Abstract
Aims/hypothesis
Hypoxic damage complicates islet isolation for transplantation and may contribute to beta cell failure in type 2 diabetes. Polymorphisms in the SLC30A8 gene, encoding the secretory granule zinc transporter 8 (ZnT8), influence type 2 diabetes risk, conceivably by modulating cytosolic Zn2+ levels. We have therefore explored the role of ZnT8 and cytosolic Zn2+ in the response to hypoxia of pancreatic islet cells.
Methods
Human, mouse or rat islets were isolated and exposed to varying O2 tensions. Cytosolic free zinc was measured using the adenovirally expressed recombinant targeted zinc probe eCALWY4. Gene expression was measured using quantitative (q)RT-PCR, western (immuno-) blotting or immunocytochemistry. Beta cells were identified by insulin immunoreactivity.
Results
Deprivation of O2 (1% vs 5% or 21%) for 24 h lowered free cytosolic Zn2+ concentrations by ~40% (p < 0.05) and ~30% (p < 0.05) in mouse and human islet cells, respectively. Hypoxia similarly decreased SLC30A8 mRNA expression in islets, and immunoreactivity in beta cells. Implicating lowered ZnT8 levels in the hypoxia-induced fall in cytosolic Zn2+, genetic ablation of Slc30a8 from mouse islets lowered cytosolic Zn2+ by ~40% (p < 0.05) and decreased the induction of metallothionein (Mt1, Mt2) genes. Cell survival in the face of hypoxia was enhanced in small islets of older (>12 weeks) Slc30a8 null mice vs controls, but not younger animals.
Conclusions/interpretation
The response of pancreatic beta cells to hypoxia is characterised by decreased SLC30A8 expression and lowered cytosolic Zn2+ concentrations. The dependence on ZnT8 of hypoxia-induced changes in cell survival may contribute to the actions of SLC30A8 variants on diabetes risk in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic beta cells are highly dependent on oxidative metabolism for ATP synthesis, particularly at elevated glucose concentrations [1, 2]. Correspondingly, hypoxia has been shown to influence islet survival and function during transplantation [3, 4]. Moreover, as many as 25% of islets are exposed in vivo to low oxygenation [5], suggesting that hypoxia acts as a regulator of islet function under physiological conditions. Indeed, glucose-induced oxygen consumption creates intracellular hypoxia sufficient to activate hypoxia-inducible factors (HIFs) in rat beta cells [6], an effect that is increased in diabetic animals [7] and which may contribute to defective insulin secretion in some forms of type 2 diabetes.
Hypoxic stress induces genes such as metallothionein (MT1/2) as a defence against large changes in free metal ion concentration [8], which may affect the activity of anti-oxidative enzymes [9]. Whether genetic factors influence the susceptibility of pancreatic beta cells to hypoxia has not previously been explored. Suggesting this as a possibility, genome-wide association studies (GWAS) have revealed that a non-synonymous single nucleotide polymorphism (rs13266634) in the SLC30A8 gene (encoding the secretory granule-resident zinc transporter 8 [ZnT8]) is associated with a ~20% increase in disease risk per allele [10–12]. SLC30A8 expression is largely confined to pancreatic beta and alpha cells [13], and is required for the accumulation of zinc into the secretory granule, where it binds to insulin [14, 15]. Consequently, mice inactivated systemically for Slc30a8 display profound changes in insulin crystal formation and secretory granule morphology [14, 15], consistent with the lower zinc-transporting activity of the transporter isoform encoded by the risk allele [15]. Defective insulin secretion is seen in global ZnT8 −/− mice on some [15], but not all, backgrounds [14, 16, 17], and mice with selectively deleted beta cell Slc30a8 display marked changes in insulin secretion and glucose tolerance [18]. Whether Slc30a8 influences cytosolic, as well as granular, Zn2+ concentrations has not previously been examined because of the uncertain subcellular targeting of the probes used in earlier work [15].
Monitoring cytosolic Zn2+ with the molecularly targeted recombinant probe eCALWY4 [19], the present study aimed to explore the impact of hypoxia on Zn2+ homeostasis, and the expression of SLC30A8/ZnT8 and other zinc transporters and importers, in human and rodent beta cells.
Methods
Reagents
RPMI and CMRL medium, ZnCl2, N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN), 2-mercaptopyridine N-oxide (pyrithione), poly-l-lysine and dimethyloxalylglycine (DMOG) were from Sigma (Gillingham, UK), TRIzol reagent was from Applied Biosystems / Life Technologies (Paisley, UK).
Mouse and rat strains and maintenance
Female CD1 mice and male Wistar rats were purchased from Harlan (Bicester, UK/Itingen, Switzerland). Global ZnT8 −/− mice [15] on a mixed SV129/C57BL/6 background and 129S7/SvEvBrd-Mt1 tm1Bri Mt2 tm1Bri/J mice (purchased from Jackson Laboratory, Bar Harbor, ME, USA) have previously been described [20]. Mice were killed at 10–12 weeks (−15 weeks for cell death analysis) of age by cervical dislocation as approved by the UK Home Office Animal Scientific Procedures Act, 1986 (PPL 70/7349), with ethics approvals.
Islet isolation, culture, infection and dissociation
Human islets were isolated from six beating-heart donors (electronic supplementary material [ESM] Table 1) with appropriate local ethical permissions (Charing Cross Research Ethics Committee reference 07/H0711/114) in Oxford, UK [21] or in Geneva, Switzerland [22], and maintained in RPMI medium containing 5.5 mmol/l glucose and 10% (vol./vol.) FCS. Mouse and rat islets were prepared as in Ravier and Rutter [23]. Islets were dissociated by 10 min incubation in Hanks’-based enzyme-free cell dissociation buffer (Gibco, Invitrogen, Paisley, UK), plated onto 24 mm sterile coverslips treated with 0.1% poly-l-lysine and allowed to recover overnight in RPMI medium containing 11 mmol/l glucose.
Hypoxia exposure
Pancreatic islets or beta cells were exposed to hypoxia (1% O2, 5% CO2, 94% N2), normoxia (21% O2, 5% CO2, 74% N2) or other oxygen concentrations using a tissue culture incubator with adjustable O2 or a modular incubator chamber (Billups-Rothenberg, del Mar, CA, USA).
Imaging of free cytosolic Zn2+ concentrations
Adenovirus expressing the recombinant Zn2+ probe eCALWY-4 was generated as previously described [24]. Virus was added to dissociated islet cells for 4 h. The medium was then changed, and cells were allowed to express the protein for 48 h. For imaging, cells were washed twice in Krebs Hepes-bicarbonate (KHB) buffer (140 mmol/l NaCl, 3.6 mmol/l KCl, 0.5 mmol/l NaH2PO4, 0.2 mmol/l MgSO4, 1.5 mmol/l CaCl2, 10 mmol/l Hepes [pH 7.4], 2 mmol/l NaHCO3), 11 mmol/l glucose, pre-equilibrated with 74:21:5 N2:O2:CO2 (normoxia) or 94:1:5 N2:O2:CO2 (hypoxia). Zn2+ imaging was carried out as described previously [19], with perifusion of the infected beta cell with buffer subsequently supplemented with 50 μmol/l TPEN and then 5 μmol/l pyrithione/100 μmol/l ZnCl2 (ZnPyr) (Fig. 1a, b). For hypoxia-treated cells, care was taken to keep oxygen tension low during microscopy by perifusing cells with oxygen-depleted solutions.
Imaging the effect of hypoxia on [Zn2+]cyt in dissociated islets. (a) [Zn2+]cyt was measured in dissociated CD1 mouse islets as described in the Methods section. Scale bar, 10 μm. (b) A representative trace showing changes in citrine/cerulean fluorescence ratio; ZnPyr, 5 μmol/l pyrithione/100 μmol/l ZnCl2. (c, d) Analysis of [Zn2+]cyt in dissociated (c) CD1 mouse and (d) human islets exposed for 24 h to normoxia (white bars) or hypoxia (black bars). Bars represent mean ± SE. * p < 0.05
RNA extraction and qRT-PCR
Total RNA from ~50 islets was obtained using TRIzol reagent and reverse-transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). cDNA (equivalent to 10–20 ng of RNA) was subject to qRT-PCR using Power SYBR Green master mix (Applied Biosystems/Life Technologies) in a 7500 fast real-time PCR system (Applied Biosystems/Life Technologies) and analysed by the comparative Ct method (primers are shown in ESM Table 2). The expression of target genes was normalised to the expression of cyclophilin A.
Protein extraction and western (immuno-) blotting analysis
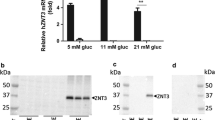
After exposure to normoxia or hypoxia for 24 h, ~200 islets were washed and lysed in ice-cold radioimmune precipitation assay buffer. Total protein extracts (30 μg) were resolved by SDS-PAGE (12% vol./vol. acrylamide) and transferred to PVDF membranes, followed by immunoblotting with rabbit anti-rodent ZnT8 antibody (1:200, Mellitech, Grenoble, France), and mouse monoclonal anti-tubulin (1:5,000, Sigma clone B-5-1-2) antibodies. Secondary horseradish peroxidase (HRP)-linked anti-rabbit antibodies (1:10,000, GE Healthcare, Little Chalfont, UK) were revealed by using ECL detection reagent (GE Healthcare).
Immunofluorescence
Cells were fixed in 3.7% paraformaldehyde and permeabilised in 0.1% Triton X-100 before immunostaining with a polyclonal anti-swine insulin antibody (1:200, DakoCytomation, Ely, UK), anti-glucagon antibody (G2654, Sigma) and anti-rodent human/rodent ZnT8 antibody overnight at 4°C. Alexa-coupled secondary antibodies (Invitrogen) were used to reveal the primary antibody staining. Confocal imaging was performed as described elsewhere [15].
Overexpression experiments
INS-1 (832/13) rat beta cells were plated onto 24 mm cover slips and transfected using Lipofectamine 2000 (Invitrogen) with 0.5 μg/well of plasmid encoding eCALWY-4 and 1 μg/well of a construct containing ZnT8 fused in-frame with mCherry (A. Pramatarova, McGill University, Montreal, Canada). At 48 h after transfection, cytosolic Zn2+ was imaged as above.
Dead:live cell assay
Islets were incubated for 15 min in PBS containing 3 μmol/l calcein-AM (Life Technologies) and 2.5 μmol/l propidium iodide (PI; Sigma-Aldrich) before detection of absorbance/emission at 491/525 nm and 561/620 nm, respectively. The islet area occupied by dead cells (PI) was calculated and expressed as a unitary ratio vs that occupied by all (live and dead) cells (PI plus calcein) using ImageJ (http://imagej.nih.gov/ij/) [25].
Transmission electron microscopy
Isolated islets were fixed and analysed as previously described [26].
Insulin secretion and content
Total and secreted insulin were measured after batch incubation of islets in KHB buffer as previously described [23].
Statistics
Data are given as mean ± SE or relative frequency. For comparison of continuous variables in two independent groups, Student’s two-tailed t test was used. Bonferroni correction was applied for multiple comparisons. Multiple linear regression was used to assess the effect of different factors on islet cell death. A value of p < 0.05 was considered significant. Statistical analyses were performed using SPSS 18.0 software (SPSS, Chicago, IL, USA) and Excel 2010 software (Microsoft, Redmond, WA, USA).
Results
Exposure of pancreatic beta cells to hypoxia decreases cytosolic free Zn2+ concentrations and Slc30a8/ZnT8 expression
Given the previously described role of Zn2+ ions in the responses of other tissues to hypoxia and ischaemia [27], we first determined whether lowered oxygen tensions might affect cytosolic free Zn2+ concentrations ([Zn2+]cyt) in mouse or human islet cells by using eCALWY4. This recombinantly expressed probe is confined exclusively to the cytosolic compartment, and is expected chiefly to report changes in beta cells which predominate in the islet preparations used (>70% for rodent islets [28], ~60% in human islets [29]). A significant lowering in steady-state [Zn2+]cyt of 30–40% in preparations from either species maintained under hypoxic conditions (Fig. 1c, d) was detected.
To determine whether changes in the expression of key regulators of intracellular Zn2+ homeostasis (zinc transporter [Slc30/ZnT] and zinc importer [Slc39/ZIP] [30] families) are involved in the hypoxia-induced changes in [Zn2+]cyt, their expression was measured in control or hypoxia-exposed islets. Whereas exposure to hypoxia for 24 h exerted no significant effect on the expression of members of the latter family in both mice and rats (ESM Fig. 1a, b), marked decreases were observed in levels of mRNA encoding Slc30a8 in both mouse and human islets (Fig. 2a, b), as well as rat islets (ESM Fig. 1c). These changes were accompanied by the expected increase in the expression of the major islet metallothionein genes, MT1 (also known as MT1A) (Mt1) and MT2 (also known as MT2A) (Mt2) (Fig. 2b, c). Western blot analysis revealed a significant decrease in ZnT8 protein abundance during hypoxia exposure in mouse islets (Fig. 2d). Confirming that the above hypoxia-induced changes were unlikely to require the more severely hypoxic conditions that may exist in the islet core [3], or paracrine intra-islet signalling (e.g. by inflammatory signals) [31], similar changes in the expression of Slc30a8 and Mt1/2 were apparent in dissociated mouse and human islet cells after exposure to hypoxia for 24 h (Fig. 3a, b). Correspondingly, a hypoxia-induced decrease in the expression of ZnT8 at the protein level was readily revealed by immunocytochemical analysis of individual insulin-positive mouse beta cells (Fig. 3c). To exclude hyperoxia-induced Slc30a8 overexpression, we compared the effects of 1%, 5% and 21% oxygen concentrations. Exposure to 1%, but not 5%, ambient oxygen altered the expression of Slc30a8 compared with 21% ambient oxygen (ESM Fig. 2a). The hypoxia-induced changes in Slc30a8 mRNA levels, as well as cytosolic free Zn2+ concentrations in mouse islets/islet cells, were largely reversible after 24 h of re-oxygenation (ESM Fig. 2b, c).
The effect of hypoxia on the expression of genes implicated in Zn2+ homeostasis in pancreatic islets. (a, c) CD1 mouse and (b) human islets were incubated for 24 h at normoxia (white bars) or hypoxia (black bars). Expression of (a) Slc30a1–10, (b) SLC30A8, MT1, MT2 and (c) Mt1–Mt3 was determined (normalisation to the expression in normoxia [b]). Bars represent mean ± SE. * p < 0.05 and ** p < 0.01. (d) Western blot analysis of CD1 mouse islets after incubation for 24 h at normoxia (21%) or hypoxia (1%)
The effect of hypoxia on the expression of the genes implicated in Zn2+ homeostasis in dissociated islets. (a) Dissociated CD1 mouse and (b) human islet cells were incubated for 24 h at normoxia (white bars) or hypoxia (black bars). qRT-PCR analysis of (a) Slc30a8, Mt1, Mt2 and (b) SLC30A8 was performed (normalisation to the expression in normoxia). (c) Dispersed CD1 mouse islet cells were incubated for 24 h at normoxia (21%) or hypoxia (1%). Scale bar, 10 μm. The level of ZnT8 was quantified by calculating the fluorescence ratio of ZnT8 vs insulin (white bar, 21%; black bar, 1%). Bars represent mean ± SE. * p < 0.05, ** p < 0.01 and *** p < 0.001
To exclude a decrease in cell viability as the chief reason for the reduced Slc30a8/ZnT8 expression, cell death was quantified 24 h after exposure to hypoxia. This analysis revealed a dead:live cell ratio of 12.4 ± 1.9% and 3.9 ± 1.2% in mouse and human islets, respectively, maintained under hypoxic conditions, compared with values of 0.7 ± 0.2 and 0.4 ± 0.1% in normoxia (ESM Fig. 3a, b). Correspondingly, hypoxia did not cause major changes in the morphology or ultrastructure of surviving islet cells as examined by electron microscopy (ESM Fig. 3c). As expected [32, 33], characterisation of hypoxic islets revealed reduced glucose-stimulated insulin secretion: insulin secretion ratio (release at high [16.7 mmol/l] glucose vs low [3.3 mmol/l] glucose) was only 4% of the ratio in normoxia (ESM Fig. 3d). Similarly, KCl-induced insulin secretion was also reduced in hypoxia (ESM Fig. 3d).
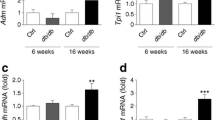
We sought next to determine whether the hypoxia-induced changes in the expression of Slc30a8/ZnT8 might reflect more global cellular dedifferentiation and/or alterations in the ratio of different islet cell types. Arguing against these possibilities, the expression of beta cell-enriched genes including insulin 1 (Ins1) and glucokinase (Gck) (also present in alpha cells) [34] did not change significantly during hypoxia and re-oxygenation treatment of mouse islets (Fig. 4a, b). By contrast, and as expected, large changes in the expression of the HIF1α targets GLUT1 (Slc2a1) and solute carrier family 16 (monocarboxylic acid transporters), member 3 (MCT4) (Slc16a3) were observed (Fig. 4c, d). Similar to the expression of Slc30a8 (Fig. 4e), expression of pancreatic duodenum homeobox-1 (Pdx1), a key transcription factor involved in the regulation of insulin genes and of beta cell differentiation [35], was markedly decreased by hypoxia (Fig. 4f), and this change was not as rapidly reversed as those for the mainly HIF1α-controlled genes (Slc2a1 and Slc16a3) or the metallothioneins (Mt1, Mt2) (Fig. 4g, h).
The effect of hypoxia and re-oxygenation on the expression of genes implicated in beta cell differentiation and function as well as Zn2+ homeostasis. CD1 mouse islets were incubated at normoxia, at hypoxia for 24 h or hypoxia for 24 h followed by normoxia for 8 h. The qRT-PCR analysis was performed for (a) Ins1, (b) Gck, (c) Slc2a1, (d) Slc16a3, (e) Slc30a8, (f) Pdx1, (g) Mt1 and (h) Mt2. Hypox, hypoxia; normox, normoxia; and reox, re-oxygenated
To further explore a possible role for HIF1α in controlling Slc30a8/ZnT8 expression, pancreatic islets were exposed to the HIF1α-stabilising agent DMOG for 24 h. DMOG treatment did not significantly affect Slc30a8 expression (ESM Fig. 4).
Regulation of Slc30a8/ZnT8 by hypoxia does not require changes in metallothionein gene expression
The expression of Slc30a8/ZnT8 has previously been shown to be regulated by changes in intracellular free Zn2+ [36], which in turn are expected to be influenced by alterations in metallothionein expression. We therefore determined whether the regulation of Slc30a8 expression by hypoxia might be diminished in islets from mice null for Mt1 and Mt2 [20]. Hypoxia still resulted in a marked decrease in Slc30a8 expression in Mt1 −/− :Mt2 −/− mice, to a similar extent to that observed in wild-type (WT) mouse islets (Fig. 5a). Further supporting a metallothionein-independent effect of hypoxia on Slc30a8 expression in CD1 mouse islets, Slc30a8 mRNA levels tended to be decreased as early as 5 h after the initiation of hypoxia (significant decrease after 10 h) (Fig. 5b).
The effect of hypoxia on the expression of Slc30a8 and cell death in mouse pancreatic islets deficient for metallothioneins 1 and 2. Islets from WT 129S1/SvImJ and 129S7/SvEvBrd-Mt1 tm1Bri Mt2 tm1Bri/J mice were incubated for 24 h at normoxia (white bars) or hypoxia (black bars). (a) The qRT-PCR analysis of Slc30a8 was performed. (b) CD1 mouse islets were incubated for 0 h (white bars), 5 h (grey bars) or 10 h (black bars) at hypoxia. qRT-PCR analysis of Slc30a8, Mt1 and Mt2 was performed (normalisation to the expression in normoxia). (c) The percentage of dead cells within intact islets after exposure for 24 h to normoxia (white bars, 21%) or hypoxia (black bars, 1% ambient oxygen). Scale bar, 100 μm. Bars represent mean ± SE. * p < 0.05 and ** p < 0.01. Mt1/2 −/−, 129S7/SvEvBrd-Mt1 tm1Bri Mt2 tm1Bri/J
While Slc30a8 expression was not affected by the loss of Mt1 and Mt2, cell survival after exposure to hypoxia was markedly (~twofold) decreased in Mt1 −/− :Mt2 −/− islets compared with controls (Fig. 5c).
Hypoxia-induced metallothionein gene induction and cell death is modulated by ZnT8
To further explore the signalling pathway(s) involved in regulating metallothionein expression and free cytosolic Zn2+ in response to hypoxia, we next asked whether inactivation of ZnT8 affected these responses. Induction of Mt1 and Mt2 by hypoxia was significantly impaired in ZnT8 −/− mouse islets, consistent with the lower [Zn2+]cyt in the latter vs WT mouse islet cells (Fig. 6a, b). As expected, Mt1/Mt2 expression was induced by exogenously added Zn2+ ions in hypoxia (Fig. 6c). Similar to the observations in hypoxia, [Zn2+]cyt was lower in islet cells from mice lacking ZnT8 vs WT islets (Fig. 6d). This difference between WT and ZnT8 −/− islets was no longer observed in hypoxia (Fig. 6e). Conversely, overexpression of Slc30a8 in the clonal INS1 (832/13) beta cell line increased [Zn2+]cyt (Fig. 6f). ZnCl2 was able to raise the expression of the most abundant metallothionein, Mt2, in hypoxic ZnT8 −/− mouse islets to levels similar to those of hypoxic WT islets (Fig. 6g). In addition to the regulation of metallothioneins, the expression of other genes important in the response of the beta cell to hypoxia was assessed (ESM Fig. 5). In contrast to metallothionein, there was a tendency for enhanced expression of these genes in ZnT8 −/− islets, reaching statistical significance for Slc16a3 (MCT4) when compared in WT and ZnT8 −/− islets during hypoxia. Insulin content did not significantly differ between WT and ZnT8 −/− mouse islets in normoxia or hypoxia (ESM Fig. 6).
The effect of deficiency for the zinc transporter ZnT8 on the expression of Mt1 and Mt2 and [Zn2+]cyt as well as cell death rate in mouse pancreatic islets. Islets from ZnT8 +/+ (WT) and ZnT8 −/− mice were incubated for 24 h at normoxia (white bars) or hypoxia (black bars). qRT-PCR analysis of (a) Mt1 and (b) Mt2 was performed. (c) CD1 mouse islets were incubated for 24 h at normoxia or hypoxia, with or without 30 μmol/l extracellular ZnCl2 (white bars, normoxia without ZnCl2; grey bars, normoxia with ZnCl2; dark grey bars, hypoxia without ZnCl2; black bars, hypoxia with ZnCl2); qRT-PCR analysis of Mt1 and Mt2 was performed. (d, e) [Zn2+]cyt was measured in dissociated islets of ZnT8 +/+ (WT) and ZnT8 −/− (knockout) mice after incubation for 24 h at (d) normoxia or (e) hypoxia. (f) Analysis of free [Zn2+]cyt in INS-1(832/13) cells expressing plasmid constructs encoding ZnT8 carrying either the at-risk R325 or the protective W325 polymorphism, compared with control (Ctrl). (g) Islets from ZnT8 +/+ (WT, white bars) and ZnT8 −/− mice (black bars) were incubated for 24 h at hypoxia, with or without 30 μmol/l extracellular ZnCl2 (Zn). qRT-PCR of Mt2 was performed. (h, i) The proportion of dead cells in islets of ZnT8 +/+ (white bars) and ZnT8 −/− mice (black bars) is depicted (h) after incubation of islets of all sizes for 24 h at normoxia or hypoxia in mice aged 10–15 weeks, and (i) for islets <120 μm in older mice (>12 weeks). Bars represent mean ± SE. * p < 0.05, ** p < 0.01 and *** p < 0.001. Hypox, hypoxia; normox, normoxia; Zn, extracellular ZnCl2
In contrast to Mt1 −/− :Mt2 −/− islets, cell survival was not affected in a large sample of ZnT8 −/− mouse islets (Fig. 6h) exposed to hypoxia. Multiple linear regression revealed a significant effect of mouse age (p = 0.001) and islet size (p < 0.001), but not genotype, on islet cell survival. However, when assessed in a defined population of smaller islets (<120 μmol/l) from older mice (12–15 weeks), we observed a significant difference in islet cell survival with a survival advantage of ZnT8 −/− mouse islets after 24 h (p < 0.001) or 48 h of hypoxia exposure (p < 0.01) (Fig. 6i).
Discussion
We demonstrate here that hypoxia strongly, but reversibly, regulates the expression of Slc30a8/ZnT8 in islets and beta cells from human and two rodent species. These findings thus extend the list of pathophysiological factors, currently including cytokines [31, 37] and fatty acids [36], which regulate the expression of this type 2 diabetes risk gene in islets. Hypoxia might therefore contribute to the downregulation of Slc30a8 previously observed in human type 2 diabetes islets [38].
The present findings extend to the islet beta cell those of a recent report [39] showing that hypoxia lowers the expression of Slc30a8/ZnT8 in the retinal pigment epithelium of the eye. The latter studies [39] provided evidence for control of ZnT8 levels via HIF1 stabilisation. By contrast, in our hands, the HIF1α-stabilising agent DMOG tended only slightly to reduce ZnT8 mRNA levels. Instead, our results suggest that the effects of hypoxia on Slc30a8/ZnT8 expression in pancreatic islets are more complex and may conceivably involve changes in the expression of Pdx1, recently shown to control Slc30a8 expression in clonal beta cells [40].
Recently, Lefebvre and colleagues [36] reported that depletion of intracellular zinc reduced Slc30a8 expression in human islets, confirming findings in INS-1E cells [41]. These earlier observations thus raise the possibility that the lowered cytosolic Zn2+ concentrations reported here during hypoxia may contribute to the lowering of Slc30a8/ZnT8 expression. Arguing against this view, addition of extracellular Zn2+ (30 μmol/l ZnCl2) did not rescue the lowering of Slc30a8 mRNA levels after hypoxia (ESM Fig. 7). Moreover, any direct or indirect (e.g. by alterations in cytosolic Zn2+) effect of hypoxia-driven metallothionein gene induction also seems unlikely, as hypoxia-induced changes in Slc30a8 mRNA levels were not different in Mt1/Mt2 −/− mice compared with control animals (Fig. 5a).
We propose instead a model by which hypoxia initially depresses Slc30a8 transcription and/or mRNA stability by mechanisms which remain to be elucidated (as discussed below). This then leads to a lowering of cytosolic free Zn2+, and a consequent drop in Mt1/Mt2 expression. The above sequence of events is supported by the observation of reduced cytosolic Zn2+ in ZnT8 −/− mice compared with controls (Fig. 6d), and by an increased cytosolic Zn2+ concentration in clonal pancreatic beta cells when Slc30a8 was overexpressed (Fig. 6f).
The observation that cytosolic free Zn2+ is lowered by ablating Slc30a8, a mediator of Zn2+ uptake into granules, may at first glance appear surprising. We would stress that the use here of a molecularly targeted cytosolic Zn2+ probe excludes uncertainties over the subcellular compartment in which Zn2+ concentrations are interrogated. So how might this apparent paradox be explained? First, our data suggest that ZnT8 may, under normal circumstances, catalyse Zn2+ efflux from granules, in line with bidirectional transport by ZnT5 [42]. Alternatively, gradual release of Zn2+ from granules by exocytosis may elevate the extracellular Zn2+ concentration in the medium surrounding WT, but not ZnT8 −/−, cells for which granule Zn2+ content is near zero (see Chimienti et al and Li et al [13, 43]). The released Zn2+ may then be recaptured, at least in part, and taken into the cytosol, by plasma-membrane-located Zn2+-uptake systems (e.g. ZIP1, voltage-gated Ca2+ channels) [30]. However, we calculate that release of Zn2+ ions into the medium is likely to increase total external Zn2+ concentration to only a miniscule extent (<50 nmol/l) vs a total extracellular concentration of >6 μmol/l [44], although local concentrations at the cell surface [14, 43] may be higher.
One of the effects of ZnT8 inhibition and consequently lowered cytosolic Zn2+ levels was impaired metallothionein induction in response to hypoxia (Fig. 6a, b). However, this did not affect islet cell survival under the conditions examined, despite the fact that islets lacking metallothionein-1 and -2 exhibited a twofold higher frequency of beta cell death compared with control islets (Fig. 5c). The latter finding is consistent with earlier data showing that overexpression of metallothionein protects islets against hypoxia, leading to improved islet cell survival [45]. We assume, therefore, that residual levels of metallothionein (~50%) in ZnT8 −/− mouse islets are sufficient to prevent an increase in cell death.
Unexpectedly, in small islets from older mice, we observed a significantly lower rate of cell death in ZnT8 −/− compared with WT islets. This finding is in line with our previous report on glucose homeostasis in these mice, where a compromising effect of ZnT8 deficiency disappeared with age [15], and suggests that, with ageing, a deleterious effect of ZnT8 deficiency may revert to one of protection. On the other hand, the lack of a genotype effect in large islets may be due to the variance of oxygen concentration in these islets, where the core is relatively anoxic as a consequence of larger diffusion distance when exposed to hypoxia in vitro. Nonetheless, the expression of hypoxia-inducible genes (with the exception of Mt1/Mt2) tended to be enhanced in ZnT8 −/− mice, possibly reflecting regulation of HIF1α by Zn2+ [46]. Importantly, the current results may provide an explanation for the recent finding that rare loss-of-function mutations in the SLC30A8 gene in man are associated with protection against type 2 diabetes [47].
Reduced expression of ZnT8 in hypoxia may thus reflect an ‘adaptive’ response of beta cells to permit survival under a hypoxic/oxidative stress in a less differentiated state (as previously described after partial pancreatectomy-induced hyperglycaemia [48]). The mechanisms behind this observation remain to be elucidated, but may suggest that reduced zinc levels are beneficial in the situation of increased hypoxic stress. Indeed, it has previously been shown that high concentrations of Zn2+ are able to induce islet cell death in a dose-dependent manner [49, 50]. A similar rate of cell death after 24 h and 48 h of hypoxia, as observed here, suggests that, once adapted to hypoxia, islets are better able to survive. Further studies will be required, however, to determine whether pharmacological modification of ZnT8 function may be beneficial in islet transplantation or type 2 diabetes mellitus.
Abbreviations
- DMOG:
-
Dimethyloxalylglycine
- HIF:
-
Hypoxia-inducible factor
- KHB:
-
Krebs Hepes-bicarbonate
- MCT4:
-
Solute carrier family 16 (monocarboxylic acid transporters), member 3
- PI:
-
Propidium iodide
- TPEN:
-
N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine
- WT:
-
Wild-type
- ZIP:
-
Zinc importer
- ZnT8:
-
Zinc transporter 8
References
Pullen TJ, Rutter GA (2013) When less is more: the forbidden fruits of gene repression in the adult beta-cell. Diabetes Obes Metab 15:503–512
Sekine N, Cirulli V, Regazzi R et al (1994) Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem 269:4895–4902
Lau J, Henriksnas J, Svensson J, Carlsson PO (2009) Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant 14:688–693
Olsson R, Olerud J, Pettersson U, Carlsson PO (2011) Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 60:2350–2353
Olsson R, Carlsson PO (2011) A low-oxygenated subpopulation of pancreatic islets constitutes a functional reserve of endocrine cells. Diabetes 60:2068–2075
Bensellam M, Duvillie B, Rybachuk G et al (2012) Glucose-induced O(2) consumption activates hypoxia inducible factors 1 and 2 in rat insulin-secreting pancreatic beta-cells. PLoS One 7:e29807
Sato Y, Endo H, Okuyama H et al (2011) Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J Biol Chem 286:12524–12532
Gunther V, Lindert U, Schaffner W (2012) The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta 1823:1416–1425
Bosco MD, Mohanasundaram DM, Drogemuller CJ, Lang CJ, Zalewski PD, Coates PT (2010) Zinc and zinc transporter regulation in pancreatic islets and the potential role of zinc in islet transplantation. Rev Diabet Stud 7:263–274
Boesgaard TW, Zilinskaite J, Vanttinen M et al (2008) The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients—the EUGENE2 study. Diabetologia 51:816–820
Kirchhoff K, Machicao F, Haupt A et al (2008) Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 51:597–601
Sladek R, Rocheleau G, Rung J et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885
Chimienti F, Favier A, Seve M (2005) ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals 18:313–317
Lemaire K, Ravier MA, Schraenen A et al (2009) Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci U S A 106:14872–14877
Nicolson TJ, Bellomo EA, Wijesekara N et al (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58:2070–2083
Pound LD, Sarkar SA, Ustione A et al (2012) The physiological effects of deleting the mouse slc30a8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS One 7:e40972
Rutter GA (2010) Think zinc: new roles for zinc in the control of insulin secretion. Islets 2:1–2
Wijesekara N, Dai FF, Hardy AB et al (2010) Beta cell specific ZnT8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53:1656–1668
Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M (2009) Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods 6:737–740
Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD (1994) Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci U S A 91:584–588
Cross SE, Hughes SJ, Partridge CJ, Clark A, Gray DW, Johnson PR (2008) Collagenase penetrates human pancreatic islets following standard intraductal administration. Transplantation 86:907–911
Bosco D, Armanet M, Morel P et al (2010) Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59:1202–1210
Ravier MA, Rutter GA (2005) Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 54:1789–1797
Bellomo EA, Meur G, Rutter GA (2011) Glucose regulates free cytosolic Zn2+ concentration, Slc39 (ZiP) and metallothionein gene expression in primary pancreatic islet beta-cells. J Biol Chem 286:25778–25789
Klee P, Allagnat F, Pontes H et al (2011) Connexins protect mouse pancreatic beta cells against apoptosis. J Clin Invest 121:4870–4879
da Silva Xavier G, Loder MK, McDonald A et al (2009) TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes 58:894–905
Sensi SL, Jeng JM (2004) Rethinking the excitotoxic ionic milieu: the emerging role of Zn(2+) in ischemic neuronal injury. Curr Mol Med 4:87–111
Meur G, Qian Q, da Silva Xavier G et al (2011) Nucleo-cytosolic shuttling of FOXO1 directly regulates mouse INS2 but not INS1 gene expression in pancreatic beta cells (min6). J Biol Chem 286:13647–13656
Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A (2006) The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103:2334–2339
Liuzzi JP, Cousins RJ (2004) Mammalian zinc transporters. Annu Rev Nutr 24:151–172
El MM, Billings LK, Raja MR et al (2010) Acute cytokine-mediated downregulation of the zinc transporter ZnT8 alters pancreatic beta-cell function. J Endocrinol 206:159–169
Dionne KE, Colton CK, Yarmush ML (1993) Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42:12–21
O'Sullivan ES, Johnson AS, Omer A et al (2010) Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia 53:937–945
Heimberg H, de Vos A, Moens K et al (1996) The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proc Natl Acad Sci U S A 93:7036–7041
Melloul D, Marshak S, Cerasi E (2002) Regulation of insulin gene transcription. Diabetologia 45:309–326
Lefebvre B, Vandewalle B, Balavoine AS et al (2012) Regulation and functional effects of ZNT8 in human pancreatic islets. J Endocrinol 214:225–232
Egefjord L, Jensen JL, Bang-Berthelsen CH et al (2009) Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in beta-cell apoptosis? BMC Endocr Disord 9:7
Marselli L, Thorne J, Dahiya S et al (2010) Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One 5:e11499
Deniro M, Al-Mohanna FA (2012) Zinc transporter 8 (ZnT8) expression is reduced by ischemic insults: a potential therapeutic target to prevent ischemic retinopathy. PLoS One 7:e50360
Pound LD, Hang Y, Sarkar SA et al (2011) The pancreatic islet beta-cell-enriched transcription factor Pdx-1 regulates Slc30a8 gene transcription through an intronic enhancer. Biochem J 433:95–105
Smidt K, Jessen N, Petersen AB et al (2009) SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One 4:e5684
Valentine RA, Jackson KA, Christie GR, Mathers JC, Taylor PM, Ford D (2007) ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J Biol Chem 282:14389–14393
Li D, Chen S, Bellomo EA et al (2011) Imaging dynamic insulin release using a fluorescent zinc probe, ZIMIR. Proc Natl Acad Sci U S A 108:21063–21068
Vallee BL, Gibson JG (1948) The zinc content of normal human whole blood, plasma, leucocytes, and erythrocytes. J Biol Chem 176:445–457
Li X, Chen H, Epstein PN (2004) Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem 279:765–771
Nardinocchi L, Pantisano V, Puca R et al (2010) Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS One 5:e15048
Flannick J, Thorleifsson G, Beer NL et al (2014) Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet 46:357–363
Jonas JC, Sharma A, Hasenkamp W et al (1999) Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem 274:14112–14121
Chang I, Cho N, Koh JY, Lee MS (2003) Pyruvate inhibits zinc-mediated pancreatic islet cell death and diabetes. Diabetologia 46:1220–1227
Kim BJ, Kim YH, Kim S et al (2000) Zinc as a paracrine effector in pancreatic islet cell death. Diabetes 49:367–372
Gerber PA, Bellomo EA, Meur G et al (2013) Hypoxia alters the expression of the type 2 diabetes-associated Zn2+ transporter Slc30a8/ZnT8 and cytosolic Zn2+ levels in human and rodent islets. Diabetologia 56(Suppl 1):S16
Acknowledgements
We thank L. Zhao and E. Oliver Perez (Imperial College London, London, UK) for assistance with the hypoxia experiments. We would also like to thank P. Chabosseau (Imperial College London, London, UK) for assistance with measurements of cytosolic Zn2+ and R. Zullig (University of Zurich, Zurich, Switzerland) for assistance with islet preparation.
Preliminary reports of these findings have previously been published in abstract form [51] and reported at the 49th EASD Annual Meeting in Barcelona in 2013.
Funding
This study was supported by: Wellcome Trust Senior Investigator (WT098424AIA) and Royal Society Wolfson Research Merit Awards to GAR; grants from the MRC (Programme MR/J0003042/1), European Association of the Study of Diabetes (EFSD) and Diabetes UK (studentships to GAR and AS); a Diabetes UK R. D. Lawrence Research Fellowship (12/0004431) to DJH; and a University of Zurich Academic Career Development Award, a Swiss Life Jubilee Foundation Grant and a Swiss National Science Foundation Grant (SNF IZK0Z3_150520) to PAG. The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 155005 (IMIDIA), resources of which comprise financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution (GAR). The isolation of human islets in Geneva was supported by a Juvenile Diabetes Research Foundation (JDRF) award (31-2008-416) to DB. The isolation of human islets was supported by the Oxford NIHR Biomedical Research Centre and a Juvenile Diabetes Research Foundation (JDRF) award (31-2008-617) to PRVJ).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
PAG and GAR designed the studies. PAG, EAB, DJH, GM, AS, RKM and MH performed all the procedures and analyses. DB, SJH and PRVJ provided human islets and analysed human islet data. FC provided anti-ZnT8 antibodies and analysed the ZnT8 expression data. GAR and PAG wrote the manuscript. All authors contributed to the discussion and revised the manuscript and all approved the final version. GAR is responsible for the integrity of the work as a whole.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 3 kb)
ESM Table 2
(PDF 9 kb)
ESM Fig. 1
(PDF 68 kb)
ESM Fig. 2
(PDF 8 kb)
ESM Fig. 3
(PDF 79 kb)
ESM Fig. 4
(PDF 12 kb)
ESM Fig. 5
(PDF 14.3 kb)
ESM Fig. 6
(PDF 4 kb)
ESM Fig. 7
(PDF 11 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gerber, P.A., Bellomo, E.A., Hodson, D.J. et al. Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia 57, 1635–1644 (2014). https://doi.org/10.1007/s00125-014-3266-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3266-0