1c as a surrogate endpoint was questioned by the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, where HbA1c lowering may have had detrimental effects on the risk of premature mortality [14]. Therefore, ‘the lower the better’ may not universally hold for HbA1c, and additional (bio)markers might be useful to individualise treatment targets and risk prediction [15].
The haemoglobin glycation index (HGI) quantifies the variation in the relation between HbA1c and the plasma glucose concentration [16]. For any individual within a study population, HGI is defined as the difference between the observed HbA1c and the fitted value from a simple linear model that predicts HbA1c from the fasting plasma glucose (FPG) concentration, i.e. the residual from the fitted linear regression line. In previous studies, HGI was normally distributed, stable over time and consistent over a wide range of blood glucose concentrations [17,18,19,20]. In an analysis in individuals with type 1 diabetes in the DCCT, a high HGI was associated with the risk for and progression of retino- and nephropathy [21]. In an analysis of the ACCORD trial, only participants in the highest HGI third were at higher risk for mortality and those with high HGI showed no benefit on cardiovascular outcomes after intensive glucose lowering, in contrast to participants with a low or intermediate HGI [16]. The use of HGI is not without controversy, as in the DCCT population it was shown that the effect of HGI on microvascular complications disappeared after adjustment for the effect of HbA1c [22]. However, the use of HbA1c in type 1 diabetes is undisputed, whereas in individuals with type 2 diabetes, HbA1c seems to have shortcomings, as demonstrated by the ACCORD trial.
The aim of this study was to assess whether HGI is a predictor of adverse outcomes of intensive glucose-lowering therapy and a predictor of diabetes-related complications in the cohort of individuals with type 2 diabetes recruited for the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial (ClinicalTrials.gov registration no. NCT00145925) [23]. Additionally, we aimed to compare the predictive values of HGI and HbA1c to assess the possible added value of HGI beyond HbA1c.
Methods
ADVANCE trial
In the ADVANCE trial, 11,140 individuals with type 2 diabetes and a history of, or a risk factor for, vascular disease were randomised in a factorial design between two BP-lowering strategies and two glucose-lowering strategies [24]. Glucose-lowering treatment was either standard (based on local guidelines) or intensive, starting with gliclazide (30–120 mg daily, modified release) and adding other medication as necessary (based on the study protocol and discretion of the treating physician) to achieve a HbA1c level of ≤48 mmol/mol (≤6.5%). Primary endpoints were a composite of major macro- and microvascular events. Study participants were, on average, 66 years old, with a mean diabetes duration of 8 years and a mean baseline HbA1c of 59 mmol/mol (7.5%). After a follow-up of 5 years, mean HbA1c was 48 mmol/mol (6.5%) in the intensively treated group vs 56 mmol/mol (7.3%) in the standard group. Intensive glucose-lowering treatment reduced the combination of macro- and microvascular events, mainly due to a 20% reduction in nephropathy, at the cost of an 86% increase in the risk of severe hypoglycaemia.
Present study
HGI
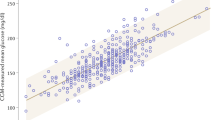
We excluded 57 individuals with missing baseline HbA1c (n = 54) or baseline FPG (n = 3). For the 11,083 remaining individuals, we fitted a linear regression. The linear regression equation describing the relation between baseline HbA1c and FPG in our population was HbA1c (%) = 4.5 + 0.356 × FPG (mmol/l) (r2 = 0.40, Fig. 1a). Results from simple linear regression were comparable with a cubic spline model, so we chose the simpler linear model. We derived predicted HbA1c by inserting FPG values into this regression equation. Baseline HGI was calculated by subtracting predicted HbA1c from observed HbA1c (equalling the residual from the regression line, Fig. 1b). In this way, individuals with high HGI will have a higher measured HbA1c than anticipated from the FPG value. In accordance with existing literature, we divided the population in three equally sized HGI groups (low, intermediate and high). This study was conducted according to the principles of the declaration of Helsinki [25] and in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO). All participants provided written informed consent for the original study.
(a) Regression of FPG on HbA1c. The dotted line represents the simple linear regression line of the equation: HbA1c (%) = 4.5 + 0.356 × FPG (mmol/l), r2 = 0.40. (b) Plot of the residuals (HGI) vs the fitted (predicted HbA1c) values (p=1.00). To convert values for HbA1c in % into mmol/mol, subtract 2.15 and multiply by 10.929
Outcomes
We analysed four predefined outcome measures: (1) major macrovascular events, defined as death from cardiovascular cause, non-fatal myocardial infarction or non-fatal stroke; (2) major microvascular events, defined as new or worsening nephropathy or retinopathy; (3) total mortality; and (4) severe hypoglycaemia. Severe hypoglycaemia was defined as transient dysfunction of the central nervous system that could not be self-treated.
Statistical analysis
We compared baseline characteristics between the low, intermediate and high HGI groups using ANOVA or Kruskal–Wallis tests for continuous variables, depending on the distribution, and χ2 tests for categorical variables. We used Cox proportional hazard regression models to analyse the association between baseline HGI and time to event for all four outcomes, with adjustment for covariates. We studied separately: (1) the treatment-modifying effect of HGI; and (2) the effect of HGI independent of treatment allocation—the first to make a comparison with the results from the ACCORD reanalysis, the latter as it seems more relevant for use in clinical practice where individuals will undergo variable treatment regimens. To assess whether HGI was better than time-matched (baseline) HbA1c we added HbA1c as a covariate to our models and used HbA1c instead of HGI as predicting variable to compare the effect on outcomes.
Treatment-modifying effect of HGI
We investigated the effect of intensive glucose control on outcomes across HGI groups, by adding the interaction term between HGI group and treatment allocation (intensive or standard glucose-lowering therapy) to the model with HGI and treatment. We adjusted the model for age, sex, ethnic origin (Asian or non-Asian), BMI, duration of type 2 diabetes, history of macro- and microvascular events, current drinking and smoking habits, use of glucose-lowering medication, use of BP-lowering drugs, systolic and diastolic BP, haemoglobin, renal function (eGFR), LDL-, HDL- and total cholesterol and triacylglycerol. We added baseline HbA1c to the before-mentioned covariates in a separate analysis. Finally, we assessed the associations between HbA1c (instead of HGI) as the exposure variable and outcomes, adjusted for the same covariates and studied the interaction between treatment and HbA1c.
Effect of HGI independent of treatment allocation
We assessed the association between HGI and outcomes, irrespective of treatment, for the low, intermediate and high HGI group, as well as for HGI as a continuous variable. The low HGI group served as the reference group in the comparison. We adjusted for the identical set of standard covariates (with and without HbA1c). Again, we assessed the associations between HbA1c (instead of HGI) as the exposure variable and outcomes, adjusted for the same covariates.
As only 1.5% of data were missing, no imputation was carried out. Significance levels were set at a p < 0.05. Statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS STAT 9.4; SAS Institute, Cary, NC, USA).
Results
In this population, HGI showed a normal distribution, ranging from −6.46 to 8.99, with a mean of 0 (SD 1.20). When dividing the study population into thirds, HGI cut-off points were ≤−0.53 for the low, −0.52 to 0.28 for the intermediate and ≥0.29 for the high HGI group. Mean baseline HGI was −1.14 (SD 0.57) in the low, −0.14 (SD 0.23) in the intermediate and 1.29 (SD 0.99) in the high HGI group (Table 1).
There were some significant differences in baseline characteristics between the HGI groups (Table 1). Individuals in the high HGI group had the longest duration of diabetes and a higher proportion used glucose-lowering medication. More of those in the high HGI group were smokers, but fewer consumed alcohol. The prevalence of previous microvascular events was highest in the high HGI group. Almost half of the individuals in the high HGI group were of Asian origin, in contrast to a third in the low and intermediate group. HbA1c levels increased from low to high HGI group and FPG was lowest in the intermediate HGI group.
Treatment-modifying effect of HGI
Table 2 shows the adjusted HRs based on HGI group and treatment allocation (intensive or standard glucose-lowering therapy). The effect of treatment allocation on macro- and microvascular complications was similar across the three HGI groups. However, the effect of intensive therapy on mortality risk differed between HGI groups (p for interaction = 0.011). In the high HGI group the mortality risk was significantly lower with intensive therapy (adjusted HR 0.74 [95% CI 0.61, 0.91]; p = 0.003), whereas intensive treatment did not diminish risk for mortality in the low and intermediate groups. This effect remained after additional adjustment for baseline HbA1c level (electronic supplementary material (ESM) Table 1). With regard to severe hypoglycaemia, absolute rates increased progressively as HGI rose, regardless of whether individuals received intensive or standard treatment. The effect of intensive treatment on the risk for severe hypoglycaemia was not different between HGI thirds, as indicated by the non-significant p value for interaction (0.228). To compare the predictive value of HGI with HbA1c, we assessed the interaction between treatment and HbA1c groups using the same multivariable model (ESM Table 2). Here, there was no difference in effect of intensive treatment on mortality risk across the three HbA1c groups (p for interaction = 0.530). Individuals with intermediate and high HbA1c were at greater risk for severe hypoglycaemia when intensively treated, contrary to individuals with low HGI.
Effect of HGI independent of treatment allocation
Table 3 shows HRs for outcomes by HGI group irrespective of treatment allocation, with the low HGI group as reference group. In the multivariable analysis, individuals in the high HGI group had a significantly higher risk for major macrovascular events, compared with the low HGI group (HR 1.26 [95% CI 1.09, 1.46]; p = 0.002). Further, the risk for major microvascular events and mortality was also higher in the high HGI group compared with the low HGI group (HR 1.46 [95% CI 1.26, 1.69], p < 0.0001 and HR 1.36 [95% CI 1.17, 1.59], p < 0.0001, respectively). The risk of severe hypoglycaemia did not differ between HGI groups. The effect of HGI on these complications disappeared after additional adjustment for HbA1c (ESM Table 3). Again, we assessed the association between HbA1c groups and adverse outcomes using the same multivariable model (ESM Table 4). Likewise, the high HbA1c group had a higher risk for macro- and microvascular complications and mortality compared with the low HbA1c group. HRs exceeded those seen for HGI. HbA1c was not associated with the risk of severe hypoglycaemia.
When we considered HGI as a continuous variable, every 1 SD (=1.20 HGI) increase resulted in a 14% risk increase for microvascular complications, a 17% risk increase for macrovascular complications and a 16% risk increase for mortality (p < 0.0001) (Fig. 2). Continuous HGI was not associated with severe hypoglycaemia (HR 1.10 [95% CI 0.97, 1.25]; p = 0.123). Continuous HbA1c (1 SD = 1.56% HbA1c) was a stronger predictor for the risk of microvascular events (HR 1.19 [95% CI 1.13, 1.26]; p < 0.0001), macrovascular events (HR 1.31 [95% CI 1.24, 1.38]; p < 0.0001) and mortality (HR 1.14 [95% CI 1.14, 1.28]; p < 0.0001) than HGI.
Multivariable Cox proportional hazard regression analysis for major macrovascular events, major microvascular events, total mortality and severe hypoglycaemia predicted by continuous HGI and HbA1c per SD increase (1 SD of HGI is 1.20. 1 SD of HbA1c is 1.56%). Major macrovascular events were defined as death from a cardiovascular cause, non-fatal myocardial infarction or stroke. Major microvascular events were defined as new or worsening nephro- or retinopathy. Severe hypoglycaemic episodes were defined as transient dysfunction of the central nervous system with the inability to treat oneself. Model was adjusted for age, sex, ethnic origin (Asian vs non-Asian), BMI, duration of type 2 diabetes, history of macro- and microvascular events, current drinking and smoking, use of glucose-lowering drugs, use of BP-lowering drugs, systolic BP, diastolic BP, haemoglobin, renal function (eGFR), LDL-, HDL- and total cholesterol, triacylglycerol. Diamonds, HGI; circles, HbA1c
Discussion
With this analysis of the ADVANCE trial we showed that HGI predicts diabetes-related complications, but no better than HbA1c. Irrespective of treatment allocation, the high HGI group (i.e. individuals with higher HbA1c levels than would be expected for their given fasting glucose levels) was at higher risk for macro- and microvascular complications and mortality compared with the low HGI group. Every SD increase in HGI gave a significant 14–17% risk increase for these three outcomes. Hypothetically, this could be explained by a higher propensity for glycation of membrane proteins and lipids other than haemoglobin, with these glycation products leading to microvascular complications and atherogenesis. This effect disappeared after additional adjustment for HbA1c, which is in line with results on the effect of HGI in individuals with type 1 diabetes in the DCCT [22]. However, this might be considered over-adjustment, as HGI is so strongly related to HbA1c (HGI is HbA1c corrected for FPG). Therefore, we separately assessed the effect of HbA1c on outcomes using the same model and found that HbA1c was just as strong or even stronger for predicting complications in this cohort of individuals with type 2 diabetes. To our knowledge, this is the first time the predictive value of HGI has been compared with that of HbA1c in this way. As HbA1c does not need a population regression equation as does HGI, this makes HbA1c more straightforward and convenient to use.
In our study, intensive treatment carried a lower risk for mortality in individuals with a high HGI, whereas it had no effect on mortality in individuals with a low or intermediate HGI. Thus, a high HGI identified a group of people who benefitted most from intensive HbA1c-lowering treatment in terms of mortality. This finding remained after additional correction for baseline HbA1c, but was not replicated by using HbA1c as the predicting variable, as we found no interaction between treatment and HbA1c groups. Overall, the estimates for the effect of intensive treatment as shown in Table 2 remained unchanged after additional adjustment for HbA1c (ESM Table 1), which is not surprising given that HGI is just a linear function of HbA1c and FPG (i.e. it is HbA1c – (a + b × FPG), where a and b are regression coefficients). The above directly opposes the results of an analysis of the ACCORD trial, where intensive treatment was associated with a significantly higher, instead of lower, risk for mortality in participants with a high HGI [16]. Thus, the hypothesis put forward that a high HGI results in more complications due to more intensive treatment to lower HbA1c than is necessary to lower plasma glucose is not supported by our study.
The inconsistency between the effect of HGI on outcomes in these two large outcome studies might be explained by important differences between ACCORD and ADVANCE. First, glucose-treatment strategies were different, although both took HbA1c as predominant measure of glycaemia and both took glucose into account. Glycaemic treatment in ACCORD was intensified when HbA1c level was ≥42 mmol/mol (≥6%) or when >50% of the self-monitored pre- or 2 h post-meal capillary glucose values were above a certain threshold [26].The treatment algorithm of the ADVANCE trial took discrepancies between HbA1c levels and blood glucose values into account simultaneously [23]. When HbA1c level was >47 mmol/mol (>6.5%) but fasting glucose was relatively low, mealtime interventions were optimised and the reliability of the tests was checked. Also ACCORD had participants who started with a higher HbA1c and had a lower target HbA1c in the intensive group. Further, 30% more individuals under intensive treatment received insulin in ACCORD compared with ADVANCE [5]. This is in agreement with the observation that FPG was treated more aggressively in ACCORD, with a decrease of 3.3 mmol/l from baseline to end of trial, compared with 1.9 mmol/l in ADVANCE. Moreover, in ADVANCE all participants received a sulfonylurea derivative at the start, while in ACCORD thiazolidinedione treatment was frequently used. The additional treatments differed between studies (i.e. use of aspirin and statins was substantially higher in ACCORD). Second, ACCORD was terminated prematurely, limiting the follow-up, and potentially misrepresenting estimates (no adjustment to standard errors was made for early stopping). Third, ADVANCE and ACCORD were discordant in the major findings, including mortality. In ACCORD, mortality rates were significantly higher in the intervention arm compared with the control arm [14], whereas in ADVANCE there were no significant differences in mortality between arms [23]. Post hoc, it was shown that intensively treated ACCORD participants with a high average on-treatment HbA1c (>53 mmol/mol [>7%]) were at greater risk for mortality than intensively treated participants with average HbA1c <53 mmol/mol (<7%) or standard-treated individuals with average HbA1c >53 mmol/mol (>7%) [27]. The number of individuals experiencing severe hypoglycaemia was significantly higher with intensive treatment in both studies, but the event rates per person-year were higher in the ACCORD trial (3.5% per year with intensive treatment vs 1.0% per year in the control arm), whereas in ADVANCE rates were 0.7% per year in intensive treatment vs 0.4% per year in the control arm. We found no observable difference in the effect of HGI on severe hypoglycaemia due to intensive treatment, although the absolute rates and adjusted hazard ratios increased as HGI rose. With regard to HbA1c, individuals with intermediate and high HbA1c were at greater risk for severe hypoglycaemia when intensively treated compared with individuals with low HbA1c, a finding consistent with previous literature [28].
Baseline characteristics of individuals with high HGI accorded with previous studies [16, 29]. The ethnic differences (i.e. more Asians in the high HGI group) are consistent with the observation that ethnicity influences the haemoglobin glycation, with, in general, relative higher HbA1c levels in non-whites [17, 30,31,32,33]. However, regression equations in Asian (HbA1c = 4.6 + 0.373 × FPG, r2 = 0.41) and non-Asian (HbA1c = 4.5 + 0.340 × FPG, r2 = 0.40) participants were very similar. The combination of a slightly younger age, longer duration of diabetes (on average 2 years), more use of glucose-lowering medication and higher rates of microvascular complications suggests that individuals with high HGI might have a form of diabetes that is more difficult to treat. This in itself can be the cause of diabetes-related complications, but there is also potential for confounding, as these characteristics could well be explained by the higher HbA1c levels in individuals with high HGI [22]. The HGI concept is based on the proposed inter-individual variation in haemoglobin glycation, while an adequate method for measuring glycation rate is lacking. Erythrocyte lifespan is a major determinant of the variation in haemoglobin glycation and subtle natural variation in senescence of erythrocytes is complex to quantify [34, 35]. To our knowledge, there are no studies focusing on the pathophysiological mechanism explaining both the biological variation in haemoglobin glycation as well as the reason for the possible increased risk for complications associated with higher glycation rates. This study was limited by a single FPG measurement to determine the relationship with HbA1c, so we could not take diurnal changes in plasma glucose into account. A measure of average glucose would have been preferred, but was not available in our data and might not be in clinical practice where individuals often use oral glucose-lowering medication only. The DCCT used seven-point glucose profiles to assess HGI [21], while ACCORD used FPG only [16].
In conclusion, we found that HGI predicted macro- and microvascular complications and mortality, but was no better than HbA1c, which was a stronger predictor for these outcomes. Moreover, HbA1c is simpler than HGI. High HGI does predict risk for mortality with intensive treatment, but results are the opposite of those from ACCORD. Bringing all this together, the evidence does not support the clinical relevance and usefulness of HGI above HbA1c.
Data availability
The datasets analysed are available upon reasonable request and with permission of the ADVANCE Collaborative Group.
Abbreviations
- ACCORD:
-
Action to Control Cardiovascular Risk in Diabetes
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- FPG:
-
Fasting plasma glucose
- HGI:
-
Haemoglobin glycation index
References
ADA (2016) Standards of medical care in diabetes. Available from http://care.diabetesjournals.org/content/suppl/2015/12/21/39.Supplement_1.DC2/2016-Standards-of-Care.pdf. Accessed 1 July 2017
The DCCT Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Abraira C, Colwell J, Nuttall F et al (1997) Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Arch Intern Med 157:181–188
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Turnbull FM, Abraira C, Anderson RJ et al (2009) Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52:2288–2298
Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ (1990) Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 33:208–215
Gould BJ, Davie SJ, Yudkin JS (1997) Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta 260:49–64
Rohlfing C, Wiedmeyer HM, Little R et al (2002) Biological variation of glycohemoglobin. Clin Chem 48:1116–1118
McCarter RJ, Hempe JM, Chalew SA (2006) Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care 29:352–355
Kilpatrick ES, Rigby AS, Atkin SL (2007) Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clin Chem 53:897–901
Leslie RD, Cohen RM (2009) Biologic variability in plasma glucose, hemoglobin A1c, and advanced glycation end products associated with diabetes complications. J Diabetes Sci Technol 3:635–643
Hempe JM, Soros AA, Chalew SA (2010) Estimated average glucose and self-monitored mean blood glucose are discordant estimates of glycemic control. Diabetes Care 33:1449–1451
Wilson DM, Xing D, Cheng J et al (2011) Persistence of individual variations in glycated hemoglobin: analysis of data from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Randomized Trial. Diabetes Care 34:1315–1317
Gerstein HC, Miller ME, Byington RP et al (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
Hare MJ, Shaw JE, Zimmet PZ (2012) Current controversies in the use of haemoglobin A1c. J Intern Med 271:227–236
Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V (2015) The Hemoglobin Glycation Index Identifies Subpopulations With Harms or Benefits From Intensive Treatment in the ACCORD Trial. Diabetes Care 38:1067–1074
Hempe JM, Gomez R, McCarter RJ Jr, Chalew SA (2002) High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complicat 16:313–320
Wilson DM, Kollman (2008) Relationship of A1C to glucose concentrations in children with type 1 diabetes: assessments by high-frequency glucose determinations by sensors. Diabetes Care 31:381–385
Soros AA, Chalew SA, McCarter RJ, Shepard R, Hempe JM (2010) Hemoglobin glycation index: a robust measure of hemoglobin A1c bias in pediatric type 1 diabetes patients. Pediatr Diabetes 11:455–461
Kamps JL, Hempe JM, Chalew SA (2010) Racial disparity in A1C independent of mean blood glucose in children with type 1 diabetes. Diabetes Care 33:1025–1027
McCarter RJ, Hempe JM, Gomez R, Chalew SA (2004) Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 27:1259–1264
Lachin JM, Genuth S, Nathan DM, Rutledge BN (2007) The hemoglobin glycation index is not an independent predictor of the risk of microvascular complications in the Diabetes Control and Complications Trial. Diabetes 56:1913–1921
Patel A, MacMahon S, Chalmers J et al (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
ADVANCE Management Committe (2001) Study rationale and design of ADVANCE: action in diabetes and vascular disease--preterax and diamicron MR controlled evaluation. Diabetologia 44: 1118–1120
The World Medical Association (2013) Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 64th WMA General Assembly, Fortaleza, Brazil
Gerstein HC, Riddle MC, Kendall DM et al (2007) Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 99:34i–43i
Riddle MC, Ambrosius WT, Brillon DJ et al (2010) Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 33:983–990
Mitchell BD, Vietri J, Zagar A, Curtis B, Reaney M (2013) Hypoglycaemic events in patients with type 2 diabetes in the United Kingdom: associations with patient-reported outcomes and self-reported HbA1c. BMC Endocr Disord 13:59
van Steen SC, Schrieks IC, Hoekstra JB et al (2017) The haemoglobin glycation index as predictor of diabetes-related complications in the AleCardio trial. Eur J Prev Cardiol 24:858–866
Herman WH, Ma Y, Uwaifo G et al (2007) Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 30:2453–2457
Dagogo-Jack S (2010) Pitfalls in the use of HbA(1)(c) as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol 6:589–593
Wolffenbuttel BH, Herman WH, Gross JL, Dharmalingam M, Jiang HH, Hardin DS (2013) Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care 36:2931–2936
Herman WH, Cohen RM (2012) Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab 97:1067–1072
Cohen RM, Franco RS, Khera PK et al (2008) Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112:4284–4291
Soranzo N, Sanna S, Wheeler E et al (2010) Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes 59:3229–3239
Acknowledgements
Some of the data were presented as an abstract at the 53rd EASD Annual Meeting in 2017 (number 1135).
Funding
We received no funding for this study. The ADVANCE trial was supported by grants from Servier (the major financial sponsor) and the National Health and Medical Research Council of Australia (211086 and 358395). Servier manufactures gliclazide (modified release) and the fixed combination of perindopril and indapamide. The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Consortia
Contributions
SCS, MW, JC, DEG and JHDV conceived the study, interpreted the data, and drafted and edited the manuscript. MW and QL performed statistical analysis, interpreted the data and edited the manuscript. All other authors interpreted the data and critically reviewed the manuscript for important intellectual content. All authors gave final approval of the version to be published. SCS and QL are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
MW received consulting fees from Amgen. JC received research grants and speaker fees from Servier. MM received personal fees from Novo Nordisk, Sanofi, Eli Lilly, Merck Sharp and Dohme, Abbott, Novartis and AstraZeneca and grant support from Novo Nordisk, Sanofi, Eli Lilly, Merck Sharp and Dohme and Novartis. MEC received consulting fees from Merck, GlaxoSmithKline, Amgen and AstraZeneca and lecture fees from Servier. PH received consulting fees from Servier. GM received lecture fees from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Novartis, Menarini International, Recordati, Servier and Takeda. SC received fees for serving on advisory boards and lecture fees from Servier. BW received lecture fees from Novartis, Boehringer Ingelheim and Merck Sharpe and Dohme. DEG received lecture fees from Servier and consulting and lecture fees and grant support from Pfizer, AstraZeneca, Novartis and Sanofi-Aventis. JHDV received speaker fees from Novo Nordisk and Senseonics, research support from Abbott, Dexcom, Medtronic, Novo Nordisk, Sanofi and Senseonics, and fees for serving on advisory boards from Merck Sharpe and Dohme, Novo Nordisk, Roche and Sanofi. The remaining author declares that there is no duality of interest associated with their contribution to this manuscript.
Electronic supplementary material
ESM Tables
(PDF 199 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Steen, S.C., Woodward, M., Chalmers, J. et al. Haemoglobin glycation index and risk for diabetes-related complications in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia 61, 780–789 (2018). https://doi.org/10.1007/s00125-017-4539-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-017-4539-1