Abstract
Aims/hypothesis
The association between sodium–glucose cotransporter 2 (SGLT2) inhibitors and the risk of cancer in individuals with type 2 diabetes remains uncertain. This study aimed to evaluate the risk of cancer associated with SGLT2 inhibitor treatment of type 2 diabetes.
Methods
We systematically searched PubMed, EMBASE, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov from inception to 15 February 2017 to identify eligible randomised controlled trials (RCTs) that report cancer events in individuals with type 2 diabetes treated with SGLT2 inhibitors for at least 24 weeks. We performed pairwise and network meta-analyses as well as a cumulative meta-analysis to calculate ORs and 95% CIs.
Results
In total, 580 incidences of cancer among 34,569 individuals were identified from 46 independent RCTs with a mean trial duration of 61 weeks. When compared with comparators (placebo or other active glucose-lowering treatments), SGLT2 inhibitors were not significantly associated with an increased risk of overall cancer (OR 1.14 [95% CI 0.96, 1.36]). For pre-specified cancer types, the risk of bladder cancer might be increased with SGLT2 inhibitors (OR 3.87 [95% CI 1.48, 10.08]), especially empagliflozin (OR 4.49 [95% CI 1.21, 16.73]). Interestingly, canagliflozin might be protective against gastrointestinal cancers (OR 0.15 [95% CI 0.04, 0.60]).
Conclusions/interpretation
Current evidence from short-term RCTs did not indicate a significantly increased risk of overall cancer among individuals with type 2 diabetes using SGLT2 inhibitors. Given the short-term trial durations and uncertainty of evidence, future long-term prospective studies and post-marketing surveillance studies are warranted.
Similar content being viewed by others
Introduction
Growing evidence suggests that people with type 2 diabetes are at elevated risk for cancer [1, 2]. Though the mechanisms remain unknown, several carcinogenic processes involving the pathophysiology of type 2 diabetes may explain the increased cancer risk in these individuals. Certain diabetes risk factors (e.g. obesity) play a significant role in increasing cancer risk [3]. Furthermore, several glucose-lowering drugs have the potential to affect cancer risk [1]. For example, metformin therapy has been shown to decrease the risk of cancer, while other drugs may increase the risk of specific cancers [4]. Recently, concern was raised about a potential link between thiazolidinediones (e.g. pioglitazone) and bladder cancer [5]. However, no clear conclusions have been drawn regarding a causal relationship [6].
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are a novel class of oral glucose-lowering drugs for treating type 2 diabetes [7]. They decrease plasma glucose levels by selectively inhibiting renal glucose reabsorption and increasing urinary glucose excretion [8, 9]. In addition to their hypoglycaemic effects, SGLT2 inhibitors also offer additional benefits for weight loss and reduction of BP [10]. In clinical practice, SGLT2 inhibitors are recommended in combination with metformin and/or other agents as second- or third-line therapy if an individual fails to achieve the target level of glycaemic control with one or more other agents [11].
In 2011, a regulatory submission presented to the US Food and Drug Association (FDA) raised concerns regarding the risk of bladder and breast cancer associated with dapagliflozin [12]. An imbalance between dapagliflozin and comparators in the risk of bladder and breast cancer was observed in the 2011 report [12]. However, a recent pooled analysis of 21 clinical trials suggested that the increased risk of bladder and breast cancers might be an absence of detailed diagnosis prior to randomisation rather than a causal relationship [13]. An elevated risk of bladder or breast cancer has not been reported for other SGLT2 inhibitors in humans [14], although it was indicated that they might induce tumours in rats [15] and male mice [16]. Given conflicting results regarding possible associations with rare cancers, individual trials are not powerful enough to clarify the cancer risk associated with the use of SGLT2 inhibitors. We therefore performed a pairwise meta-analysis of all available head-to-head randomised controlled trial (RCT) data to test the hypothesis that SGLT2 inhibitors affect cancer risk by comparing SGLT2 inhibitors with placebo in individuals with type 2 diabetes. We also carried out a network meta-analysis to evaluate the comparative effects of SGLT2 inhibitors on cancer risk using a combination of direct and indirect evidence based on a common comparator (e.g. placebo).
Methods
The network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting of systematic reviews incorporating network meta-analyses of healthcare interventions [17] and was registered with PROSPERO (number CRD42016045707).
Search strategy and study selection
We comprehensively searched PubMed, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) from inception to 15 February 2017 to identify eligible RCTs using the following search terms: random*, RCTs, sodium–glucose cotransporter, SGLT2, SGLT-2 and the names of ten individual SGLT2 inhibitors. No restrictions were applied in terms of language, date or publication. In addition, we identified other published and unpublished trials by manually searching the references of included trials and relevant meta-analyses as well as ClinicalTrials.gov. Detailed information about our search strategy is presented in the electronic supplementary material (ESM) Table 1. Furthermore, we reviewed the submission documents provided to the US FDA or European Medicines Agency (EMA) for more data. Two reviewers independently selected the studies according to the following inclusion criteria: (1) RCTs that compared SGLT2 inhibitors with placebo or other active glucose-lowering treatments in adults with type 2 diabetes; (2) trial duration ≥24 weeks; and (3) studies reporting any cancer as an outcome. Our primary outcome measure was risk of overall cancer and the secondary outcomes included risk of pre-specified cancer types including skin, breast, respiratory, gastrointestinal, bladder, prostate and renal (ESM Table 2). Any cancer event was reported by investigators as a serious adverse event identified in the database using pre-specified lists from the Medical Dictionary for Regulatory Activities (MedDRA). Conference abstracts were excluded because of the lack of detailed information on the trials’ characteristics, definition of outcome and trial quality.
Data extraction and quality assessment
Two reviewers (H. Tang and W. Shi) independently extracted the following data: first author, publication year, study characteristics (country of origin, funding and follow-up), characteristics of participants (inclusion criteria, background treatments, mean age, proportion of men, duration of type 2 diabetes, baseline HbA1c [%] and BMI), interventions (type and dose of SGLT2 inhibitors), comparators and the incidence of cancer.
If multiple reports from the same population were retrieved, only the most complete and/or most recently reported data were used. If cancer events were not reported in the manuscripts, data from regulatory submissions or the ‘Serious adverse events’ section on ClinicalTrials.gov were extracted. In addition, if pre-specified cancer outcomes were not reported on ClinicalTrials.gov, the incidence of the events was assumed to be zero. If two different comparison groups of non-overlapping participants (i.e. A vs B and C vs D) were included in the same report, each comparison was considered separately. If three arms (i.e. A vs B vs A+B) were evaluated in the RCTs, only two arms (A vs B) were included.
The Cochrane risk of bias tool was used to assess the quality of RCTs based on the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias) [18]. Two reviewers (H. Tang and W. Shi) independently reviewed and judged each domain as low risk of bias, high risk of bias or unclear risk of bias.
Statistical analysis
Both pairwise and network meta-analyses were performed to calculate the ORs and 95% CIs of overall cancer or pre-specified types of cancer. All meta-analyses were performed with Stata (version 14; Stata, College Station, TX, USA).
For the pairwise meta-analysis, Peto’s method was used to calculate the ORs for direct comparisons between therapeutic regimens to account for low event rates [19]. An I 2 statistic was used to evaluate the presence of between-study heterogeneity, with an I 2 of <25%, ≥25 and <75%, and ≥75% indicating low, medium and high heterogeneity, respectively [20]. The source of heterogeneity was further explored in the following pre-specified subgroups: (1) type of SGLT2 inhibitors (canagliflozin vs dapagliflozin vs empagliflozin); (2) type of control groups (placebo vs other active treatment); (3) length of trial duration (<52 vs ≥52 weeks); (4) mode of therapy (SGLT2 inhibitor monotherapy vs SGLT2 inhibitor add-on therapy); (5) race/ethnicity (white vs Asian); (6) mean age (≥60 years vs <60 years); (7) mean BMI (≥30 kg/m2 vs <30 kg/m2); and (8) mean percentage of male participants (≥50% vs <50%). Additionally, a meta-regression was performed to explore whether the above variables influenced the size of intervention effects. A sensitivity analysis was carried out by comparing two statistical methods (Peto vs Manthel–Haenszel method), comparing two effect measures (OR vs RR) or excluding the largest trial (EMPA-REG OUTCOME Trial) [21]. In addition, a cumulative meta-analysis was performed to explore the evolution of the evidence with the accumulation of data over time. Finally, potential publication bias was assessed by the Begg’s and Egger’s tests, as well as visual inspection of the funnel plots.
For indirect and mixed comparisons, a network meta-analysis with a random-effects model using the ‘mvmeta’ command and programmed Stata routines was used to compare different interventions [22, 23]. For zero-event RCT, a 0.5 zero-cell correction was applied before meta-analysis [24]. To rank the SGLT2 inhibitors for a specified outcome, we estimated the relative ranking probabilities of each treatment using the surface under the cumulative ranking curve (SUCRA) and mean ranks. For incidence of cancer, large SUCRA probability and lower mean rank indicate a safer intervention [25]. The heterogeneity variance (tau) estimated by a restricted maximum likelihood method was employed to investigate between-study heterogeneity in the network meta-analysis [26].
To check for the presence of inconsistency, a loop inconsistency–specific approach was introduced to evaluate the difference between direct and indirect estimates for a specific comparison [27]. To check the assumption of consistency in the entire network, a design-by-treatment interaction model using the χ2 test was used [28]. In addition, a comparison-adjusted funnel plot was used to assess small-study effects within a network of interventions [29].
Results
Study selection and study characteristics
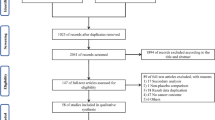
A total of 2450 citations were retrieved through electronic search. Of which, 201 potentially eligible reports were identified by reviewing study titles and abstracts. After fully reviewing the potential trials and searching lists of references and ClinicalTrials.gov, finally, 45 articles with 46 independent RCTs were eligible and included in this meta-analysis [21, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] (ESM Fig. 1). Two articles provided two independent datasets for two different comparisons which we considered separately [42, 58]. Because data from two trials were presented together on ClinicalTrials.gov, we included the combined data as one independent trial [70, 71].
The study characteristics are summarised in ESM Table 3. In total, 34,569 participants from 46 independent trials were randomly assigned to one of three SGLT2 inhibitors (canagliflozin, dapagliflozin and empagliflozin) or comparators (placebo or other active glucose-lowering treatment). Sample sizes of individual trials were between 180 and 7020 participants, and the mean trial duration was 61 weeks (range 24–160 weeks). The spread of trial duration for each SGLT2 inhibitors is presented on ESM Fig. 2.
The risk of bias for the 46 RCTs is summarised as follows (ESM Fig. 3): 36 RCTs reported adequate random sequence generation; 33 RCTs reported adequate allocation concealment; masking conditions were high in three RCTs, of which two RCTs were open-label in their extended periods and one RCT set one arm with open-label; finally, all RCTs were judged as unclear for selective reporting because none included cancer events as outcomes of interest. All of the trials were funded by industrial companies.
Pairwise meta-analysis
Forty-six trials reported the incidence of overall cancer with a total of 580 events among 34,569 participants (a crude event rate of 1.68%). Cancer rates were 1.78% in the SGLT2 inhibitor treatment groups and 1.55% in the comparator groups. The results of overall and subgroup pairwise meta-analysis are presented in Fig. 1. There was no significant difference between SGLT2 inhibitors and comparators in overall cancer risk (OR 1.14 [95% CI 0.96, 1.36]), with low statistical heterogeneity (I 2 = 19.2%) (ESM Fig. 4). The pre-specified subgroup analyses showed that SGLT2 inhibitors were significantly associated with increased risk of overall cancer only in obese participants with a mean BMI ≥30 kg/m2 (OR 1.23 [95% CI 1.02, 1.48]) (Fig. 1). We found no significant difference between SGLT2 inhibitors and placebo (OR 1.17 [95% CI 0.96, 1.41]) and between SGLT2 inhibitors and other active treatments (OR 1.03 [95% CI 0.67, 1.57]). Our meta-regression analysis indicated that none of the pre-specified factors, significantly influenced the sizes of treatment effects (all p > 0.05). There was low heterogeneity among studies (I 2 range 0–53.1%). Our cumulative meta-analysis based on publication year showed that SGLT2 inhibitors were not significantly associated with increased risk of overall cancer (Fig. 2).
In the sensitivity analysis, the results remained robust to different pairwise meta-analysis methods and the exclusion of the largest trial (EMPA-REG OUTCOME Trial) (OR 1.03 [95% CI 0.81, 1.33]) (ESM Table 4 and ESM Fig. 5). Moreover, our analysis yielded no evidence of substantial publication bias, based on the Egger’s test (p = 0.31), Begg’s test (p = 0.72), and a visual inspection of the funnel plot (ESM Fig. 6).
When pre-specified types of cancer were analysed, SGLT2 inhibitors were significantly associated with increased risk of bladder cancer (OR 3.87 [95% CI 1.48, 10.08]), particularly in the comparison of empagliflozin vs comparators (OR 4.49 [95% CI 1.21, 16.73]) (Fig. 3). Canagliflozin was significantly associated with lower risk of gastrointestinal cancers than comparators (OR 0.15 [95% CI 0.04, 0.60]) (Fig. 3). No significant differences between SGLT2 inhibitors and comparators were observed in the risks of other pre-specified cancer types (Fig. 3). For bladder cancer risk, a further subgroup analysis indicated a significantly increased risk in the trials with durations ≥52 weeks (OR 4.80 [95% CI 1.74, 13.29]), mean BMI ≥30 kg/m2 (OR 4.65 [95% CI 1.40, 15.48]), or mean age ≥ 60 years (OR 3.57 [95% CI 1.09, 11.66]) (ESM Fig. 7). In addition, there was low to medium heterogeneity among studies (I 2 range 0–52.1%).
Network meta-analysis
The trial network plot and the results of network meta-analysis for overall cancer risk are presented in ESM Fig. 8 and Fig. 4, respectively. Compared with placebo, none of canagliflozin (OR 0.74 [95% CI 0.35, 1.55]), dapagliflozin (OR 1.02 [95% CI 0.68, 1.53]) and empagliflozin (OR 1.03 [95% CI 0.65, 1.64]) were significantly associated with increased risk of overall cancer; the incidence of overall cancer was similar among these three SGLT2 inhibitors. In the generated hierarchies of treatment effects based on the SUCRA probabilities, canagliflozin was ranked the lowest risk for overall cancer among these SGLT2 inhibitors (ESM Table 5). There was low between-study heterogeneity (tau = 0.25) (ESM Table 6), no inconsistency between direct and indirect estimates (all 95% CIs across zero) (ESM Table 7) and no global inconsistency within any network (p = 0.83) (ESM Table 8). In addition, the comparison-adjusted funnel plot indicated the absence of small-study effects (ESM Fig. 9).
When different types of cancer were analysed (ESM Figs 10–16), canagliflozin was significantly associated with a decreased risk of gastrointestinal cancer compared with placebo (OR 0.31 [95% CI 0.11, 0.88]). Empagliflozin (OR 4.01 [95% CI 1.34, 11.96]) or other active treatments (OR 3.56 [95% CI 1.14, 11.13]) were significantly associated with higher risk than canagliflozin (ESM Fig. 11). Among these medications, canagliflozin was placed as the safest intervention for its largest SUCRA probability and lowest mean rank (ESM Table 5). In contrast to the results from pairwise meta-analysis, empagliflozin was not significantly associated with an increased risk of bladder cancer compared with placebo (OR 0.52 [95% CI 0.14, 1.90]) (ESM Fig. 12). There was low between-study heterogeneity (tau ≈ 0) (ESM Table 6), no inconsistency between direct and indirect estimates (all 95% CIs across zero) (ESM Table 7) and no global inconsistency within any network (p > 0.05) (ESM Table 8).
Discussion
Our meta-analysis included 46 RCTs that reported 580 incidences of cancer among 34,569 people with type 2 diabetes. We found that SGLT2 inhibitors were not significantly associated with an increased risk of overall cancer during a mean trial duration of 61 weeks. Our meta-regression analysis identified that none of the pre-specified factors significantly influenced the sizes of the treatment effects. However, there was some evidence to suggest that SGLT2 inhibitors might increase the cancer risk in obese participants (BMI ≥30 kg/m2). For pre-specified cancer types, SGLT2 inhibitors might significantly increase bladder cancer risk, particularly empagliflozin. The increased risk was observed in the trials with a duration ≥52 weeks and in obese participants (BMI ≥30 kg/m2). Interestingly, there was suggestive evidence that canagliflozin was significantly associated with decreased risk of gastrointestinal cancer. However, given the short durations of the included RCTs, estimates of cancer caused by longer exposure to SGLT2 inhibitors are not possible. Thus, our results should be interpreted with caution.
Our meta-analysis of current available evidence from RCTs indicates that SGLT2 inhibitor treatment is not associated with a significantly increased risk of overall cancer. Our results are consistent with one previous meta-analysis of data from regulatory submissions and scientific reports, which also showed no effect on risk of cancer [74]. One pooled analysis of 21 phase 2b/3 clinical trials showed that the overall incidence of malignancies was balanced between a dapagliflozin group and comparator groups [13]. Additionally, the overall incidence of bladder, breast and renal cancers was not increased by canagliflozin relative to comparators in a pooled analysis of eight phase 3 clinical trials [14]. Furthermore, preclinical studies did not find increased hyperplasia or neoplasia in the urinary bladder mucosa, urogenital tract or kidney in SGLT2 knockout mice compared with wild-type mice [75]. However, our results included only 580 incidences from 46 short-term RCTs with a mean trial duration of 61 weeks (range 24–160 weeks). Furthermore, we observed a non-significant risk increase among individuals using SGLT2 inhibitors with a lower border of CI of 0.96 (OR 1.17 [95% CI 0.96, 1.41]). We cannot completely rule out the possibility of an increased cancer risk. Our findings need to be confirmed in large trials such as CANVAS (canagliflozin; NCT01032629) and DECLARE-TIMI58 (dapagliflozin; NCT01730534), as well as in long-term observational studies.
Interestingly, our meta-analysis of direct and indirect evidence showed that canagliflozin was significantly associated with a decreased risk of gastrointestinal cancer. SGLT1 has been found to be overexpressed in many cancers [76] and SGLT2 is functionally expressed in pancreatic and prostate adenocarcinomas [77]. SGLTs, especially SGLT1, have been shown to play an important role in cancer cell survival through glucose uptake [77]. Canagliflozin is not only a potent SGLT2 inhibitor but also possesses potent SGLT1 inhibitory activity [76]. SGLT1 is expressed mainly in the gastrointestinal tract, but also in the kidneys and heart, while SGLT2 is highly selectively expressed in the kidneys and less so in the gastrointestinal tract [78]. Therefore, these findings suggest that canagliflozin may protect against gastrointestinal cancer by suppressing the expression of both SGLT1 and SGLT2 in the gastrointestinal tract. In a study of human colon cancer cells not expressing UGT1A9, which encodes the enzyme for metabolising SGLT2 inhibitors, dapagliflozin significantly reduced the number of colon cells [79]. However, our meta-analysis did not detect a decreased risk of gastrointestinal cancer with the use of dapagliflozin or empagliflozin. This might reflect the higher selectivity for SGLT2 vs SGLT1 exhibited by empagliflozin and dapagliflozin compared with canagliflozin [76], or the small number of incidences of gastrointestinal cancer observed. Further prospective studies are needed to determine the potential effects of SGLT2 inhibitors on the risk of gastrointestinal cancer.
An increased risk of bladder and breast cancer remains a safety issue associated with SGLT2 inhibitors. Our pairwise meta-analysis showed that SGLT2 inhibitors (particularly empagliflozin) were significantly associated with bladder cancer; although this was not confirmed in the network meta-analysis. Most incidences of bladder cancer were identified from the EMPA-REG OUTCOME Trial (empagliflozin: six incidences of bladder cancer, two incidences of bladder transitional cell carcinoma and one incidence of bladder cancer recurrent; placebo: zero incidences) [21]. An increased risk of bladder cancer was observed in the individuals taking empagliflozin compared with placebo in this trial [21], which was consistent with the findings on dapagliflozin in the regulatory report submitted to the US FDA [12]. However, our meta-analysis did not find a significantly increased risk of bladder cancer with dapagliflozin or canagliflozin. One pooled analysis of eight phase 3 clinical trials based on regulatory submissions (canagliflozin: five incidences; comparators: four incidences) showed that the incidence of bladder cancer was no higher with canagliflozin than with comparators [14]. The mechanisms underlying the elevated risk of bladder cancer associated with SGLT2 inhibitors remain unclear. Diabetes and obesity are indeed risk factors for bladder cancer, and increased rates of glycosuria and urinary tract infections related to SGLT2 inhibitor use may be responsible for the observed increased risk [14]. We found a significantly increased risk of bladder cancer among obese participants (BMI ≥30 kg/m2) or the trials with a duration ≥52 weeks. Our meta-analysis did not detect a significantly increased risk of breast cancer with the use of SGLT2 inhibitors compared with comparators. However, the possibility of an increased risk cannot be excluded, as the duration of the included RCTs is probably insufficient to address these safety issues conclusively. Future large long-term RCTs and real-world data are required to clarify the association between SGLT2 inhibitors and the risk of pre-specified cancer types (especially bladder cancer).
Several pre-specified risk factors (e.g. ethnicity, sex, BMI and age) were further explored in our meta-regression analysis. None of the results were significant. However, in the subgroup analysis, we found that, compared with comparators, SGLT2 inhibitors were significantly associated with an increased risk of overall cancer and bladder cancer in obese participants (BMI ≥30 kg/m2) but not in normal weight/overweight participants. These disparate findings may be explained by imbalanced sample sizes. It should be noted that the significantly increased risk was largely driven by EMPA-REG OUTCOME Trial [21], which contributed over 50% of the weight to the overall results and even more weight to the subgroup results. Overweight and obesity are risk factors for several types of cancer (e.g. bladder cancer) [80, 81]. Future prospective studies are needed to clarify the subgroup findings.
Compared with the null finding regarding overall cancer risk in one previously published meta-analysis [74], our meta-analysis not only showed a non-significantly increased risk of overall cancer associated with SGLT2 inhibitors, but also suggests some novel and important findings: (1) SGLT2 inhibitors in general might increase the risk of overall cancer in obese individuals; (2) SGLT2 inhibitors (especially empagliflozin) might increase the risk of bladder cancer; and (3) canagliflozin might have a protective effect against gastrointestinal cancer.
Our meta-analysis has several advantages: (1) our research question was specific regarding incidence of cancer, including both overall cancer and pre-specific cancer types; (2) this is the first network meta-analysis to comprehensively assess the comparative effects of SGLT2 inhibitors on cancer risk; (3) RCTs from electronic databases were systematically searched and additional data from Clinicaltrials.gov were included; and (4) multiple subgroup analyses, meta-regression and sensitivity analyses were performed to test the robustness of our findings. However, several limitations of our study merit consideration. First, a large number of potentially eligible trials were not included in the meta-analysis because of lack of data on incidence of cancer; however, additional data on ClinicalTrials.gov and regulatory reports submitted to the US FDA and EMA were searched and retrieved to minimise publication bias and outcome-reporting bias. The data for canagliflozin and empagliflozin from regulatory submissions were not included because they only reported the total number of incidences from several trials, which made it difficult to assign these outcomes to each trial. However, these results were considered in the discussion. Second, the exposure or follow-up time in most trials (mean trial duration 61 weeks, range 24–160 weeks) were not adequate to detect incidence of cancer given the long latency period of cancer. The evidence at this point is far from convincing and, therefore, it is likely that the observed associations may be caused by chance and may reflect their effects on late stage carcinogenesis. Third, the quality of our evidence is relatively low as a result of indirect comparisons, inadequate power and wide CIs according to the GRADE system [82]. Furthermore, we cannot rule out any heterogeneity and inconsistency due to sparse cancer events among the trials. It is premature to apply the results of the analyses to clinical practice and guideline development. Fourth, background treatments and participant characteristics varied among the RCTs and might contribute to heterogeneity, although multiple subgroup analyses were performed to minimise clinical heterogeneity. Finally, the risk of cancer associated with other novel SGLT2 inhibitors remains uncertain as RCT data are lacking.
In conclusion, the current evidence from RCTs does not show a significant association between SGLT2 inhibitors and an increased risk of overall cancer. There is some evidence suggesting that SGLT2 inhibitors (especially empagliflozin) might increase the risk of bladder cancer, while canagliflozin might offer a protective effect against gastrointestinal cancer. However, given the relatively short-term design of the RCTs include in the analysis, the long-term effects of SGLT2 inhibitors on cancer remain uncertain. Future long-term prospective studies and post-marketing surveillance studies are warranted.
Abbreviations
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- RCT:
-
Randomised controlled trial
- SGLT2:
-
Sodium–glucose cotransporter 2
- SUCRA:
-
Surface under the cumulative ranking curve
References
Giovannucci E, Harlan DM, Archer MC et al (2010) Diabetes and cancer: a consensus report. Diabetes Care 33:1674–1685
Mayor S (2016) Cancer risk is higher in years before and shortly after type 2 diabetes diagnosis, study shows. BMJ 354:i3832
Klil-Drori AJ, Azoulay L, Pollak MN (2017) Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol 14:85–99
Lutz SZ, Staiger H, Fritsche A, Haring HU (2014) Antihyperglycaemic therapies and cancer risk. Diab Vasc Dis Res 11:371–389
Tuccori M, Filion KB, Yin H, Yu OH, Platt RW, Azoulay L (2016) Pioglitazone use and risk of bladder cancer: population based cohort study. BMJ 352:i1541
Lewis JD, Habel LA, Quesenberry CP et al (2015) Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 314:265–277
Fujita Y, Inagaki N (2014) Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: clinical data and mechanism of action. J Diabetes Invest 5:265–275
Ferrannini E, Solini A (2012) SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 8:495–502
Marsenic O (2009) Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 53:875–883
Monami M, Nardini C, Mannucci E (2014) Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 16:457–466
Inzucchi SE, Bergenstal RM, Buse JB et al (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38:140–149
US Food and Drug Administration (2011) FDA briefing document, NDA 202293 Dapagliflozin tablets, 5 and 10 mg. Advisory Committee Meeting. Available from http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm262994.pdf. Accessed 25 Aug 2016
Ptaszynska A, Cohen SM, Messing EM, Reilly TP, Johnsson E, Johnsson K (2015) Assessing bladder cancer risk in type 2 diabetes clinical trials: the dapagliflozin drug development program as a 'case study'. Diabetes Ther 6:357–375
Lin HW, Tseng CH (2014) A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol 2014:719578
De Jonghe S, Proctor J, Vinken P et al (2014) Carcinogenicity in rats of the SGLT2 inhibitor canagliflozin. Chem Biol Interact 224:1–12
Taub ME, Ludwig-Schwellinger E, Ishiguro N et al (2015) Sex-, species-, and tissue-specific metabolism of empagliflozin in male mouse kidney forms an unstable hemiacetal metabolite (M466/2) that degrades to 4-hydroxycrotonaldehyde, a reactive and cytotoxic species. Chem Res Toxicol 28:103–115
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of healthcare interventions: checklist and explanations. Ann Intern Med 162:777–784
Higgins JPT, Altman DG, Sterne AC (2011) Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions 5.1.0. [updated March 2011]. The Cochrane Collaboration. Available from http://handbook.Cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm. Accessed 25 Aug 2016
Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26:53–77
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Zinman B, Wanner C, Lachin JM et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128
White IR, Barrett JK, Jackson D, Higgins JP (2012) Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 3:111–125
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G (2013) Graphical tools for network meta-analysis in STATA. PLoS One 8:e76654
Keus F, Wetterslev J, Gluud C, Gooszen HG, van Laarhoven CJ (2009) Robustness assessments are needed to reduce bias in meta-analyses that include zero-event randomized trials. Am J Gastroenterol 104:546–551
Salanti G (2012) Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 3:80–97
Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP (2012) Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 41:818–827
Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G (2013) Evaluation of inconsistency in networks of interventions. Int J Epidemiol 42:332–345
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3:98–110
Chaimani A, Salanti G (2012) Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 3:161–176
Stenlof K, Cefalu WT, Kim KA et al (2013) Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 15:372–382
Wilding JP, Charpentier G, Hollander P et al (2013) Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract 67:1267–1282
Lavalle-González FJ, Januszewicz A, Davidson J et al (2013) Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 56:2582–2592
Schernthaner G, Gross JL, Rosenstock J et al (2013) Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 36:2508–2515
Yale JF, Bakris G, Cariou B et al (2014) Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 16:1016–1027
Forst T, Guthrie R, Goldenberg R et al (2014) Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 16:467–477
Leiter LA, Yoon KH, Arias P et al (2015) Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care 38:355–364
Bode B, Stenlof K, Harris S et al (2015) Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab 17:294–303
Inagaki N, Kondo K, Yoshinari T, Kuki H (2015) Efficacy and safety of canagliflozin alone or as add-on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: a 52-week open-label study. J Diabetes Investig 6:210–218
Rosenstock J, Chuck L, Gonzalez-Ortiz M et al (2016) Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naive type 2 diabetes. Diabetes Care 39:353–362
Nauck MA, Del Prato S, Meier JJ et al (2011) Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 34:2015–2022
Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S (2011) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 13:928–938
Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF (2012) Dapagliflozin, metformin XR, or both: Initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract 66:446–456
Rosenstock J, Vico M, Wei L, Salsali A, List JF (2012) Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 35:1473–1478
Bailey CJ, Iqbal N, T'Joen C, List JF (2012) Dapagliflozin monotherapy in drug-naive patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab 14:951–959
Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF (2013) Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med 11:43
Kohan DE, Fioretto P, Tang W, List JF (2014) Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85:962–971
Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S (2014) Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab 16:124–136
Bolinder J, Ljunggren O, Johansson L et al (2014) Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 16:159–169
Jabbour SA, Hardy E, Sugg J, Parikh S (2014) Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care 37:740–750
Leiter LA, Cefalu WT, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ (2014) Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc 62:1252–1262
Ji L, Ma J, Li H et al (2014) Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther 36:84–100.e109
Kaku K, Kiyosue A, Inoue S et al (2014) Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab 16:1102–1110
Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF (2015) Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med 32:531–541
Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ (2015) Dapagliflozin's effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care 38:1218–1227
Matthaei S, Bowering K, Rohwedder K, Sugg J, Parikh S, Johnsson E (2015) Durability and tolerability of dapagliflozin over 52 weeks as add-on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab 17:1075–1084
Rosenstock J, Hansen L, Zee P et al (2015) Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 38:376–383
Mathieu C, Ranetti AE, Li D et al (2015) A randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care 38:2009–2017
Ferrannini E, Berk A, Hantel S et al (2013) Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 36:4015–4021
Häring HU, Merker L, Seewaldt-Becker E et al (2013) Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 36:3396–3404
Roden M, Weng J, Eilbracht J et al (2013) Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 1:208–219
Häring HU, Merker L, Seewaldt-Becker E et al (2014) Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 37:1650–1659
Barnett AH, Mithal A, Manassie J et al (2014) Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2:369–384
Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC (2014) Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2:691–700
Kovacs CS, Seshiah V, Swallow R et al (2014) Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 16:147–158
Rosenstock J, Jelaska A, Frappin G et al (2014) Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 37:1815–1823
Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ (2015) Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 17:936–948
Kadowaki T, Haneda M, Inagaki N et al (2015) Efficacy and safety of empagliflozin monotherapy for 52 weeks in Japanese patients with type 2 diabetes: a randomized, double-blind, parallel-group study. Adv Ther 32:306–318
Araki E, Tanizawa Y, Tanaka Y et al (2015) Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab 17:665–674
Tikkanen I, Narko K, Zeller C et al (2015) Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 38:420–428
Lewin A, DeFronzo RA, Patel S et al (2015) Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care 38:394–402
DeFronzo RA, Lewin A, Patel S et al (2015) Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 38:384–393
Hadjadj S, Rosenstock J, Meinicke T, Woerle HJ, Broedl UC (2016) Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care 39:1718–1728
NCT01734785 (2016) Safety and efficacy of the combination of empagliflozin and linagliptin compared to linagliptin alone over 24 weeks in patients with type 2 diabetes. Available from https://clinicaltrials.gov/ct2/show/NCT01734785. Accessed 15 July 2016
Wu JH, Foote C, Blomster J et al (2016) Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 4:411–419
Reilly TP, Graziano MJ, Janovitz EB et al (2014) Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus. Diabetes Ther 5:73–96
Cangoz S, Chang YY, Chempakaseril SJ et al (2013) The kidney as a new target for antidiabetic drugs: SGLT2 inhibitors. J Clin Pharm Ther 38:350–359
Scafoglio C, Hirayama BA, Kepe V et al (2015) Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A 112:E4111–E4119
Chao EC, Henry RR (2010) SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov 9:551–559
Saito T, Okada S, Yamada E et al (2015) Effect of dapagliflozin on colon cancer cell [rapid communication]. Endocr J 62:1133–1137
Wang J, Yang DL, Chen ZZ, Gou BF (2016) Associations of body mass index with cancer incidence among populations, genders, and menopausal status: a systematic review and meta-analysis. Cancer Epidemiol 42:1–8
Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB (2015) Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One 10:e0119313
Puhan MA, Schunemann HJ, Murad MH et al (2014) A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 349:g5630
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
The project described was supported by the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
HT, YS and JH designed the study. HT and WS identified and acquired reports of trials and extracted data. HT, QD, WS, SZ, YS and JH performed all data analyses, checked for statistical inconsistency, and interpreted data. HT, QD, WS, SZ, YS and JH contributed to data interpretation. HT drafted the report and all other authors critically reviewed the report. All authors approved the final version of manuscript. JH is the guarantor of this work.
Electronic supplementary material
ESM
(PDF 1615 kb)
Rights and permissions
About this article
Cite this article
Tang, H., Dai, Q., Shi, W. et al. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia 60, 1862–1872 (2017). https://doi.org/10.1007/s00125-017-4370-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-017-4370-8