Abstract
Aims/hypothesis
Pancreatic beta cell dysfunction is a prerequisite for the development of type 2 diabetes. Histone deacetylases (HDACs) may affect pancreatic endocrine function and glucose homeostasis through alterations in gene regulation. Our aim was to investigate the role of HDAC7 in human and rat pancreatic islets and clonal INS-1 beta cells (INS-1 832/13).
Methods
To explore the role of HDAC7 in pancreatic islets and clonal beta cells, we used RNA sequencing, mitochondrial functional analyses, microarray techniques, and HDAC inhibitors MC1568 and trichostatin A.
Results
Using RNA sequencing, we found increased HDAC7 expression in human pancreatic islets from type 2 diabetic compared with non-diabetic donors. HDAC7 expression correlated negatively with insulin secretion in human islets. To mimic the situation in type 2 diabetic islets, we overexpressed Hdac7 in rat islets and clonal beta cells. In both, Hdac7 overexpression resulted in impaired glucose-stimulated insulin secretion. Furthermore, it reduced insulin content, mitochondrial respiration and cellular ATP levels in clonal beta cells. Overexpression of Hdac7 also led to changes in the genome-wide gene expression pattern, including increased expression of Tcf7l2 and decreased expression of gene sets regulating DNA replication and repair as well as nucleotide metabolism. In accordance, Hdac7 overexpression reduced the number of beta cells owing to enhanced apoptosis. Finally, we found that inhibiting HDAC7 activity with pharmacological inhibitors or small interfering RNA-mediated knockdown restored glucose-stimulated insulin secretion in beta cells that were overexpressing Hdac7.
Conclusions/interpretation
Taken together, these results indicate that increased HDAC7 levels caused beta cell dysfunction and may thereby contribute to defects seen in type 2 diabetic islets. Our study supports HDAC7 inhibitors as a therapeutic option for the treatment of type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Data from genome-wide association studies point towards pancreatic beta cell dysfunction as a key defect causing type 2 diabetes [1]. However, the genetic variants identified so far only explain a modest proportion of the estimated heritability of type 2 diabetes, implying that additional factors remain to be discovered [2]. These may include epigenetic mechanisms. Indeed, we and others have identified epigenetic modifications in pancreatic islets, adipose tissue, skeletal muscle and liver from individuals with type 2 diabetes that might be important in the disease pathogenesis [3–8]. Numerous enzymes, including histone deacetylases (HDACs), regulate epigenetic modifications and may thereby affect gene expression and cellular function. A growing body of evidence suggests that HDACs control mammalian pancreatic endocrine cell function and glucose homeostasis [9–11]. For example, mice lacking Hdac5 exhibit increased beta cell mass [9]. We recently reported decreased DNA methylation and increased gene expression of HDAC7 in pancreatic islets from human donors with type 2 diabetes [3]. However, the role of HDAC7 in beta cells has not been explored. In the present study, we investigated the functional consequences of Hdac7 overexpression in beta cells and islets in an effort to dissect its potential role in diabetic islets.
Methods
RNA sequencing
Pancreatic islets from 85 non-diabetic and 16 type 2 diabetic donors were obtained from the Human Tissue Lab at EXODIAB/Lund University Diabetes Centre through the Nordic Network for Clinical Islet Transplantation. The selection criteria for non-diabetic donors were no diagnosis of type 2 diabetes and an HbA1c level below 6.0% (52 mmol/mol), as determined by the Mono-S method. The clinical characteristics of the islet donors are shown in Table 1. Parts of this islet cohort have been described previously [12]. High-quality RNA extracted from human islets was used for sequencing with the TruSeq RNA sample preparation kit (Illumina, San Diego, CA, USA) as previously described [12]. This study was approved by the local ethics committee. Informed consent was obtained from pancreatic donors or their relatives.
Rat islet isolation and culture
Pancreatic islets from 8- to 10-week-old male Wistar rats (Taconic, Lille Skensved, Denmark) were isolated by collagenase digestion and hand-picked under a stereo microscope [13]. The isolated islets were precultured for 24 h before adenoviral transduction in RPMI 1640 with UltraGlutamine (Lonza, Vallensbaek, Denmark) supplemented with 10% newborn calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Paisley, UK) in 5% CO2 at 37°C. All animal experiments were approved by the local ethics committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals [14].
Overexpression of Hdac7 in rat islets and clonal beta cells
An adenoviral vector for Hdac7 overexpression, Ad-GFP-CMV-ratHdac7, and a control vector conferring only green fluorescent protein expression, Ad-GFP-CMV, were made by Vector Biolabs (Philadelphia, PA, USA). Isolated rat islets were infected with 50,000 virus particles/islet. The rat clonal beta cell line INS-1 832/13 was transfected with a pcDNA3.1 expression vector containing the cDNA sequence of rat Hdac7 (Genscript, Piscataway, NJ, USA) or the empty vector (control) by using Lipofectamine LTX (Life Technologies). Experiments were performed 48 h after transduction/transfection, unless stated otherwise.
PCR and western blot
mRNA expression of Hdac7 and Tcf7l2 was analysed using TaqMan assays and related to expression of Ppia (Life Technologies) by quantitative real-time (q)PCR and the ΔΔCt method. To verify overexpression of HDAC7 protein, clonal beta cells were transfected with haemagglutinin-tagged cDNA for Hdac7 and lysed in RIPA buffer (50 mmol/l Tris, pH 7.6, 150 mmol/l NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton-X100, protease inhibitor cocktail; Sigma-Aldrich, St Louis, MO, USA), and boiled with sample buffer (60 mmol/l Tris, pH 6.8, 10% glycerol, 2% SDS, 10% β-mercaptoethanol, bromophenol blue). Samples were separated on Mini-PROTEAN TGX gels (Bio-Rad, Hercules, CA, USA) and transferred onto Hybond-LFP PVDF membranes (GE Healthcare, Piscataway, NJ, USA). Protein expression was detected using a rabbit haemagglutinin tag (Abcam, Cambridge, UK; diluted 1:4000) and mouse β-actin (Sigma-Aldrich; diluted 1:10,000) antibodies, and secondary DyLight 680/800 conjugated goat antibodies (Thermo Scientific, Rockford, IL, USA; diluted 1:15,000), all validated by the respective suppliers. Blots were scanned using an Odyssey imaging system (LI-COR, Lincoln, NE, USA).
Insulin secretion and content
Glucose-stimulated insulin secretion (GSIS) was examined in isolated rat islets. For each condition (Hdac7 overexpression and control), 24 islets (three islets per well) were transferred to Krebs–Ringer HEPES buffer (115 mmol/l NaCl, 4.7 mmol/l KCl, 2.6 mmol/l CaCl2, 1.2 mmol/l KH2PO4, 1.2 mmol/l MgSO4, 10 mmol/l HEPES, 0.2% BSA, 2 mmol/l glutamine, 5 mmol/l NaHCO3, 100 U/ml penicillin, 100 μg/ml streptomycin, pH 7.4) containing 2.8 mmol/l glucose and incubated 30 min prior to the GSIS experiment. Islets were then exposed to basal (2.8 mmol/l) or stimulatory (16.7 mmol/l) glucose for 60 min. The buffer was then collected and insulin secretion was determined using ELISA (Mercodia, Uppsala, Sweden) and normalised to insulin content.
Insulin secretion was also measured in transfected clonal beta cells at 2.8 and 16.7 mmol/l glucose levels in a secretion assay buffer (SAB) with normal (5.9 mmol/l) as well as elevated (35 mmol/l) K+ concentrations, as previously described [15]. In addition, two HDAC inhibitors, MC1568 (1 μmol/l; Sigma-Aldrich) [16] and trichostatin A (TSA) (0.625 μmol/l; Sigma-Aldrich) [17] were added to the culture medium 24 h before secretion experiments, as indicated. Insulin was measured in the supernatant fraction using an insulin RIA (Siemens Diagnostics, Erlangen, Germany) or insulin ELISA and normalised to total protein, as determined by a bicinchoninic acid assay (Thermo Scientific). Total insulin content was determined after acid ethanol extraction and normalised to protein content.
Mitochondrial respiration
The Extracellular Flux Analyzer XF24 (Seahorse Bioscience, North Billerica, MA, USA) was used to monitor the oxygen consumption rate (OCR) as described elsewhere [18]. Clonal beta cells were cultured and transfected as above on XF24 microplates coated with poly-d-lysine (10 μg/ml) at 200,000 cells/well prior to analysis. Data were normalised to total protein.
ATP measurement
Clonal beta cells were starved for 2 h in SAB containing 2.8 mmol/l glucose. Next, the cells were treated with SAB containing either 2.8 or 16.7 mmol/l glucose for 15 min, washed in PBS, lysed in water and kept on dry ice for 15 min. Lysates were thawed, sonicated for 15 s and used for ATP and total protein measurements. The level of ATP was monitored using an ATP Kit SL (BioThema, Handen, Sweden) according to the manufacturer’s instructions.
Microarray mRNA expression analysis
RNA was extracted from transfected clonal beta cells and the quantity and quality were assessed using the NanoDrop spectrophotometer ND-1000 (Thermo Fisher Scientific, Wilmington, DE, USA) and 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA), respectively. Whole-transcript microarray analysis was conducted on the WT Gene 2.0 ST Array (Affymetrix, Santa Clara, CA, USA) by the Swegene Center for Integrative Biology at Lund University. mRNA expression data were obtained from a total of 30,619 probe sets, representing 20,173 annotated transcripts and 19,603 unique genes. The Robust Multi-array Average method was used for background correction, data normalisation and probe summarisation.
Gene Set Enrichment Analysis
Gene Set Enrichment Analysis (GSEA) [19, 20] (www.broad.mit.edu/GSEA) version 4.0 was used to identify enriched gene sets of the microarray data using the Kyoto Encyclopedia of Genes and Genomes (KEGG). Probe sets pertaining to transcripts were ranked based on t statistics values from paired t tests.
Cell number, apoptosis and proliferation assays
Clonal beta cells were seeded and transfected on 96-well plates. Cell number was determined by crystal violet staining, as previously described [3]. Caspase-3 and -7 activity as a measure of apoptosis were assessed using Apo-ONE Homogeneous Caspase-3/7 Assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Cell proliferation was measured with the Cell Proliferation ELISA, BrdU kit (Roche Applied Sciences, Mannheim, Germany). BrdU labelling solution was added to the cells 24 h post-transfection and cells were cultured for another 24 h prior to absorbance measurement with a Tecan Infinite M200 Pro plate reader (Tecan Group, Männedorf, Switzerland).
Silencing of Hdac7 and Tcf7l2 in INS-1 832/13 cells
INS-1 832/13 beta cells were transfected with Hdac7 plasmid as described above and cotransfected with small interfering (si)RNAs targeting Hdac7 (siHdac7, 12.5 nmol/l) or Tcf7l2 (siTcf7l2, 25 nmol/l) (ON-TARGETplus siRNA–SMARTpool; Dharmacon, Heidelberg, Germany) using the DharmaFECT I siRNA Transfection Reagent (Thermo Scientific). A non-targeting siRNA (5ʹ-GAGACCCUAUCCGUGAUUAUU-3ʹ) was used as negative control. Silencing was validated by qPCR, as described above.
Chromatin immunoprecipitation (ChIP)
ChIP followed by ChIP-qPCR was performed on Hdac7- and control-transfected INS-1 832/13 beta cells to test for enrichment of histone 3 lysine 27 acetylation (H3K27ac) at the Tcf7l2, Gapdh and Ldha genes (see electronic supplementary material [ESM] Methods and ESM Table 1).
Statistics
RNA sequencing data were analysed using a Mann–Whitney U test. Wilcoxon signed-rank tests were used to analyse the rat islet and clonal beta cell data. False discovery rate analysis was used to correct for multiple testing.
Results
HDAC7 expression is increased in pancreatic islets from human donors with type 2 diabetes
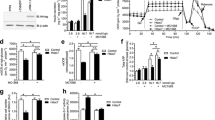
Using RNA sequencing, we found increased HDAC7 expression in pancreatic islets from donors with type 2 diabetes compared with non-diabetic controls (Fig. 1a), confirming our previous finding [3]. There were also significant differences in GSIS at 16.7 mmol/l glucose (p = 0.05), HbA1c (p < 0.0001) and BMI (p = 0.03) between the non-diabetic and diabetic donors (Table 1). However, there was no significant difference in age (p = 0.24) between the two groups. In addition, the expression level of HDAC7 correlated negatively with GSIS in human islets (r = –0.41, p < 0.004).
Hdac7 overexpression impaired insulin secretion and content. (a) HDAC7 expression, as measured by RNA sequencing, was higher in human pancreatic islets from donors with type 2 diabetes (n = 16) compared with non-diabetic controls (n = 85). (b) Transduction of isolated rat islets with an adenoviral vector encoding Hdac7 led to significant overexpression of Hdac7 mRNA. (c, d) Transfection of clonal beta cells with a pcDNA3.1 expression plasmid containing Hdac7 resulted in elevated mRNA (c) and protein (d) levels (n = 6 and n = 3, respectively). (e) Hdac7 overexpression in rat islets resulted in reduced GSIS (n = 6). White bars, control; black bars, Hdac7. (f) Insulin secretion in response to 2.8 and 16.7 mmol/l glucose with or without depolarising concentrations of K+ in clonal beta cells overexpressing Hdac7 compared with control transfected cells (n = 9). White bars, control; black bars, Hdac7. (g) Hdac7 overexpression resulted in reduced insulin content in clonal beta cells (n = 6). Data are presented as means ± SEM. *p < 0.05, **p < 0.01. RNA sequencing data were analysed with a Mann–Whitney U test, and Wilcoxon signed-rank tests were used to analyse the rat islet and clonal beta cell data. HA-tag, haemagglutinin-tag; IB, immunoblot antibody; T2D, type 2 diabetes
Impaired GSIS in rat islets and clonal beta cells overexpressing Hdac7
The expression data on HDAC7 from human islets do not resolve whether changes are a primary or secondary phenomenon. To address this question, and mimic the situation in human type 2 diabetic islets, we overexpressed Hdac7 in isolated rat islets and in INS-1 832/13 beta cells (Fig. 1b–d). Increased HDAC7 expression resulted in reduced GSIS at 16.7 mmol/l glucose in both rat islets and clonal beta cells (Fig. 1e,f). In addition, Hdac7 overexpression had nominal effects on insulin secretion in response to only the membrane-depolarising agent KCl (p = 0.054 at 2.8 mmol/l glucose), but there was no effect on the basal secretion at 2.8 mmol/l glucose (Fig. 1f). Furthermore, the insulin content was reduced in Hdac7-overexpressing beta cells (Fig. 1g), but unaffected in rat islets (data not shown).
Hdac7 overexpression impairs mitochondrial function
A possible explanation for the reduced GSIS in Hdac7-overexpressing beta cells is deficient mitochondrial function. Indeed, we found mitochondrial respiration at elevated glucose levels to be reduced in Hdac7-overexpressing cells (Fig. 2a, b). This was reflected by reduced glucose-stimulated ATP levels in these cells (Fig. 2c), as well as by reduced oligomycin-sensitive respiration (i.e. ATP turnover; Fig. 2a).
Hdac7 overexpression resulted in mitochondrial dysfunction. (a) The OCR in clonal beta cells overexpressing Hdac7 and control cells (n = 5). The OCR was measured in the presence of 2.8 mmol/l glucose (basal respiration, BR) and then after the sequential addition of 16.7 mmol/l glucose (Glc; glucose-stimulated respiration, GSR), 4 μg/ml oligomycin (oligo), 4 μmol/l carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) and 1 μmol/l rotenone. White circles, control; black squares, Hdac7. (b) The glucose-stimulated OCR was significantly decreased in Hdac7-overexpressing beta cells compared with control cells (n = 5). *p < 0.001 as analysed by a paired t test. (c) Cellular ATP levels were reduced in Hdac7-overexpressing clonal beta cells (n = 6). White bars, control; black bars, Hdac7. Data are presented as means ± SEM. *p < 0.05, as analysed by Wilcoxon signed-rank tests
Altered gene expression and cell number in beta cells overexpressing Hdac7
As HDAC7 may alter gene expression, we investigated the genome-wide expression pattern in Hdac7-overexpressing and control clonal INS-1 832/13 beta cells. We found 1171 differentially expressed genes at p < 0.05 (548 genes upregulated and 623 genes downregulated), but no individual gene, except Hdac7, had a false discovery rate of less than 5% (ESM Table 2). However, based on the impaired mitochondrial function in Hdac7-overexpressing beta cells, we specifically examined whether the expression of genes with known roles in mitochondrial metabolism was decreased (p < 0.05) in these cells. Indeed, Sdhc, Ndufa7 and Atp5g3, which are involved in the tricarboxylic acid cycle and the electron transport chain, exhibited decreased expression (ESM Table 2). Furthermore, the expression of Pcsk1, which is involved in the processing of proinsulin to insulin, was slightly reduced in Hdac7-overexpressing cells.
To identify biological pathways with altered expression in Hdac7-overexpressing beta cells, we next used GSEA. This analysis yielded eight significant gene sets with downregulated expression in beta cells overexpressing Hdac7 (q < 0.05, Table 2). These included pathways involved in DNA replication and repair, transcription and nucleotide metabolism, as well as protein folding, sorting and degradation. However, we found no significant gene sets with upregulated expression.
In addition, Tcf7l2 was one of the most significant genes in the microarray analysis and was upregulated in beta cells overexpressing Hdac7 (ESM Table 2). Interestingly, TCF7L2 single-nucleotide polymorphisms have shown the strongest association with type 2 diabetes in genome-wide association studies, and TCF7L2 has been proposed to regulate islet function [21]. We used qPCR to technically validate the upregulation of Tcf7l2 in RNA isolated from the Hdac7-overexpressing beta cells used for the microarray analysis (Fig. 3a). We could also biologically replicate these data in a different set of transfected cells. (Fig. 3b). We next tested whether restoring the expression of Tcf7l2 in Hdac7-overexpressing cells, by cotransfecting these cells with an siRNA against Tcf7l2, had any impact on insulin secretion. Interestingly, knockdown of Tcf7l2 partially reversed the insulin secretion impairment in Hdac7-overexpressing cells (Fig. 3c, d).
Hdac7 overexpression impaired insulin secretion partly through increased Tcf7l2 expression. qPCR was used to technically (a, n = 6) and biologically (b, n = 4) validate the increased expression of Tcf7l2 in Hdac7-overexpressing clonal beta cells. *p < 0.05. Silencing Tcf7l2 (c) partially restored GSIS in Hdac7-overexpressing beta cells (d). White bars, 2.8 mmol/l glucose; black bars, 16.7 mmol/l glucose; n = 6. *p < 0.05 vs siRNA negative control (siNC); † p < 0.05 vs Hdac7 siNC. Data are presented as means ± SEM
Based on the altered expression of gene sets regulating DNA replication and nucleotide metabolism, we next tested whether beta cell proliferation and/or apoptosis were altered when Hdac7 was overexpressed. Increased HDAC7 levels slightly, but significantly, reduced the number of beta cells (ESM Fig. 1a). Moreover, caspase-3 and -7 activity was increased in Hdac7-overexpressing cells, reflecting increased apoptosis, whereas BrdU-incorporation was unchanged, suggesting that cell proliferation was not affected (ESM Fig. 1b,c). In isolated rat islets, however, short-term overexpression of Hdac7 did not result in enhanced apoptosis (ESM Fig. 1d).
Histone acetylation
To investigate whether histone acetylation was affected by Hdac7 overexpression, we performed ChIP for the H3K27ac mark, which is associated with promoters and enhancer regions of active genes. We performed ChIP-qPCR for Tcf7l2, as well as for Gapdh and Ldha as positive and negative controls, respectively, since Gapdh is highly expressed and Ldha is expressed at very low levels, if at all, in beta cells. As expected, the Gapdh promoter was highly enriched for H3K27ac, whereas the mark was virtually absent in Ldha. There was no significant difference in H3K27ac enrichment between the Hdac7-overexpressing and control cells for Gapdh or Ldha (ESM Fig. 2). Furthermore, Tcf7l2 also failed to show significant differences in H3K27ac enrichment between control and Hdac7-overexpressing cells (ESM Fig. 2), suggesting other regulatory mechanisms for the differential Tcf7l2 expression.
HDAC7 inhibition restores GSIS in beta cells overexpressing Hdac7
In an attempt to rescue the Hdac7-associated beta cell dysfunction, and to test if HDAC7 may be a potential target for novel type 2 diabetes therapies, we treated Hdac7-overexpressing beta cells with two different HDAC inhibitors: TSA (an inhibitor of class I and class II HDACs) and MC1568 (an inhibitor of class II HDACs) [16, 17]. TSA treatment nominally increased GSIS in the beta cells transfected with the control vector (p = 0.0625). However, the effect was greater on Hdac7-overexpressing beta cells and the negative impact of HDAC7 on GSIS was no longer apparent (Fig. 4a). MC1568 treatment also completely reversed the negative effect of Hdac7 overexpression on GSIS, but without affecting insulin secretion in the control cells (Fig. 4b).
Inhibition of HDAC7 rescued impaired insulin secretion. (a) TSA (0.625 μmol/l) restored GSIS in Hdac7-overexpressing clonal beta cells, while the effect on control cells did not reach significance (p = 0.0625) (n = 6). White bars, control; black bars, Hdac7. (b) MC1568 (1 μmol/l) restored GSIS in Hdac7-overexpressing clonal beta cells (n = 6). White bars, control; black bars, Hdac7. (c) Hdac7 silencing (60%) in Hdac7-overexpressing clonal beta cells was confirmed by qPCR (n = 6). (d) Silencing Hdac7 in cotransfected Hdac7-overexpressing beta cells partially restored GSIS (n = 6). White bars, 2.8 mmol/l glucose; black bars, 16.7 mmol/l glucose. Data are presented as means ± SEM. *p < 0.05; † p < 0.05 vs Hdac7 siRNA negative control (siNC)
Finally, as a more specific approach, we tested whether cotransfecting the Hdac7-overexpressing cells with an siRNA targeting Hdac7 could rescue the secretory defect seen in Fig. 1f. The siRNA resulted in 60% lower Hdac7 expression compared with cells transfected with the Hdac7 overexpression plasmid alone (Fig. 4c). This resulted in a partial rescue of the decreased GSIS in Hdac7-overexpressing beta cells (Fig. 4d). The fact that the rescue was not complete is very likely explained by Hdac7 still being significantly overexpressed compared with control transfected cells (Fig. 4c).
Discussion
In this study, we have shown that HDAC7 is upregulated in type 2 diabetic human islets, and impairs insulin secretion and mitochondrial function and induces apoptosis when overexpressed in clonal beta cells. Importantly, insulin secretion was restored when Hdac7-overexpressing beta cells were treated with HDAC inhibitors, supporting the use of such compounds in the treatment of diabetes.
HDAC inhibitors are currently used for the treatment of epilepsy and cancer [22, 23], and HDACs might also be interesting pharmacological targets for type 2 diabetes. Indeed, work from Mandrup-Poulsen and colleagues has shown that several HDACs, including HDAC1–3, HDAC6 and HDAC11, regulate beta cell function [24–26]. Although these studies imply that increased HDAC levels may contribute to diabetes via hyperglycaemia and cytokine-induced toxicity, they did not identify HDAC7 as a target for the disease. Using microarray techniques, we recently found decreased DNA methylation and increased expression of HDAC7 in pancreatic islets from donors with type 2 diabetes [3], supporting a possible role for this enzyme in beta cell dysfunction and diabetes. Importantly, through the use of RNA sequencing in a larger islet cohort, we also found increased HDAC7 levels in diabetic islets in the present study. We also found a negative correlation between HDAC7 expression and insulin secretion from human islets cultured in vitro. Using functional experiments in clonal beta cells, we could further dissect how HDAC7 contributes to impaired insulin secretion. These experiments suggest that HDAC7 impairs mitochondrial function, increases beta cell apoptosis and increases Tcf7l2 expression. An impaired capacity to increase ATP production in beta cells in response to elevated glucose levels is indeed an important defect seen in diabetes, resulting in reduced insulin secretion [27–29]. The effect of Hdac7 overexpression on mitochondrial function may be mediated by the altered transcription of genes involved in metabolic processes. Indeed, we found decreased expression of genes involved in the tricarboxylic acid cycle and electron transport chain. It could also be due to interactions with and/or deacetylation of non-histone proteins affecting mitochondrial function. For example, HDAC7 has been shown to interact with and increase the transcriptional activity of hypoxia-inducible factor 1α [30], a known regulator of mitochondrial metabolism [31]. TCF7L2 is known as the ‘top’ diabetes gene, and the genotype increasing the risk for type 2 diabetes is also associated with increased TCF7L2 expression in human islets [21]. Interestingly, in the current study we found reduced insulin secretion and content in beta cells with increased Hdac7 and Tcf7l2 levels. Our findings are also in line with those of a previous study, showing that inhibition of HDACs enhances mitochondrial function and oxidative metabolism in muscle and adipose tissue [32]. Furthermore, a reduced number of functional beta cells can contribute to type 2 diabetes. Our data showing a decreased beta cell number due to increased apoptosis with Hdac7 overexpression are also supported by a previous study in cancer cells, which demonstrated that ectopic expression of HDAC7 promotes apoptosis and inhibits tumour growth [33].
In addition, there is evidence that HDACs may be promising pharmacological targets in multifactorial diseases [34]. To investigate if HDAC7 inhibition restores insulin secretion, we treated Hdac7-overexpressing beta cells with two different HDAC inhibitors. Indeed, TSA treatment restored insulin secretion in Hdac7-overexpressing beta cells. However, it also nominally increased insulin secretion in control cells, potentially due to effects on enzymes other than HDAC7. We therefore also used MC1568, a selective inhibitor of class II HDACs [16]. Importantly, MC1568 restored insulin secretion in Hdac7-overexpressing beta cells, but had no effect on control cells. These experiments support the development of a HDAC7-specific inhibitor for potential use in the treatment of diabetes.
The reduced methylation and increased expression of HDAC7 seen in type 2 diabetic islets may be due to environmental and/or genetic factors. We have previously published studies on clonal beta cells and human islets treated with elevated levels of palmitate (i.e. a type 2 diabetes-like treatment), but found no differences in HDAC7 expression in these studies [18, 35]. This does not, however, exclude elevated palmitate levels as a cause of increased HDAC7 expression, as such changes may need longer than the 48 h treatment we used to be established. Furthermore, we recently performed a methylation quantitative trait loci study in human pancreatic islets to identify genetic variants that influence DNA methylation and expression [36]. However, we did not find any single-nucleotide polymorphisms associated with altered expression or methylation of HDAC7. Other environmental factors may, however, alter the methylation and expression of HDAC7 in islets.
In addition, the decreased methylation and increased expression of HDAC7 may occur as a result of environmental insults during embryonic development. This may potentially predispose individuals to diabetes by affecting the number of beta and/or alpha cells in the mature pancreas. Interestingly, it is known that the development of endocrine cells is controlled by HDACs, and HDAC inhibition by TSA has been reported to increase the number of neurogenin-3-expressing progenitor cells [37]. In addition, selective inhibition of class IIa HDACs (HDAC4, -5, -7 and -9) has been reported to increase the pool of beta and delta cells [9].
Pancreatic islets contain cell types other than beta cells, and it is possible that the increased expression of HDAC7 in diabetic islets stems from differential expression of HDAC7 in the different islet cell types, in combination with altered cellular composition of the islets. Unfortunately, to our knowledge, no data that can conclusively resolve this issue exists, and we have been unsuccessful in our attempts to generate this type of data owing to limitations in islet material. However, publicly available expression data on isolated human alpha and beta cells, the two major islet cell types, show that the expression of HDAC7 does not differ between the two cell types [38], and our own published data found that islet cell composition did not differ between diabetic and non-diabetic donors in a subset of our cohort [3].
Furthermore, other HDACs may contribute to type 2 diabetes. In fact, the expression of HDAC1 and HDAC11 was increased and decreased, respectively, in our previously analysed cohort of islets from diabetic and control donors (T. Dayeh and C. Ling, unpublished data). However, these changes were of smaller relative magnitude compared with the changes in HDAC7, and these enzymes have already been somewhat investigated in beta cells [25, 26]. It is also possible that increased HDAC7 levels may alter the expression of other HDACs. In our Hdac7-overexpressing beta cells, we could see that the expression of Hdac5 was slightly reduced (p = 0.045). The present study did not investigate whether this has any functional effects on beta cells.
In conclusion, our study identifies HDAC7 as an enzyme that regulates beta cell function and number, and which is upregulated in human diabetic islets. It also supports further development of HDAC7 inhibitors for diabetes therapy.
Abbreviations
- ChIP:
-
Chromatin immunoprecipitation
- GSEA:
-
Gene Set Enrichment Analysis
- GSIS:
-
Glucose-stimulated insulin secretion
- H3K27ac:
-
Histone 3 lysine 27 acetylation
- HDAC:
-
Histone deacetylase
- OCR:
-
Oxygen consumption rate
- qPCR:
-
Quantitative real-time PCR
- SAB:
-
Secretion assay buffer
- siRNA:
-
Small interfering RNA
- TSA:
-
Trichostatin A
References
Rosengren AH, Braun M, Mahdi T et al (2012) Reduced insulin exocytosis in human pancreatic β-cells with gene variants linked to type 2 diabetes. Diabetes 61:1726–1733
Groop L, Pociot F (2014) Genetics of diabetes-are we missing the genes or the disease? Mol Cell Endocrinol 382:726–739
Dayeh T, Volkov P, Salo S et al (2014) Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet 10:e1004160
Ling C, Del Guerra S, Lupi R et al (2008) Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51:615–622
Nilsson E, Jansson PA, Perfilyev A et al (2014) Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 63:2962–2976
Nilsson E, Matte A, Perfilyev A et al (2015) Epigenetic alterations in human liver from subjects with type 2 diabetes in parallel with reduced folate levels. J Clin Endocrinol Metab 100:E1491–E1501
Yang BT, Dayeh TA, Kirkpatrick CL et al (2011) Insulin promoter DNA methylation correlates negatively with insulin gene expression and positively with HbA(1c) levels in human pancreatic islets. Diabetologia 54:360–367
Yang BT, Dayeh TA, Volkov PA et al (2012) Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol 26:1203–1212
Lenoir O, Flosseau K, Ma FX et al (2011) Specific control of pancreatic endocrine β- and δ-cell mass by class IIa histone deacetylases HDAC4, HDAC5, and HDAC9. Diabetes 60:2861–2871
Lundh M, Galbo T, Poulsen SS, Mandrup-Poulsen T (2015) Histone deacetylase 3 inhibition improves glycaemia and insulin secretion in obese diabetic rats. Diabetes Obes Metab 17:703–707
Mihaylova MM, Vasquez DS, Ravnskjaer K et al (2011) Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145:607–621
Fadista J, Vikman P, Laakso EO et al (2014) Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci U S A A111:13924–13929
Eliasson L, Ma X, Renstrom E et al (2003) SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 121:181–197
National Research Council of the National Academies (2011) Guide for the Care and Use of Laboratory Animals. The National Academies Presss, Washington, DC
Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430
Wang J, Gong B, Zhao W et al (2014) Epigenetic mechanisms linking diabetes and synaptic impairments. Diabetes 63:645–654
Tiernan AR, Champion JA, Sambanis A (2015) Trichostatin A affects the secretion pathways of beta and intestinal endocrine cells. Exp Cell Res 330:212–221
Malmgren S, Spegel P, Danielsson AP et al (2013) Coordinate changes in histone modifications, mRNA levels, and metabolite profiles in clonal INS-1 832/13 β-cells accompany functional adaptations to lipotoxicity. J Biol Chem 288:11973–11987
Mootha VK, Lindgren CM, Eriksson KF et al (2003) PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273
Subramanian A, Tamayo P, Mootha VK et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550
Lyssenko V, Lupi R, Marchetti P et al (2007) Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Investig 117:2155–2163
Hoffmann K, Czapp M, Loscher W (2008) Increase in antiepileptic efficacy during prolonged treatment with valproic acid: role of inhibition of histone deacetylases? Epilepsy Res 81:107–113
Waibel M, Christiansen AJ, Hibbs ML et al (2015) Manipulation of B-cell responses with histone deacetylase inhibitors. Nat Commun 6:6838
Christensen DP, Gysemans C, Lundh M et al (2014) Lysine deacetylase inhibition prevents diabetes by chromatin-independent immunoregulation and β-cell protection. Proc Natl Acad Sci U S A 111:1055–1059
Lundh M, Christensen DP, Damgaard Nielsen M et al (2012) Histone deacetylases 1 and 3 but not 2 mediate cytokine-induced beta cell apoptosis in INS-1 cells and dispersed primary islets from rats and are differentially regulated in the islets of type 1 diabetic children. Diabetologia 55:2421–2431
Lundh M, Christensen DP, Rasmussen DN et al (2010) Lysine deacetylases are produced in pancreatic beta cells and are differentially regulated by proinflammatory cytokines. Diabetologia 53:2569–2578
Koeck T, Olsson AH, Nitert MD et al (2011) A common variant in TFB1M is associated with reduced insulin secretion and increased future risk of type 2 diabetes. Cell Metab 13:80–91
Malmgren S, Nicholls DG, Taneera J et al (2009) Tight coupling between glucose and mitochondrial metabolism in clonal β-cells is required for robust insulin secretion. J Biol Chem 284:32395–32404
Olsson AH, Yang BT, Hall E et al (2011) Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes. Eur J Endocrinol 165:589–595
Kato H, Tamamizu-Kato S, Shibasaki F (2004) Histone deacetylase 7 associates with hypoxia-inducible factor 1α and increases transcriptional activity. J Biol Chem 279:41966–41974
Semenza GL (2011) Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta 1813:1263–1268
Galmozzi A, Mitro N, Ferrari A et al (2013) Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes 62:732–742
Barneda-Zahonero B, Collazo O, Azagra A et al (2015) The transcriptional repressor HDAC7 promotes apoptosis and c-Myc downregulation in particular types of leukemia and lymphoma. Cell Death Dis 6:e1635
Treppendahl MB, Kristensen LS, Gronbaek K (2014) Predicting response to epigenetic therapy. J Clin Investig 124:47–55
Hall E, Volkov P, Dayeh T et al (2014) Effects of palmitate on genome-wide mRNA expression and DNA methylation patterns in human pancreatic islets. BMC Med 12:103
Olsson AH, Volkov P, Bacos K et al (2014) Genome-wide associations between genetic and epigenetic variation influence mRNA expression and insulin secretion in human pancreatic islets. PLoS Genet 10:e1004735
Haumaitre C, Lenoir O, Scharfmann R (2009) Directing cell differentiation with small-molecule histone deacetylase inhibitors: the example of promoting pancreatic endocrine cells. Cell Cycle 8:536–544
Dorrell C, Schug J, Lin CF et al (2011) Transcriptomes of the major human pancreatic cell types. Diabetologia 54:2832–2844
Acknowledgements
We thank SCIBLU at Lund University for analysing gene expression, the Nordic Network for Clinical Islet Transplantation (JDRF award 31-2008-413) and the tissue isolation teams for providing human pancreatic islets as well as U. Krus, P. Storm, L. Jacobsson, B.-M. Nilsson and E. Nilsson (Lund University Diabetes Centre, Malmö, Sweden) for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by grants from the Swedish Research Council, a Linnaeus grant (LUDC: Dnr. 349–2008–6589) and a strategic research area grant (EXODIAB: Dnr. 2009–1039), ALF, the Novo Nordisk Foundation, the Swedish Diabetes Foundation, the Påhlsson Foundation, the Royal Physiographic Society and the Sigurd och Elsa Goljes Minne Foundation, as well as equipment grants from Wallenberg KAW (2009–0243).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript. The study sponsors were not involved in the design of the study; the collection, analysis or interpretation of data; writing the report; or the decision to submit the report for publication.
Author contribution statement
MD designed the study, generated and analysed data, and wrote the manuscript. KB designed the study, analysed data and wrote the manuscript. AB designed experiments and analysed data. MB, EOL and PV generated and analysed data. HM and LE designed experiments and provided research material. CL designed the study, analysed data and wrote the manuscript. All authors read and commented on the manuscript, and approved the final version to be published. CL is the guarantor of this work and, as such, had full access to all study data, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Electronic supplementary material
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Daneshpajooh, M., Bacos, K., Bysani, M. et al. HDAC7 is overexpressed in human diabetic islets and impairs insulin secretion in rat islets and clonal beta cells. Diabetologia 60, 116–125 (2017). https://doi.org/10.1007/s00125-016-4113-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-016-4113-2