Abstract
Autoantibodies to islet cell proteins currently provide the only reliable indication that the process leading to type 1 diabetes has started. The period from the first detection of islet autoantibodies to clinical onset of diabetes can last months or years. Longitudinal birth cohort family studies give crucial information concerning the natural history of islet autoimmunity and have already shown that islet autoantibodies, which precede diabetes development, often appear in early infancy. In this issue of Diabetologia, Ziegler et al (DOI:10.1007/s00125-012-2472-x) and Parikka et al (DOI:10.1007/s00125-012-2523-3) report findings from their birth cohort studies after numerous children have entered adolescence, allowing a more complete picture of islet autoimmunity in childhood to be revealed. Both groups are in accord that, between 6 months and 3 years of age, there is an explosion of islet autoimmunity in susceptible children and that the great majority (approximately 80%) of genetically at-risk children who present with diabetes before adolescence develop islet autoimmunity at this young age. These findings emphasise the importance of early life events in disease pathogenesis and have major implications for efforts aimed at preventing type 1 diabetes.
Similar content being viewed by others
The fall of the Berlin Wall in 1989 marked the end of an era and the beginning of a new chapter in diabetes research, with the establishment in Munich of BABYDIAB, the first longitudinal birth cohort family study of type 1 diabetes [1]. In this issue of Diabetologia, both the BABYDIAB/BABYDIET [2] and the Finnish population-based Type 1 Diabetes Prediction and Prevention (DIPP) [3] studies publish their long awaited findings on the natural history of islet autoimmunity and diabetes, extending into adolescence and early adulthood—information that is vital to our understanding of the pathogenesis of the disease.
Although destruction of the insulin-producing beta cells in type 1 diabetes is largely mediated by T cells, circulating autoantibodies to islet cell proteins currently provide the only reliable indication that the process leading to type 1 diabetes has started. The period from the first detection of autoantibodies to clinical onset of diabetes can last months or years; one individual in the Bart’s–Windsor family study [4] developed diabetes 25 years after the first detection of islet autoantibodies. Islet autoantibody measurement therefore allows at-risk individuals to be identified—particularly if multiple antibodies are detected in childhood—and offers the opportunity to monitor the disease process and potentially intervene to prevent or delay the onset of hyperglycaemia.
BABYDIAB has followed 1,650 children of parents with type 1 diabetes recruited between 1989 and 2004, and its offshoot, BABYDIET, enrolled 150 high-risk children between 2000 and 2004, to investigate the effect of early exposure to dietary gluten [5]. DIPP screened 122,636 newborn infants for HLA class II type 1 diabetes susceptibility genes between 1994 and 2007, and recruited 11,689 of those considered to be at high or moderate genetic risk for prospective study. In BABYDIAB, blood samples were collected at 9 months and at 2, 5, 8, 11, 14, 17 and 20 years of age; in BABYDIET, samples were collected at 3 month intervals until age 3 years; and in DIPP, samples were collected at 3–12 month intervals until the children reached the age of 15 years. BABYDIAB/BABYDIET tested all samples for autoantibodies to insulin (IAA), GAD (GADA), islet antigen-2 (IA-2A) and zinc transporter 8 (ZnT8-A) [6], and also assessed evidence of thyroid autoimmunity by repeated measurement of thyroid peroxidase autoantibodies (TPOA). In DIPP, prior to 2003, samples were pre-screened for islet cell autoantibodies (ICA). Any samples found to be ICA-positive were assayed for IAA, GADA and IA-2A, as were all the samples collected from children born after 2003. Both studies tested for maternal antibodies if a child was positive in their first postnatal sample.
Since their inception, BABYDIAB and DIPP, along with the Diabetes Autoimmunity Study in the Young (DAISY) in the USA [7], have yielded important new findings on the timing, quality and quantity of the islet autoantibody response and their relationship to the development of type 1 diabetes. The studies have been in general agreement that: (1) islet autoantibodies can be detected from 6 months of age; (2) IAA tend to appear first, closely followed by GADA and then IA-2A and ZnT8-A; (3) progression to diabetes is usually preceded by spreading of autoantibody reactivity to several different islet antigens and epitopes; and (4) progression to multiple islet autoantibodies and diabetes is faster in children with high-risk HLA genotypes.
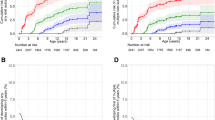
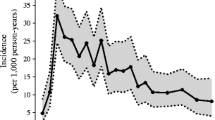
What these new papers reveal is a much clearer picture of autoantibody development throughout childhood, since it is only now that sufficient children have entered adolescence to allow reliable estimates of the incidence of individual antibodies and how these relate to diabetes progression. BABYDIAB, BABYDIET and DIPP have all now been running for more than 10 years, with median follow-ups from birth to the most recent sample of 11.1, 5.2 and 7.7 years, respectively. All three studies are in accord that, between 6 months and 3 years of age, there is an explosion of islet autoimmunity in susceptible children, and that the great majority (approximately 80%) of genetically at-risk children who present with diabetes before adolescence, develop autoimmunity at this young age. The reasons for this early peak in islet autoreactivity remain to be identified, but could be related to peri- or postnatal pancreatic development, maturation of the immune system, infections or dietary factors (Fig. 1).
In BABYDIAB, the early peak in islet autoimmunity contrasted with the incidence of TPOA, which peaked in adolescence. One intriguing explanation offered by the authors for differences in the peak incidence of islet and thyroid autoimmunity is that these are periods when the respective organs undergo remodelling, which could increase susceptibility to the initiation of autoreactivity [2]. The DIPP group observed that progression to diabetes was associated with autoantibody concentration, particularly with IAA levels at seroconversion or within the following 6 months [3]. A similar observation was made in the DAISY cohort [8], suggesting that the intensity of the early humoral response to (pro)insulin may be more closely linked to initiation of beta cell destruction than the early responses to other islet antigens. One subtle difference in the findings between the studies concerned the effect of sex. In BABYDIAB, although the cumulative incidence of islet autoantibodies was similar between sexes, the incidence peak for IAA was at 9 months in boys and 2 years in girls. That this sex difference was not seen in the DIPP study could be the result of differences between the populations, sampling frequency, or assay sensitivity and thresholds. It will be interesting to know whether the male excess of type 1 diabetes in young adults seen in many countries [9] is reflected by differences in the natural history of islet autoimmunity.
Both BABYDIAB and DIPP showed that initial seroconversion, with subsequent rapid development of multiple islet autoantibodies and/or diabetes, is relatively uncommon after the age of 5 years. There is recent evidence from the Belgian Diabetes Registry, however, that relatives of patients with diabetes who seroconvert after the age of 10 years still constitute an important minority of those who eventually develop diabetes [10]. Continued follow-up of the BABYDIAB and DIPP cohorts should further clarify the relationship between the onset of humoral islet autoimmunity and progression to diabetes, particularly during adolescence and young adulthood.
There are a number of limitations to these studies, as the authors acknowledge. Both have investigated children at higher risk of type 1 diabetes, either because of their family history (BABYDIAB) or HLA class II-determined genetic susceptibility (DIPP and BABYDIET). The great majority of children who progress to type 1 diabetes before the age of 15 years do not have an affected first-degree relative and, although just over half of these children would be expected to carry high- or moderate-risk HLA class II genotypes, as originally defined in DIPP [3, 11], the natural history of islet autoimmunity in children at lower genetic risk cannot necessarily be inferred from these studies. Gaps between sampling have also required some assumptions to be made concerning the time of seroconversion. Further, all children in BABYDIET and a proportion in DIPP were involved in clinical trials, although neither intervention is likely to have affected the natural history of islet autoimmunity.
The findings of these studies have several important implications. Primary intervention to modulate factors related to initiation of islet autoimmunity will need to be offered at a very early age, if diabetes is to be prevented in the great majority of children. Screening to allow secondary intervention targeting established autoimmunity will also need to be started early, to benefit as many children as possible. It will also be necessary to make the first few years of life the focus of efforts aimed at identifying the important triggers of islet autoimmunity, as exemplified by The Environmental Determinants of Diabetes in the Young (TEDDY) study [12]. There are many obstacles to studying children at this age since blood sampling and invasive testing are difficult or impossible in young infants. There are, however, some potential advantages. Lifestyle factors may be easier to control and there is likely to be more regular contact with healthcare providers during this period. Further, it appears that relatively small delays in the onset of islet autoimmunity during this period could have far-reaching consequences, as progression is slower in children who seroconvert after 2 years of age. Even short-term therapies implemented at an early age may therefore prove beneficial. Recent data from BABYDIAB and TEDDY indicate that the rapid increase in the incidence of type 1 diabetes in young children seen in many countries recently is caused by accelerated progression to disease rather than increases in the prevalence of islet autoimmunity [13]. Understanding the factors that cause variations in the rate of progression will be of the utmost importance in designing secondary prevention strategies.
With the continued assistance of study participants, further important insights into the natural history of type 1 diabetes will continue to emerge from these studies. Like type 1 diabetes, these results have had a long prodrome, but the papers are well worth the wait.
Abbreviations
- DAISY:
-
Diabetes Autoimmunity Study in the Young
- DIPP:
-
Type 1 Diabetes Prediction and Prevention
- GADA:
-
GAD autoantibodies
- IAA:
-
Insulin autoantibodies
- IA-2A:
-
Islet antigen-2 autoantibodies
- ICA:
-
Islet cell autoantibodies
- TEDDY:
-
The Environmental Determinants of Diabetes in the Young
- TPOA:
-
Thyroid peroxidase autoantibodies
- ZnT8-A:
-
Zinc transporter 8 autoantibodies
References
Ziegler AG, Hummel M, Schenker M, Bonifacio E (1999) Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 48:460–468
Ziegler AG, Bonifacio E, the BABYDIAB–BABYDIET Study Group (2012) Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. doi:10.1007/s00125-012-2472-x
Parikka V, Näntö-Salonen K, Saarinen M et al (2012) Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia. doi:10.1007/s00125-012-2523-3
Tarn AC, Smith CP, Spencer KM, Bottazzo GF, Gale EA (1987) Type I (insulin dependent) diabetes: a disease of slow clinical onset? BMJ 294:342–345
Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG (2011) Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET Study. Diabetes Care 34:1301–1305
Vermeulen I, Weets I, Asanghanwa M et al (2011) Contribution of antibodies against IA-2β and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care 34:1760–1765
Rewers M, Norris JM, Eisenbarth GS et al (1996) Beta-cell autoantibodies in infants and toddlers without IDDM relatives: Diabetes Autoimmunity Study in the Young (DAISY). J Autoimmun 9:405–410
Steck AK, Johnson K, Barriga KJ et al (2011) Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: Diabetes Autoimmunity Study in the Young. Diabetes Care 34:1397–1399
Gale EA, Gillespie KM (2001) Diabetes and gender. Diabetologia 44:3–15
Vermeulen I, Weets I, Costa O et al (2012) An important minority of prediabetic first-degree relatives of type 1 diabetic patients derives from seroconversion to persistent autoantibody positivity after 10 years of age. Diabetologia 55:413–420
Lambert AP, Gillespie KM, Thomson G et al (2004) Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J Clin Endocrinol Metab 89:4037–4043
TEDDY Study Group (2008) The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 1150:1–13
Ziegler AG, Pflueger M, Winkler C et al (2011) Accelerated progression from islet autoimmunity to diabetes is causing the escalating incidence of type 1 diabetes in young children. J Autoimmun 37:3–7
Contribution statement
Both authors were responsible for the conception and design of the article, writing the manuscript and revising it critically for intellectual content, as well as approving the version to be published.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, A.J.K., Bingley, P.J. Worth the wait: type 1 diabetes prospective birth cohort studies enter adolescence. Diabetologia 55, 1873–1876 (2012). https://doi.org/10.1007/s00125-012-2583-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2583-4