Abstract
Aims/hypothesis
This meta-analysis assessed the pooled effect of each genetic variant reproducibly associated with diabetic nephropathy.
Methods
PubMed, EMBASE and Web of Science were searched for articles assessing the association between genes and diabetic nephropathy. All genetic variants statistically associated with diabetic nephropathy in an initial study, then independently reproduced in at least one additional study, were selected. Subsequently, all studies assessing these variants were included. The association between these variants and diabetic nephropathy (defined as macroalbuminuria/proteinuria or end-stage renal disease [ESRD]) was calculated at the allele level and the main measure of effect was a pooled odds ratio. Pre-specified subgroup analyses were performed, stratifying for type 1/type 2 diabetes mellitus, proteinuria/ESRD and ethnic group.
Results
The literature search yielded 3,455 citations, of which 671 were genetic association studies investigating diabetic nephropathy. We identified 34 replicated genetic variants. Of these, 21 remained significantly associated with diabetic nephropathy in a random-effects meta-analysis. These variants were in or near the following genes: ACE, AKR1B1 (two variants), APOC1, APOE, EPO, NOS3 (two variants), HSPG2, VEGFA, FRMD3 (two variants), CARS (two variants), UNC13B, CPVL and CHN2, and GREM1, plus four variants not near genes. The odds ratios of associated genetic variants ranged from 0.48 to 1.70. Additional variants were detected in subgroup analyses: ELMO1 (Asians), CCR5 (Asians) and CNDP1 (type 2 diabetes).
Conclusions/interpretation
This meta-analysis found 24 genetic variants associated with diabetic nephropathy. The relative contribution and relevance of the identified genes in the pathogenesis of diabetic nephropathy should be the focus of future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus has rapidly increased to epidemic proportions over the past few decades. The number of patients with diabetes mellitus worldwide was estimated at 173 million in 2002 and is predicted to increase to 350 million cases by 2030 [1]. Diabetes mellitus is associated with severe complications including nephropathy, neuropathy, retinopathy and accelerated cardiovascular disease.
Diabetic nephropathy is the leading cause of end-stage renal disease (ESRD) in developed countries [1]. Although glycaemic control inversely relates to the degree of microvascular complications including diabetic nephropathy [2], some patients appear to be at increased risk. The majority of patients with type 1 diabetes mellitus will either develop diabetic nephropathy within the first 15 years after diagnosis or will remain relatively protected thereafter [3]. Differential disease risk in diabetic nephropathy may be partly attributable to genetic susceptibility. Evidence for a genetic component to diabetic nephropathy comes from family studies displaying familial aggregation of diabetic nephropathy both in type 1 and in type 2 diabetes mellitus [4–6], as well as differences in the prevalence of diabetic nephropathy between ethnic groups [7, 8].
The literature involving genetic associations in complex disease has been plagued by inconsistencies [9]. Small sample sizes and false positive results were often responsible for lack of reproducibility [10]. In addition, the prior probabilities of genetic associations are low. Therefore, the number of false positive associations generated by chance alone is high, particularly when low prior probabilities were not accounted for in the statistical analyses [11]. Incorrect phenotyping may also lead to spurious results. Thus independent replication of association remains essential in order to avoid false positive associations. The aim of this meta-analysis was to assess the pooled effect of genetic variants that have reproducibly been associated with diabetic nephropathy.
Methods
Eligibility criteria
We searched for studies comparing genetic variants in diabetes mellitus patients with diabetic nephropathy, relative to diabetes mellitus patients without diabetic nephropathy. We limited our analyses to studies investigating established and advanced diabetic nephropathy. To be included, all cases in the report had to have diabetes mellitus with macroalbuminuria and/or overt proteinuria, ESRD attributed to diabetic nephropathy or biopsy-proven diabetic nephropathy. In addition, diabetic control participants had to have either: (1) normoalbuminuria; (2) normoalbuminuria or microalbuminuria after >15 years diabetes mellitus duration (microalbuminuria developing after >15 years diabetes mellitus duration is a poor predictor of diabetic nephropathy [3]); (3) stable kidney function (serum creatinine <106.1 μmol/l) after >15 years of diabetes mellitus, irrespective of albuminuria.
Studies were excluded when the control group consisted of non-diabetic participants, since in that case genetic associations ascribed to diabetic nephropathy could have been due to diabetes mellitus. Follow-up and case–control studies were both eligible for inclusion in the meta-analysis.
Literature search and data collection
A search strategy was devised in collaboration with a trained librarian. The following databases were searched: PubMed (1949 to April 2010), EMBASE (OVID-version, 1980 to April 2010) and Web of Science (1945 to April 2010). The search strategy consisted of multiple queries combining: ‘Diabetic Nephropathy’ and ‘Genes’ or ‘Polymorphisms’. For these two concepts, all relevant keyword variations were used. In addition, the names of specific genes and polymorphisms were combined with the topic ‘Diabetic Nephropathy’. This search strategy was optimised for every database consulted, taking into account differences in the various controlled vocabularies and different database-specific technical variations. The search was performed in April 2010. To ensure maximum sensitivity, limits or filters were not placed on the searches. Language restrictions were not included in the initial search. References of other narrative and systematic reviews were also checked for relevant articles. The search strategy was updated if a reference was missing. The process was performed three times to ensure that no references were omitted.
Two authors (A. L. Mooyaart and E. J. J. Valk) of this study independently reviewed the titles and abstracts of the citations to identify genetic association studies. Genetic association studies were screened for whether the study contained a positive or a negative association between the genetic variant and diabetic nephropathy (association defined as significant at p < 0.05). When a genetic variant was found to be associated with established or advanced diabetic nephropathy (either at the allelic or genotypic level, including the recessive and dominant model) in two independent studies, that variant was considered to be a reproduced genetic variant. For these replicated variants, all other genetic studies were identified to estimate the effect of the variant on diabetic nephropathy, irrespective of p values.
Data extraction and analysis
The main outcome of the meta-analysis was the pooled odds ratio for the association between reproduced genetic variants and diabetic nephropathy. Odds ratios were calculated at allele level and not at genotype level. Of the reproduced genes, allele frequencies were extracted from studies. For single nucleotide polymorphisms (SNPs), the frequency of the minor allele was compared between diabetic nephropathy cases and non-nephropathy diabetic controls. For other genetic variants such as microsatellites, we compared the allele between cases and controls, as used in the literature and other meta-analyses [12, 13]. The random-effects model was performed by default. Heterogeneity within the studies was estimated by the I 2, which is the percentage of the total variation across studies that is due to heterogeneity rather than chance. An I 2 of 25%, 50% and 75% was considered low, moderate and high, respectively [14]. Pre-specified stratified analyses were performed to explain heterogeneity or investigate whether the reported association was present in a subgroup. Stratified analysis was performed for diabetes mellitus type (type 1 or type 2), diabetic nephropathy stage (macroalbuminuria and/or overt proteinuria, established diabetic nephropathy and ESRD [advanced diabetic nephropathy]) [15] and ethnicity (European vs Asian origin). The subgroup analysis was only included in this study if the association between the genetic variant and diabetic nephropathy was reproduced in that subgroup. We tested for publication bias using the Begg and Egger tests and provided funnel plots of all genetic variants which were reproducibly associated with diabetic nephropathy. It should, however, be noted that funnel plot asymmetry can have other causes than publication bias [16]. Furthermore, the effect of ethnicity was assessed if there were sufficient data by metaregression. Most analyses were performed in Review Manager (RevMan) version 5.0 (The Nordic Cochrane Centre, Copenhagen, Denmark; The Cochrane Collaboration, 2008), except for the analysis of publication bias and metaregression, which was performed in STATA 10.0 (StataCorp, College Station, TX, USA).
Results
Initial search and results
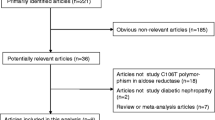
The initial literature search yielded 3,455 citations, 671 of which were genetic association studies investigating diabetic nephropathy in humans (Fig. 1). In these studies, we identified 34 reproduced genetic variants in 24 genes associated with diabetic nephropathy. Data on at least one of these 34 variants were found in 132 articles, representing 153 studies. Only three follow-up studies were included. All other studies were case–control studies. The maximum number of studies in an article was five. References of all articles and details of these studies are shown in the Electronic supplementary material (ESM) Tables 1 and 2. The 132 articles were published between 1994 and 2010. The number of cases included in these articles ranged from 4 to 1,572, and in a study from 4 to 656 cases. Of the 34 reproduced genetic variants, 21 genetic variants in or near 16 genes were significantly associated with diabetic nephropathy after random-effects meta-analysis (Fig. 2a). An overview of the pooled odds ratios of all reproduced variants in relation to diabetic nephropathy is shown in Fig. 2a, b. The odds ratios of the significant associations with diabetic nephropathy ranged between 0.48 and 0.78 for protective effects, and 1.12 to 1.70 for risk effects. Figure 3 contains an overview of the pooled odds ratios of all reproduced variants in relation to diabetic nephropathy among subgroups. Three reproduced variants were not significantly associated with diabetic nephropathy in the whole population after meta-analyses, but were associated in one subgroup: rs1799987 of CCR5 and rs741301 of ELMO1 in the Asian subgroup, and D18S880 of the CNDP1 gene in patients with type 2 diabetes mellitus. Details of analyses of all assessed genetic variants are provided in Table 1. Forest plots of all individual genetic variants and funnel plots for publication bias, as well as results of meta-regression for ethnicity are shown in the ESM (ESM Figs 1–36). If the meta-analysis revealed a positive association between a given genetic variant and diabetic nephropathy, and more than ten studies investigating that variant in relation to diabetic nephropathy were available, a meta-regression was performed. Only three genetic variants fitted the above-mentioned criteria (ACE rs179975, AKRB1 CA repeat Z−2, APOE E2/3/4). In these variants, metaregression showed that ethnicity did not explain the heterogeneity (ESM Figs 1–36).
Genetic variants reproducibly associated with diabetic nephropathy. a All genetic variants in or near a gene that were reproduced in an independent study and significantly associated with diabetic nephropathy after meta-analysis. b All genetic variants in or near a gene that were reproduced in an independent study, but were not significantly associated with diabetic nephropathy after meta-analysis. Parentheses (y-axis labelling) contain the allele used in the comparison
Genetic variants reproducibly associated with diabetic nephropathy in a subgroup. a All genetic variants in or near a gene that were reproduced in an independent study and significantly associated with diabetic nephropathy after meta-analysis in a subgroup. b All genetic variants in or near a gene that were reproduced in an independent study, but were not significantly associated with diabetic nephropathy after meta-analysis in a subgroup. Parentheses (y-axis labelling) contain the allele used in the comparison. The subgroup in which the genetic variant was reproducibly associated with diabetic nephropathy is shown in y-axis label as follows: Asian, T2D (type 2 diabetes), ESRD, T1D (type 1 diabetes), Eur (European), Prot. (proteinuria)
Genetic variants involved in the renin–angiotensin system
A variant in ACE, rs179975, was the most studied polymorphism in diabetic nephropathy, with 42 studies resulting in a pooled odds ratio of 1.24 (95% CI 1.12–1.37). The association between the deletion of the rs179975 polymorphism and diabetic nephropathy was reproduced in all subgroups. In the meta-analysis, the rs179975 polymorphism was associated with diabetic nephropathy in type 1 diabetes mellitus (OR 1.13 [95% CI 1.04–1.23]), type 2 diabetes mellitus (OR 1.33 [95% CI 1.16–1.52]), ESRD (OR 1.39 [95% CI 1.21–1.60]), proteinuria (OR 1.20 [95% CI 1.07–1.36]) and in the Asian subgroup (OR 1.28 [95% CI 1.10–1.49]), but not in Europeans. Other variants in the renin–angiotensin system that were also widely studied and reproduced, such as the rs699 variant of AGT with 21 studies and the rs5186 polymorphism of AGTR1 with 15 studies, were not associated with diabetic nephropathy in the meta-analysis.
Genetic variants involved in the polyol pathway
The CA repeat and rs759853 in AKR1B1 were studied in 19 and 9 studies, respectively. The CA repeat has a Z−2 allele thought to lead to an increased risk of diabetic nephropathy and a Z+2 allele thought to have a protective effect. The Z+2 allele and Z−2 allele were both reproducibly associated with diabetic nephropathy, but only the Z−2 allele remained associated in a combined meta-analysis with a pooled odds ratio of 1.12 (95% CI 1.02–1.24). Although reproducibly associated with diabetic nephropathy in ‘type 1 diabetes mellitus’ and ‘European’ subgroups, Z−2 was not associated with diabetic nephropathy in the meta-analysis in these subgroups. The Z+2 allele was associated with diabetic nephropathy in the ‘type 1 diabetes mellitus’ and ‘European’ subgroups (OR 0.79 [95% CI 0.68–0.92] and OR 0.81 [95% CI 0.66–0.99], respectively). The T allele in SNP rs759853 was associated with risk of diabetic nephropathy in the meta-analysis (OR 1.40 [95% CI 1.13–1.74]) and in the subgroups ‘diabetic nephropathy due to type 1 diabetes mellitus’ and ‘Europeans’ (OR 1.58 [95% CI 1.01–2.46] and OR 1.45 [95% CI 1.07–1.97], respectively).
Genetic variants involved in lipid metabolism
Two variants in genes each coding for two different apolipoproteins are reproducibly associated with diabetic nephropathy and remained associated with diabetic nephropathy in the meta-analysis: E2, E3, E4 polymorphism of APOE and rs4420638 near APOC1. The E2 allele is thought to lead to an increased risk of diabetic nephropathy and the E4 allele is thought to have a protective effect. Both the E2 and the E4 allele were associated with diabetic nephropathy in the meta-analysis (OR 1.70 [95% CI 1.12–2.58] and OR 0.78 [95% CI 0.62–0.98] respectively). The E2 allele was also reproducibly associated with diabetic nephropathy in the subgroups ‘type 2 diabetes mellitus’, ‘Asians’ and ‘European/type 1 diabetes mellitus’ (all studies investigating Europeans had type 1 diabetes mellitus and vice versa), but only associated with diabetic nephropathy in the meta-analysis in the ‘type 2 diabetes mellitus’ and ‘Asian’ subgroups (OR 2.21 [95% CI 1.22–4.00] and OR 2.35 [95% CI 1.29–4.30], respectively). rs4420638 near the APOC1 gene was studied in two studies and was associated with diabetic nephropathy in the meta-analysis (OR 1.54 [95% CI 1.29–1.83]). Both studies contained type 1 diabetic nephropathy patients of European descent.
Genetic variants involved in inflammatory cytokines and angiogenesis
rs1799987 of the CCR5 (an inflammatory cytokine) gene was only associated with diabetic nephropathy in the Asian subgroup (OR 0.58 [95% CI 0.43–0.76]) consisting of four studies (n = 1,534), but not in the total group consisting of nine studies (n = 5,527). For the total group, funnel plot asymmetry was indicated by a significant Begg test.
Two genes involved in angiogenesis, VEGFA and EPO, each had a variant that was reproducibly associated with diabetic nephropathy. rs833061 of VEGFA was associated with diabetic nephropathy in the meta-analysis in two studies (n = 543) containing only type 1 diabetes mellitus patients of European origin (OR 0.48 [95% CI 0.37–0.61]). rs1617640 of EPO was associated with diabetic nephropathy (OR 0.67 [95% CI 0.60–0.76]) in three studies (n = 2,773), also in the subgroup with type 1 diabetes mellitus patients (OR 0.67 [95% CI 0.58–0.76]).
Genetic variants involved in oxidative stress
Five genetic variants in four genes thought to be related to oxidative stress were reproducibly associated with diabetic nephropathy. The 1/2 polymorphism of HP and rs1801282 of PPARG were not associated with diabetic nephropathy in the meta-analysis. For PPARG, funnel plot asymmetry was observed (p = 0.024) suggesting publication bias. The rs3138808 and the rs2070744 variants of NOS3 were associated with diabetic nephropathy in the meta-analysis (OR 1.31 [95% CI 1.02–1.67] and OR 1.39 [95% CI 1.09–1.78] respectively). The 5L allele of CNDP1 was associated with diabetic nephropathy only in the ‘type 2 diabetes mellitus’ subgroup (OR 0.77 [95% CI 0.61–0.97]).
Genetic variants in other pathways
rs17300539 of ADIPOQ, which is believed to mitigate vascular damage, was not associated with diabetic nephropathy in the meta-analysis. rs841853 of GLUT1 (also known as SLC2A1), coding for a glucose transporter, did not show an association with diabetic nephropathy in eight studies (OR 1.10 [95% CI 0.89–1.35]). rs1129456 of GREM1, which is involved in cell growth and differentiation, was associated with diabetic nephropathy (OR 1.53 [95% CI 1.25–1.89]) in two studies (n = 1799). rs3767140 of HSPG2, which is involved in maintenance of glomerular basement membrane electrostatic charge, was also associated with diabetic nephropathy in the meta-analysis (OR 0.72 [95% CI 0.59–0.87]), and additionally with diabetic nephropathy in Europeans with type 1 diabetes mellitus (OR 0.64 [95% CI 0.49–0.84]). rs13293564 of UNC13B, thought to be involved in apoptosis, was associated with diabetic nephropathy in four studies (OR 1.23 [95% CI 1.11–1.35]).
Genetic variants identified by genome-wide association studies
Of the 14 genetic variants found to be reproducibly associated with diabetic nephropathy from genome-wide association studies (GWAS), ten remained associated in the meta-analysis. rs2268388 of ACACB, rs11993333 of PVT1, rs39075 near CPVL and CHN2, and rs6492208 (not near a gene) were not associated with diabetic nephropathy in the meta-analysis. Another variant near ‘CPVL and CHN2’, rs39059, was associated with diabetic nephropathy in two studies (n = 1,705; OR 0.74 [95% CI 0.64–0.85]). rs741301 of ELMO1 was associated with diabetic nephropathy in Asians with type 2 diabetic nephropathy (OR 1.58 [95% CI 1.28–1.94]), but not in combination with a third study of European type 1 diabetes mellitus patients. rs451041 and rs739401 of CARS were associated with diabetic nephropathy in the meta-analysis (OR 1.37 [95% CI 1.21–1.54] and OR 1.32 [95% CI 1.15–1.51] respectively).
rs1888747 and rs10868025 of FRMD3 were associated with diabetic nephropathy (OR 0.74 [95% CI 0.65–0.83] and OR 0.72 [95% CI 0.64–0.81] respectively). Another four variants, rs1041466, rs1411766, rs7989848 and rs9521445, which do not lie near a known gene, were associated with diabetic nephropathy in the meta-analysis (OR 1.38 [95% CI 1.21–1.58], OR 1.36 [95% CI 1.20–1.54], OR 1.32 [95% CI 1.16–1.51] and OR 1.35 [95% CI 1.18–1.55] respectively). The variants in CARS, FRMD3, CPVL and CHN2, and the five variants not near genes were only investigated in European participants with type 1 diabetes mellitus.
Discussion
In this meta-analysis, 21 genetic variants were associated with advanced diabetic nephropathy and three additional variants were associated within specific subgroups. Meta-analysis of several individual genetic variants in relation to diabetic nephropathy has been performed previously, but this is the first complete overview assessing for all genetic variants that are reproducibly associated with the presence of diabetic nephropathy. This information could lead to improved insight into underlying pathogenetic mechanisms. Variants in or near ACE, AKR1B1 (two variants), APOC1, APOE, EPO, NOS3 (two variants), HSPG2, VEGFA, FRMD3 (two variants), CARS (two variants), CPVL and CHN2, UNC13B and GREM1, as well as four variants not near known genes, were associated with diabetic nephropathy. ELMO1, CCR5 and CNDP1 were associated with diabetic nephropathy in a subgroup (‘Asians’ and ‘type 2 diabetes mellitus’ respectively). These results support a role for the following in the pathogenesis of diabetic nephropathy: renin–angiotensin system, polyol pathway, oxidative stress, inflammation, angiogenesis, glomerular filtration barrier defects, apoptosis, and cell growth and differentiation. Functional studies remain to be performed to establish the precise roles of these variants and pathways. Genetic variants initially identified using a genome-wide association approach in and near FRMD3, CARS, ELMO1, and CPVL and CHN2 were detected. The exact role of these genetic variants in relation to diabetic nephropathy requires further elucidation; many of these variants identified in GWAS will not prove to be causal.
Our analysis has some limitations. Publication bias is a concern in all meta-analyses. For this study, only published data in journals were used, discarding data published in congresses only. Negative studies are less likely to be published, potentially leading to an overestimation of effects. Moreover, non-significant genetic associations might have been underreported in published articles. Therefore, the effect estimates of the present study should be interpreted with caution, especially in cases where associations were based on small numbers of studies and/or small sample numbers. For example, the rs833061 variant in the VEGFA gene shows the strongest protective effect, but was investigated in two studies of moderate size. In these cases, additional studies are necessary to establish true effect sizes. It should also be acknowledged that by selecting only genetic variants that were associated with diabetic nephropathy and for which independent replication was available, genetic variants with smaller effect sizes may have been missed, an effect that may have proven significant using pooled analyses. By selecting only those genetic variants reproducibly associated with diabetic nephropathy, we have tried to reduce the chances of describing false positive associations.
The studies included in the present analysis showed heterogeneity with respect to ethnicity, study design and phenotypes. For some of the analysis, the clinical heterogeneity was accompanied by statistical heterogeneity with an I 2 statistic of up to 91%. However, there is no fully accepted statistical measure that precisely determines clinical heterogeneity [16]. To account for potential heterogeneity, random effects models were performed by default. These models assume that different studies have different true effects. To explore potential heterogeneity due to differences in ethnicity, a meta-regression was performed.
A study worth mentioning, which appeared after our inclusion date, is a paper by Maeda et al. [17], in which the authors investigated the variants found in the genome-wide association scan of the Genetics of Kidneys in Diabetes and Diabetes Control and Complications Trial studies [18] in four studies, of which three meet our criteria. We combined the data of Maeda et al. with results of the Genetics of Kidneys in Diabetes and Diabetes Control and Complications Trial studies. We found that only the rs451041 of CARS, and rs1041466, rs9521455 and rs1411766, which are not near a gene, were associated with diabetic nephropathy. In contrast to the Genetics of Kidneys in Diabetes and Diabetes Control and Complications Trial studies, which investigated Europeans with type 1 diabetes, Maeda et al. investigated diabetic nephropathy in type 2 diabetes in an Asian population. Therefore, the lack of association with diabetic nephropathy of the other variants could be explained by this difference.
The identification of diabetic nephropathy susceptibility variants can lead to novel biological insights and improved measures of individual aetiological processes, as indicated previously [19]. Individual aetiological processes (personalised medicine) could allow preventive and therapeutic interventions in complex disease to be tailored to individuals on the basis of their genetic profiles. From prediction studies with genetic variants for type 2 diabetes mellitus, it has been shown that 20 established genetic variants in type 2 diabetes mellitus have an AUC of 0.54 (0.5 means no predictive value, 1.0 is perfect prediction), in contrast to the Framingham offspring and Cambridge risk scores (AUC of 0.78 and 0.72, respectively). Interestingly, addition of genetic information to phenotype-based risk models did not improve prediction [20]. It is also possible that for diabetic nephropathy the genotypic risk does not exceed the risk contributed by conventional risk factors (e.g. BMI, age, diabetes mellitus duration), which means that the predictive value of risk variants for diabetic nephropathy would be limited [21]. Although genetic prediction and use of personalised medicine in diabetic nephropathy remains a new undertaking, prediction is likely to improve as additional disease variants are detected and replicated [22].
Novel biological insights may lead to development of new therapeutic targets, biomarkers and opportunities for disease prevention. Hypothesis-free approaches, such as GWAS, are most promising in this respect. At present, it seems wise to focus on assessing the relevance of previously detected genetic variants. As common SNPs associated with diabetic nephropathy and detected by GWAS may represent rare genetic variants with large effects, sequencing the regions surrounding highly significant and replicated genomic regions to detect rare variants appears to be reasonable. Follow-up in vitro and in vivo studies could then assess the functional relevance of these variants in diabetic nephropathy.
In summary, our meta-analysis identified 24 genetic variants (in or near 16 different genes) associated with advanced diabetic nephropathy. These genetic variants are likely to represent true associations and further investigations to elucidate their functional relationship in diabetic nephropathy should be pursued.
Abbreviations
- ESRD:
-
End-stage renal disease
- GWAS:
-
Genome-wide association studies
- SNP:
-
Single nucleotide polymorphism
References
No authors listed (2006) The World Health Report 2006: working together with health. World Health Organization, Geneva
No authors listed (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853
Krolewski AS, Warram JH, Rand LI, Kahn CR (1987) Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med 317:1390–1398
Seaquist ER, Goetz FC, Rich S, Barbosa J (1989) Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 320:1161–1165
Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC (1990) Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 33:438–443
Quinn M, Angelico MC, Warram JH, Krolewski AS (1996) Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 39:940–945
Nelson RG, Newman JM, Knowler WC et al (1988) Incidence of end-stage renal disease in type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia 31:730–736
Chandie Shaw PK, Baboe F, van Es LA et al (2006) South-Asian type 2 diabetic patients have higher incidence and faster progression of renal disease compared with Dutch-European diabetic patients. Diab Care 29:1383–1385
Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN (2003) Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 33:177–182
Zeggini E, Ioannidis JP (2009) Meta-analysis in genome-wide association studies. Pharmacogenomics 10:191–201
Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N (2004) Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst 96:434–442
Li Y, Tang K, Zhang Z et al. (2010) Genetic diversity of the apolipoprotein E gene and diabetic nephropathy: a meta-analysis. Mol Biol Rep doi:10.1007/s11033-010-9999-z
Xu M, Chen X, Yan L, Cheng H, Chen W (2008) Association between (AC)n dinucleotide repeat polymorphism at the 5′-end of the aldose reductase gene and diabetic nephropathy: a meta-analysis. J Mol Endocrinol 40:243–251
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Placha G, Canani LH, Warram JH, Krolewski AS (2005) Evidence for different susceptibility genes for proteinuria and ESRD in type 2 diabetes. Adv Chronic Kidney Dis 12:155–169
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Maeda S, Araki S, Babazono T et al (2010) Replication study for the association between four loci identified by a genome-wide association study on European American subjects with type 1 diabetes and susceptibility to diabetic nephropathy in Japanese subjects with type 2 diabetes. Diabetes 59:2075–2079
Pezzolesi MG, Poznik GD, Mychaleckyj JC et al (2009) Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58:1403–1410
McCarthy MI, Abecasis GR, Cardon LR et al (2008) Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9:356–369
Talmud PJ, Hingorani AD, Cooper JA et al (2010) Utility of genetic and non-genetic risk factors in prediction of type 2 diabetes: Whitehall II prospective cohort study. BMJ 340:b4838
Wald NJ, Hackshaw AK, Frost CD (1999) When can a risk factor be used as a worthwhile screening test? BMJ 319:1562–1565
Kraft P, Hunter DJ (2009) Genetic risk prediction – are we there yet? N Engl J Med 360:1701–1703
Acknowledgements
The authors wish to thank J. W. Schoones (Walaeus Library, Leiden University Medical Centre, the Netherlands) for his help with elaborating the systematic search strategy, and B. de Vries and R. Frants (Human Genetics, Leiden University Medical Centre, the Netherlands) for helping improve this article with their scientific contributions and discussions in relation to genetics.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00125-013-3117-4.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
The 132 articles investigating reproduced genetic variants with positive and negative results after meta-analysis (PDF 91.0 kb)
ESM Table 2
Details of the articles included in this meta-analysis study (PDF 544 kb)
ESM 1
(PDF 383 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mooyaart, A.L., Valk, E.J.J., van Es, L.A. et al. Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia 54, 544–553 (2011). https://doi.org/10.1007/s00125-010-1996-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-010-1996-1