Abstract
Insulin resistance is a hallmark of type 2 diabetes mellitus and is associated with a metabolic and cardiovascular cluster of disorders (dyslipidaemia, hypertension, obesity [especially visceral], glucose intolerance, endothelial dysfunction), each of which is an independent risk factor for cardiovascular disease (CVD). Multiple prospective studies have documented an association between insulin resistance and accelerated CVD in patients with type 2 diabetes, as well as in non-diabetic individuals. The molecular causes of insulin resistance, i.e. impaired insulin signalling through the phosphoinositol-3 kinase pathway with intact signalling through the mitogen-activated protein kinase pathway, are responsible for the impairment in insulin-stimulated glucose metabolism and contribute to the accelerated rate of CVD in type 2 diabetes patients. The current epidemic of diabetes is being driven by the obesity epidemic, which represents a state of tissue fat overload. Accumulation of toxic lipid metabolites (fatty acyl CoA, diacylglycerol, ceramide) in muscle, liver, adipocytes, beta cells and arterial tissues contributes to insulin resistance, beta cell dysfunction and accelerated atherosclerosis, respectively, in type 2 diabetes. Treatment with thiazolidinediones mobilises fat out of tissues, leading to enhanced insulin sensitivity, improved beta cell function and decreased atherogenesis. Insulin resistance and lipotoxicity represent the missing links (beyond the classical cardiovascular risk factors) that help explain the accelerated rate of CVD in type 2 diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus is a complex metabolic disorder complicated by microvascular and macrovascular disease [1]. Hyperglycaemia is the major risk factor for microvascular complications [2, 3] and reduction in HbA1c decreases the incidence of retinopathy, nephropathy and neuropathy [2, 3]. For every 1% decrement in HbA1c, the incidence of microvascular complications is reduced by ∼25% to 35%. Although microvascular complications are a major cause of morbidity, macrovascular complications represent the primary cause of mortality with heart attacks and stroke accounting for around 80% of all deaths [4]. In type 2 diabetic patients without prior history of myocardial infarction, the 7-year incidence of myocardial infarction is equal to or greater than the 7-year incidence of heart attack in non-diabetic individuals with prior myocardial infarction [5]. In diabetic patients with a previous heart attack, the 7-year incidence of subsequent myocardial infarction is more than double (45%) that of non-diabetic individuals [5]. Similarly, the recurrence rate of major atherosclerotic complications in type 2 diabetic patients with a prior cardiovascular event is very high, around 6% per year [6]. These results document that diabetes is a cardiovascular equivalent.
Hyperglycaemia and cardiovascular disease
Given the above observations and the negative results of the ACCORD [7], ADVANCE [8] and VADT [9] studies, in which intensified glycaemic control failed to reduce macrovascular complications, it is reasonable to ask what role hyperglycaemia plays in the development of cardiovascular disease (CVD). Epidemiological analysis of the UKPDS [3] demonstrated that rising HbA1c was associated with increased risk of myocardial infarction and stroke. However, the increased hazard ratio was modest and reduction in HbA1c following insulin or sulfonylurea therapy did not significantly decrease myocardial infarctions or strokes [10], although long-term follow up did demonstrate a significant reduction in atherosclerotic cardiovascular events [11]. These results suggest a ‘memory’ effect of improved glycaemic control, reminiscent of the Diabetes Control and Complications Trial in type 1 diabetic patients [12].
The negative results of the ACCORD, ADVANCE and VADT [7–9] studies have also led to questioning of the notion that improved glycaemic control prevents macrovascular complications in type 2 diabetic patients, especially in those with long-standing diabetes. What, for example, could explain the failure to observe a reduction in macrovascular events in these studies (see textbox: Insulin and atherosclerosis)?

First, the underlying hypothesis is not based on sound pathophysiological evidence. Hyperglycaemia is a weak risk factor for CVD compared with other well established risk factors, e.g. dyslipidaemia and hypertension; moreover, most patients in the above studies were being treated with lipid-lowering medications, antihypertensive drugs and anti-inflammatory agents [7–9]. Second, participants had long-standing diabetes with a prior cardiovascular event or multiple risk factors for CVD. Third, insulin may be the wrong drug for prevention of CVD. Insulin is associated with weight gain [13, 14] and weight gain (obesity) is a major risk factor for CVD [15, 16]. Moreover, even small increments in plasma insulin cause severe insulin resistance [17, 18], which is also a risk factor for CVD. Fourth, the study population, although large in the ACCORD and ADVANCE studies, was inadequate, given the low annual incidence of cardiovascular complications (∼2% per year), to demonstrate a beneficial effect of improved glycaemic control.
Insulin and atherosclerosis
Many in vivo and in vitro studies have demonstrated that insulin, especially at high doses, can promote atherogenesis [19–21] (see textbox: Insulin and atherosclerosis). Insulin promotes de novo lipogenesis and augments hepatic VLDL synthesis [22, 23] via its stimulation of sterol regulatory-element-binding protein-1c and its inhibition of acetyl-CoA carboxylase [24]. In cultured arterial smooth muscle cells, insulin augmented LDL-cholesterol transport [25]. Insulin is a potent growth factor, augments collagen synthesis [26, 27], stimulates arterial smooth muscle cell proliferation [28, 29] and turns on multiple genes involved in inflammation [27].
In vivo studies in dogs [30], rabbits [31] and chickens [32] provide further evidence that insulin promotes atherogenesis. Non-diabetic chickens fed a high-cholesterol diet develop severe atherosclerosis, which regresses when switched to low-cholesterol diet [32]. Insulin administration when the low-cholesterol diet was instituted prevented regression of coronary atherosclerosis. Alloxan-induced diabetic rabbits fed a high-cholesterol diet developed marked hypercholesterolaemia, but their aorta remained free of atherosclerotic plaque [31]. If rabbits are treated with insulin, severe atherosclerosis develops. Dogs receiving a low-dose insulin infusion into a hindlimb artery (to cause local, physiological hyperinsulinaemia) developed severe femoral atherosclerosis, whereas all other arteries remained free of atherosclerotic plaque [30]. Rats chronically (7–10 days) infused with insulin while maintaining euglycaemia became markedly resistant to the stimulation of glucose uptake and suppression of plasma NEFA by insulin [23], and became hypertensive [33].
Two other points about hyperinsulinaemia are noteworthy. In humans with normal glucose tolerance, insulin infusion to raise fasting plasma insulin from 57 to 104 pmol/l for 3 days produced severe insulin resistance [17, 18], a risk factor for CVD (see subsequent discussion). Finally, insulin therapy is invariably associated with weight gain [13, 14]. Most studies designed to reduce HbA1c <7.0% have required large insulin doses, failed to achieve the targeted HbA1c goal and resulted in weight gain [13, 14]. The study of Henry et al. [13] is especially noteworthy. HbA1c was reduced from 7.7% to 5.1%, but this was associated with weight gain of 8.6 kg. This is of major concern since the current diabetes epidemic is being driven by obesity, a major risk factor for CVD [15, 16]. Weight gain promotes atherogenesis via multiple mechanisms including dyslipidaemia and hypertension. Fat deposition in the arterial wall promotes inflammation, which directly accelerates atherogenesis [34–37]. Interestingly, 27.8% of individuals in the intensive treatment arm of the ACCORD study gained >10 kg [7].
Insulin resistance (metabolic) syndrome
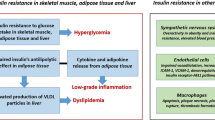
Much evidence indicates that insulin resistance per se and associated components of the insulin resistance (metabolic) syndrome (see textbox: Syndrome of insulin resistance) [38–40] contribute to development of CVD. Studies from our laboratory (Fig. 1) [1, 9, 41–44] and others [45, 46] have demonstrated that lean type 2 diabetic and obese normal glucose tolerant participants are resistant to insulin and that their insulin resistance primarily affects the glycogen synthetic pathway. Type 2 diabetes [4, 5] and obesity [15, 16] are major cardiovascular risk factors. A common thread linking all components of the insulin resistance (metabolic) syndrome is the basic cellular/molecular cause of insulin resistance [44], which not only promotes inflammation and atherogenesis, but leads to and/or aggravates other components of the syndrome, which themselves are major CVD risk factors.
Insulin-stimulated glucose disposal (40 mU m−2 min−1, euglycaemic–hyperinsulaemic clamp) in lean healthy control (CON) participants, obese normal-glucose-tolerant participants (NGT), lean drug-naive type 2 diabetic participants (T2DM), lean normal-glucose-tolerant hypertensive participants (HTN), NGT hypertriacylglycerolaemic (Hypertriacyl) participants and non-diabetic participants with coronary artery disease (CAD). White sections, non-oxidative glucose disposal (glycogen synthesis); black sections, glucose oxidation. **p < 0.01 vs CON; ***p < 0.001 vs CON. Figure adapted with permission from Kashyap et al. [19] and DeFronzo et al. [20]. To change glucose uptake into SI units, divide by 180

Hypertension is a central feature of the insulin resistance syndrome. Many studies have demonstrated that hypertensive individuals are resistant to insulin, primarily involving the glycogen synthetic pathway (Fig. 1) [47–51], and hypertension is a well established risk factor for CVD.
Type 2 diabetic and obese individuals develop dyslipidaemia characterised by hypertriacylglycerolaemia, reduced HDL-cholesterol, and small dense atherogenic LDL particles [39, 40, 52, 53], each of which is a major CVD risk factor. We and others [51, 54–57] have shown that hypertriacylglycerolaemia, but not hypercholesterolaemia is associated with insulin resistance primarily involving the glycogen synthetic pathway (Fig. 1). The frequency of hypercholesterolaemia is not increased in patients with type 2 diabetes mellitus [53]. However, if elevated, LDL-cholesterol acts synergistically with other risk factors to accelerate atherogenesis [58].
To examine the relationship between insulin resistance and CVD, we compared normal glucose-tolerant individuals with diffuse coronary artery disease with normal glucose-tolerant participants with clean coronary arteries [59]. Individuals with coronary artery disease manifested marked insulin resistance, primarily affecting the glycogen synthetic pathway in skeletal muscle [59] (Fig. 1). Studies by Reaven [40] and Paternostro et al. [60] have demonstrated that non-diabetic individuals with established coronary artery disease are resistant to insulin. Using positron emission tomography, the myocardium of non-diabetic individuals with coronary artery disease [61] and type 2 diabetic patients without coronary artery disease [62] has been shown to be resistant to insulin.
In summary, each component of the ‘metabolic’ syndrome is characterised by insulin resistance involving the glycogen synthetic pathway (Fig. 1). Insulin resistance is present at the stage of impaired glucose tolerance [63, 64] and even before any abnormality in glucose tolerance is observed [65]. Thus, we prefer to refer to the metabolic and cardiovascular cluster of factors that comprise the ‘metabolic’ syndrome as the ‘insulin resistance syndrome’ [20, 21, 66]. This focuses clearly on a specific pathogenic aetiology, rather than on a diffuse cluster of factors that may or may not be related to the same underlying pathophysiology.
Insulin resistance and the insulin resistance syndrome predict future cardiovascular disease
Multiple prospective studies have demonstrated that insulin resistance predicts future CVD. In the San Antonio Heart Study [67], insulin resistance was quantitated by HOMA of insulin resistance (HOMA-IR) in 2,564 non-diabetic participants who were followed for 8 years. Individuals in the highest quintile of insulin resistance had an approximately 2.5-fold increased incidence of CVD (Fig. 2a). After adjustment for multiple cardiovascular risk factors, individuals in the highest quintile of insulin resistance still had a twofold increased incidence of CVD. In 3,606 non-diabetic participants followed for 6.9 years in the Botnia Study [68] (Fig. 2b), the metabolic syndrome was associated with a threefold increased risk of CVD. Each component of the metabolic syndrome, as well as insulin resistance (HOMA-IR) itself, was associated with a 1.5- to 2-fold increased incidence of CVD (Fig. 2b). Similar observations have been made in the Bruneck [69], Verona Diabetes [70] and Insulin Resistance Atherosclerosis studies [71]. A strong relationship between HOMA-IR and carotid intimal media thickness has also been demonstrated [72], as has an association between insulin resistance and greater cardiovascular risk factor load [73].
a Association between insulin resistance (HOMA-IR) and 8-year incidence of CVD in non-diabetic participants in the San Antonio Heart Study before (black bars) and after (white bars) adjustment for age, sex, blood pressure, plasma lipids, smoking, exercise and waist circumference. Events, n = 187, participants, n = 2,569. Panel adapted with permission from Hanley et al. [67]. b Bar graph of 6.9 year risk of CVD in 3,606 participants with the metabolic syndrome (MS) and with individual components of the metabolic syndrome, i.e. dyslipidaemia (Dyslip), hypertension (HTN), obesity (OB) and insulin resistance (IR, highest HOMA quartile) in the Botnia Study. Panel adapted with permission from Isomaa et al. [68]
The analysis by D’Agostino et al. of six prospective studies further supports an independent role for insulin resistance in CVD [74]. It found that, using the Framingham Cardiovascular Risk Engine [75], only 69% of the observed risk for CVD could be explained, leaving 31% unaccounted for (Fig. 3a). Similarly, in the ARIC study [76] (Fig. 3b), only ∼70% of the increase in carotid intimal media thickness could be accounted for by dyslipidaemia, hypertension, glucose intolerance and obesity. We hypothesise that this unexplained risk can be attributed to insulin resistance.
a Predictive value (%) of CVD using the Framingham risk engine in Framingham Heart Study (FHS), the Atherosclerosis Risk in Community Study (ARIC), the Honolulu Heart Program (HHP), the Puerto Rico Heart Health Program (PR), the Strong Heart Study (SHS) and the Cardiovascular Health Study (CHS). On mean, the Framingham Risk engine predicts only 69% of the risk of a future cardiovascular event. Panel adapted with permission from D’Agostino et al. [74]. b Excess carotid intima–media thickness (IMT) in relation to the individual components of the insulin resistance (metabolic) syndrome as listed. Amer, American; HTN, hypertension; F, female; M, male; TG, triacylglycerol; GLU, glucose. Fields in dotted lines, unexplained risk (a 31%; b, 30%). Panel adapted with permission from Goldsen et al. [76]
Molecular causes of insulin resistance syndrome
Normal insulin signalling
As shown in Fig. 4a, for insulin to exert its biological effects, it must first bind to specific cell surface receptors [77, 78]. This activates ‘second messengers’, which initiate a phosphorylation–dephosphorylation cascade that stimulates glucose transport (via GLUT4), glucose phosphorylation (via hexokinase II), glycogen synthase (which controls glycogen synthesis) and both phosphofructokinase and pyruvate dehydrogenase (which regulate glycolysis and glucose oxidation) [79].
a Insulin signal transduction system in individuals with normal glucose tolerance (see text for a detailed discussion). NOS, nitric oxide synthase. b In type 2 diabetic participants insulin signalling is impaired at the level of IRS-1 leading to decreased glucose transport/phosphorylation/metabolism and impaired nitric oxide synthase activation/endothelial function. At the same time, insulin signalling through the MAP kinase pathway is normally sensitive to insulin. The compensatory hyperinsulinaemia (due to insulin resistance in the IRS-1/PI-3 kinase pathway) results in excessive stimulation of this pathway, which is involved in inflammation, cell proliferation and atherogenesis (see text for a detailed discussion). SHC, Src homology collagen. Reproduced from DeFronzo [1]
In muscle, insulin binding to its receptor [77, 79] leads to tyrosine phosphorylation of IRS-1, which mediates insulin’s effect on glucose metabolism. In liver, IRS-2 phosphorylation mediates the actions of insulin. IRS-1 activates phosphatidylinositol (PI)-3 kinase [80], which catalyses 3′ phosphorylation of PI, PI-4 phosphate and PI-4,5 diphosphate, and augments glucose transport and glycogen synthase [81–83]. Inhibitors of PI-3 kinase inhibit glucose transport [82], hexokinase II [84] and glycogen synthase [85].
Insulin signalling also plays a critical role in activating nitric oxide synthase, which regulates nitric oxide production [86–88]. Nitric oxide is a potent vasodilator and anti-atherogenic agent [86]. Nitric oxide deficiency activates multiple pathways involved in atherogenesis [89, 90]. Thus, a defect in insulin signalling, not only impairs glucose utilisation, but causes hypertension and accelerated atherosclerosis.
Insulin is a potent growth factor [20, 26, 27, 91, 92], whose growth-promoting effects are mediated via the mitogen-activated protein (MAP) kinase pathway [93]. After the interaction between IRS-1 and SHC, extracellular regulated kinase (ERK) is activated [77, 94], translocates into the nucleus and catalyses phosphorylation of transcription factors that promote cell growth, cell proliferation and cell differentiation [77]. Thus, this pathway plays an important role in atherogenesis. MAP kinase blockade prevents stimulation of insulin’s growth-promoting effects, but has no effect on the metabolic actions of insulin [95, 96]. Insulin resistance in the PI-3 kinase (metabolic) pathway with intact MAP kinase signalling activates multiple inflammatory pathways, including inhibitor kβ (IkB)/nuclear factor kβ (NFkB) [97] and c-Jun N-terminal kinase [98], which also cause insulin resistance. Not surprisingly, sustained physiological hyperinsulinaemia activates multiple genes involved in inflammation [27].
Insulin receptor defects in type 2 diabetes
Some studies [44, 78, 99] have demonstrated decreased insulin binding to monocytes and adipocytes in type 2 diabetic patients. However, in muscle and liver, insulin binding to solubilised insulin receptors is normal in obese normal glucose-tolerant and lean type 2 diabetic participants [100].
Insulin receptor tyrosine kinase activity
In normal-weight and obese diabetic patients, insulin-stimulated insulin receptor tyrosine kinase activity was reduced and could not be explained by decreased insulin receptor number/binding [101]. Normalisation of fasting plasma glucose with weight loss corrected the defect in insulin receptor tyrosine kinase [102], indicating that the defect is acquired.
Insulin signalling (IRS-1 and PI-3 kinase) defects
As indicated in Fig. 4b, we have shown that in skeletal muscle of lean individuals with normal glucose tolerance, physiological hyperinsulinaemia increases insulin receptor and IRS-1 tyrosine phosphorylation by 150% to 200% [94, 103]. In obese non-diabetic participants, activation of these two insulin signalling events in muscle was reduced, while in type 2 diabetic individuals insulin had no significant stimulatory effect [94]. Association of PI-3 kinase with IRS-1/IRS-2 was greatly reduced in obese non-diabetic and type 2 diabetic participants and correlated closely with impaired muscle glycogen synthase activity and with in vivo insulin-stimulated glucose disposal [94].
MAP kinase signalling
Our studies in human skeletal muscle [94] (Fig. 4b) were the first to demonstrate that in insulin-resistant type 2 diabetic and obese non-diabetic individuals the profound insulin resistance in the PI-3 kinase pathway contrasts with the normal stimulatory effect of insulin on the MAP kinase pathway (MAP kinase kinase 1 activity and ERK1/2 phosphorylation/activity) [94]. Maintained insulin stimulation of the MAP kinase pathway, with persistent insulin resistance in the PI-3 kinase pathway, represents an important pathogenic mechanism for development of CVD in insulin-resistant states (see subsequent discussion).
Molecular link between insulin resistance and atherosclerosis
Our studies in man demonstrated that impaired IRS-1 tyrosine phosphorylation/PI-3 kinase activation in lean type 2 diabetic and obese non-diabetic participants causes a profound defect in glucose transport/phosphorylation and glycogen synthesis (Fig. 4b) [104, 105]. Because nitric oxide synthase is activated by the same PI-3 kinase pathway, nitric oxide production is impaired [106, 107], resulting in endothelial dysfunction [108–110] and accelerated atherosclerosis [110–113]. This pathogenic sequence establishes the molecular basis linking insulin resistance, inflammation and accelerated atherosclerosis in people with type 2 diabetes mellitus and may help account for the missing 30% CVD risk that cannot be explained by circulating cardiovascular risk factors [74, 76] (Fig. 4b). This pathogenic sequence also explains why insulin resistance is a strong predictor of CVD [67–73].
Once the insulin signalling defect becomes established, it initiates a reverberating negative feedback cycle. Impaired glucose utilisation causes hyperglycaemia, which stimulates insulin secretion (Fig. 4b). Because the IRS-1/PI-3 kinase pathway is defective, the MAP kinase pathway is excessively stimulated since it is normally sensitive to insulin. Increased IRS-1 serine phosphorylation, induced by metabolic/inflammatory abnormalities present in the diabetic state (reviewed by Draznin [114]), further impairs insulin signalling through the PI-3 kinase pathway [115, 116]. In diabetic and obese patients, continued MAP kinase pathway stimulation [94] causes vascular smooth muscle proliferation, increased collagen formation and excessive production of growth factors/inflammatory cytokines, contributing to accelerated atherosclerosis. The same insulin signalling defects that we have demonstrated in skeletal muscle of type 2 diabetic patients have been demonstrated in arterial vascular smooth muscle cells (VSMC) in animal models of diabetes and in humans [92, 117, 118].
Draznin and colleagues [93, 114] have provided additional evidence for this ‘dual insulin signalling hypothesis’. In cultured VSMCs and endothelial cells treated with PI-3 kinase inhibitors, insulin cannot activate nitric oxide synthase to generate nitric oxide and these cells are no longer protected from the detrimental effects of vascular endothelial growth factor, platelet-derived growth factor (PDGF) and other inflammatory peptides. Insulin continues to stimulate VSMC proliferation/migration even though the PI-3 kinase pathway has been inhibited and increases prenylated Ras (rat sarcoma) and Rho (Ras related homologue), leading to augmented VSMC response to the growth-promoting effects of IGF-1, epidermal growth factor, PDGF and angiotensin II. These effects are enhanced when PI-3 kinase is inhibited [119, 120]. The sensitising effect of VSMCs to angiotensin II is of particular importance, since hyperinsulinaemia doubles the ability of angiotensin II to transactivate NF-kB [120], a powerful nuclear transcription factor that activates multiple inflammatory pathways involved in atherogenesis [121, 122] and causes IRS-1 serine phosphorylation, which inhibits insulin signalling [123]. Angiotensin II also serine phosphorylates IRS-1 in aortic smooth muscle and skeletal muscle cells [124]. This provides a pathophysiological link between insulin resistance, atherogenesis and essential hypertension.
Genetic vs acquired defects in insulin signal transduction
To examine whether the insulin signalling defect is genetic or acquired, we studied lean normal glucose-tolerant offspring of two diabetic parents [65]. These offspring are severely insulin-resistant [65, 103] and at high risk of developing diabetes. Insulin-stimulated glucose disposal was markedly reduced despite increased insulin receptor tyrosine phosphorylation [103]. Basal and insulin-stimulated IRS-1 tyrosine phosphorylation/PI-3 kinase activity were markedly reduced. From these observations five points ensue: (1) early in the natural history of type 2 diabetes insulin receptor activation is normal (Fig. 4b); (2) the rate-limiting step for insulin signalling resides at IRS-1; (3) molecular abnormalities responsible for insulin resistance are present long before onset of overt diabetes or impaired glucose tolerance; (4) insulin normally augments MAP kinase, but not PI-3 kinase, demonstrating dissociation between regulation of PI-3 kinase and MAP kinase pathways; and (5) tissues of offspring with normal glucose tolerance are being ‘incubated’ in a ‘sea’ of molecular insulin resistance and atherogenicity from an early age, explaining in part why clinically evident CVD is present in 15 to 20% of individuals at initial diagnosis [125] and why insulin resistance and CVD are closely linked [19, 20, 44, 67–72]. Only thiazolidinediones simultaneously augment the PI-3 kinase (metabolic) pathway while inhibiting the MAP kinase (atherogenic) pathway [126, 127]. Thiazolidinediones also enhance nitric oxide synthase activity, increasing nitric oxide production [128–130]; they also reduce high-sensitivity C-reactive protein levels and improve multiple cardiovascular risk factors in type 2 diabetic participants [131].
Lipotoxicity, insulin resistance and atherosclerotic CVD
The term ‘lipotoxicity’ was coined by Unger to describe the deleterious effect of tissue fat accumulation on glucose metabolism [132]. However, lipotoxicity has assumed added significance (see textbox: Lipotoxicity). Experimental NEFA elevation to reproduce levels in type 2 diabetes causes severe muscle/liver insulin resistance [133–135] and inhibits insulin secretion [136], reproducing the three basic core defects of type 2 diabetes. Elevated plasma NEFA impair glucose oxidation/glycogen synthesis [133] and decrease glucose transport/phosphorylation [104, 135]. Most importantly, lipid infusion to increase plasma NEFA levels in participants with normal glucose tolerance caused a dose–response inhibition of insulin receptor/IRS-1 tyrosine phosphorylation and PI-kinase activity, which correlate closely with reduced insulin-stimulated glucose disposal [137].

Magnetic resonance spectroscopic studies have demonstrated that intramyocellular and intrahepatic fat accumulation are closely associated with organ-specific insulin resistance [138–141]. Intracellular toxic metabolites of triacylglycerol and NEFA metabolism (fatty acyl CoA, diacylglycerol, ceramides) cause severe insulin resistance by impairing insulin signalling and multiple intracellular steps of glucose metabolism [133, 137, 142]. To examine the causality of this association, we treated type 2 diabetic patients with acipimox, an inhibitor of lipolysis. The decline in plasma NEFA (563 to 230 mmol/l) was associated with decreased muscle long-chain fatty acyl CoA [143], which correlated closely with enhanced insulin-stimulated muscle glucose uptake (Fig. 5). The deleterious effect of increased intracellular fat on insulin sensitivity is supported by the work of Kim et al. [144], who overexpressed lipoprotein lipase in muscle or liver in mice. In muscle this caused a marked increase in fat content, severe insulin resistance and impaired PI-3 kinase activity, without affecting hepatic insulin sensitivity. Lipoprotein lipase overexpression in liver increased hepatic fat content, induced severe hepatic insulin resistance and impaired hepatic insulin signalling, but had no effect on muscle [144]. Long-chain fatty acyl CoAs cause IRS-1 serine phosphorylation, thereby inhibiting the PI 3 kinase pathway [145, 146].
a Intramyocellular lipid content (measured by magnetic resonance spectroscopy) and (b) muscle long-chain fatty acyl CoA (LC-FACoA) content in control (CON) and type 2 diabetic (T2DM) participants. **p < 0.01 for T2DM vs CON. c Relationship between the increment in insulin-stimulated rate of glucose disposal (Rd) and decrement in muscle LC-FACoA content in type 2 diabetic participants after treatment with acipimox. p < 0.01; r = 0.74. Figure adapted with permission from Bajaj et al. [143]
In animals [147] and humans [148], plasma NEFA elevation by lipid infusion also increased intramyocellular diacylglycerol, a potent activator of protein kinase C (PKC) isoforms, which inhibit insulin signalling through serine phosphorylation of IRS-1 [149]. Muscle [150] and plasma [151] ceramide levels are also increased in obese and type 2 diabetic individuals, correlating closely with severity of insulin resistance.
In summary, much evidence supports the notion that increased plasma NEFA/intramyocellular levels of toxic lipid metabolites (long-chain fatty acyl CoAs, diacylglycerol, ceramides) play a role in the pathogenesis of muscle/liver insulin resistance.
NEFA, inflammation and atherosclerosis: the neglected lipid
Type 2 diabetes and obesity are characterised by low-grade, chronic inflammation [152–154], which could contribute to accelerated atherosclerosis. Increased activity of IkB/NFkB provides a molecular mechanism responsible for inflammation and insulin resistance in type 2 diabetes mellitus [91, 148, 152, 155]. Under basal conditions, NFkB is associated with IkB in the cytoplasm (Fig. 6). Upon activation by inflammatory factors, including fatty acyl CoAs, IkB kinase phosphorylates IkB, causing IkB polyubiquitination and degradation. The free NFkB translocates to the nucleus, where it binds to target genes, stimulating inflammatory mediators (TNFα, IL-1B, IL-6, PKC) involved in atherogenesis [152, 156, 157]. TNFα, IL-6 and PKC, as well as IK kinase, cause serine phosphorylation of IRS-1, inhibiting insulin signalling and causing insulin resistance [123, 158].
Skeletal muscle IkB content is decreased in type 2 diabetic patients [159], indicative of increased NFkB activity. We have confirmed this by demonstrating increased NF-kB p50–p65 DNA binding activity in type 2 diabetic and obese individuals (P Tantiwong, K Shammugasumdaran, A Monroy, S Ghosh, E Cersosimo, A Sriwijitkamol, RA DeFronzo, S Mohan, N Musi, unpublished results). Reduced IkB and increased NFkB p50–p65 binding activity were strongly correlated with reduced insulin sensitivity.
Multiple stimuli [152], including lipids, activate IkB kinase/NFkB, causing insulin resistance in cultured myocytes [160] and muscle from rodents [155, 161] and humans [162]. In human myocytes, palmitate enhances NFkB activity and increases IL6 mRNA, a downstream gene regulated by NFkB. Transfection of myocytes with an adenovirus-IkB super repressor mutant blocked the augmentation of NFkB activity and of IL6 mRNA expression by palmitate [162].
Circulating NEFA also cause inflammation and insulin resistance by directly activating plasma membrane receptors, such as toll-like receptor 4 [162] (Fig. 7). In muscle of obese and type 2 diabetic individuals, toll-like receptor 4 mRNA/protein content is increased and correlates closely with HOMA-IR [162] and IkB/NFkB activation, providing another pathway via which NEFA incite inflammation and promote atherogenesis.
Schematic representation of the toll-like receptor 4 (TLR-4) pathway and its activation by NEFA. Dissociation of NFkB from IkB allows it to diffuse into the nucleus where it can turn on genes associated with inflammation, atherosclerosis and insulin resistance (see text for a detailed discussion). CD14, cluster of differentiation 14; IRAK, interleukin-1 receptor associated kinase, MD-2, myb regulated gene; MYD88, myeloid differentiation primary response gene; TRAF, TNFa receptor associated factor
NEFA also stimulate formation of collagen, an integral component of the atherosclerotic plaque [163, 164]. Physiological NEFA elevation in participants with normal glucose tolerance increases mRNA expression of collagen, fibronectin and lumican, as well as connective tissue growth factor [134], a member of the CCN gene family, which mediates fibrotic responses [163]. Increased connective tissue growth factor levels are a characteristic finding in complicated atherosclerotic plaques and induce mononuclear cell chemotaxis [164].
Plasma NEFA exert profound effects on endothelial function. Insulin-stimulated blood flow is mediated via nitric oxide [86] and is impaired in obese and type 2 diabetic individuals [108, 165]. Experimental plasma NEFA elevation in participants with normal glucose tolerance reduced endothelium-dependent blood flow, without altering endothelium-independent blood flow [166]. Endothelial dysfunction is associated with accelerated atherosclerosis and insulin resistance [110, 111], providing another mechanism via which NEFA promote CVD in type 2 diabetes mellitus.
Adiposopathy
‘Adiposopathy’ is another form of lipotoxicity [131]. Fat cells produce adipocytokines, which travel to distant sites (muscle, liver, arterial tissue) where they exert deleterious effects on metabolism and vascular function. Adipose tissue of obese and type 2 diabetic individuals is infiltrated by mononuclear cells and is in a state of chronic inflammation [167]. The adipocytes and infiltrated macrophages secrete proinflammatory/prothrombotic cytokines (TNFα, resistin, IL-6, plasminogen activator inhibitor-1, angiotensinogen) that promote atherogenesis and cause insulin resistance (reviewed by Bays et al. [131]). Adipocytes also under-produce adiponectin, a potent insulin-sensitising, anti-atherogenic cytokine [131].
Fat topography
Accumulation of fat within the intraabdominal cavity, i.e. visceral fat (Fig. 8), is associated with insulin resistance and CVD [131, 168, 169]. Although the mechanism(s) for this association remain(s) unclear, visceral obesity is part of the insulin resistance (metabolic) syndrome [38–40]; however, the individual components of the insulin resistance syndrome, not the increase in visceral fat, may account for the increased incidence of CVD.
What therapeutic interventions reverse lipotoxicity?
Tissue fat overload is explained by: (1) excessive energy intake; (2) increased lipolysis in insulin-resistant adipocytes, leading to increased plasma NEFA and enhanced influx into muscle/liver; and (3) decreased fat oxidation. Weight loss (energy-intake restriction) has proven effective in reducing total body fat content [170]. Glucagon-like peptide-1 analogues are potent appetite suppressants and effectively promote weight loss [171].
Thiazolidinediones exert the following unique actions that ameliorate lipotoxicity (Textbox 3): (1) decrease plasma NEFA by inhibition of lipolysis; (2) reduction of muscle long-chain fatty acyl CoA levels; (3) redistribution of fat within the body; and (4) amelioration of adiposopathy.
Thiazolidinediones, insulin sensitivity and NEFA metabolism
Thiazolidinediones are potent insulin sensitisers in muscle and liver [126, 127, 140, 141, 172, 173] and improve sensitivity to the antilipolytic effect of insulin, decreasing basal NEFA turnover/plasma NEFA [172]. Thiazolidinediones also improve hepatic insulin sensitivity, leading to decreased basal hepatic glucose production and enhanced hepatic glucose production suppression by insulin [140, 172–174]. Reduced plasma NEFA levels correlate closely with improved insulin-stimulated muscle glucose disposal and decreased hepatic glucose production.
In type 2 diabetic patients, impaired insulin-stimulated IRS-1 tyrosine phosphorylation and PI 3 kinase activity [94] (Fig. 9) are improved by rosiglitazone [126], with enhanced insulin signalling correlating closely with reduced plasma NEFA. Similarly, pioglitazone increases insulin-stimulated glucose disposal by 35% in association with reduced fasting NEFA, muscle fat content and fatty acyl CoAs [127, 173] (Fig. 5). These results provide strong evidence that thiazolidinediones improve muscle insulin sensitivity via reversal of lipotoxicity.
Rosiglitazone (ROSI) treatment of type 2 diabetic patients (T2DM) for 4 months markedly augmented insulin signalling through the IRS-1/PI-3 kinase pathway without altering (a) insulin receptor tyrosine phosphorylation, (b) IRS-1 tyrosine phosphorylation, (c) association of p85 subunit of PI-3 kinase with IRS-1 and (d) association of PI-3 kinase with IRS-1. *p < 0.05; **p < 0.01. Figure adapted with permission from Miyazaki et al. [126]
Mitochondrial dysfunction, lipotoxicity and thiazolidinediones
Impaired ATP synthesis and mitochondrial dysfunction have been demonstrated in insulin-resistant states including ageing, offspring of diabetic parents, obesity and type 2 diabetes mellitus [175–179]. Using 31P nuclear magnetic resonance spectroscopy, Petersen et al. [176] have shown that decreased ATP synthesis is associated with increased intramyocellular fat. In isolated mitochondria from obese and type 2 diabetic individuals, we have demonstrated that ATP synthesis is reduced [179, 180] and correlates closely with decreased insulin-stimulated glucose disposal and increased fasting plasma NEFA. To examine whether fatty acid metabolites directly inhibit mitochondrial function, we incubated mitochondria from participants with normal glucose tolerance with palmitoyl carnitine [181]. At concentrations <2 μmol/l, palmitoyl carnitine stimulated ATP synthesis, while at concentrations >2 μmol/l it progressively decreased ATP synthesis and inner mitochondrial membrane potential, which provides the voltage gradient driving electron transport.
Type 2 diabetic individuals and insulin-resistant offspring with normal glucose tolerance have reduced expression of multiple nuclear genes that encode enzymes involved in oxidative metabolism, including peroxisome proliferator-activated receptor (PPAR)γ coactivator (PGC-1) [182], the master regulator of mitochondrial biogenesis [183]. A twofold increase of plasma NEFA in participants with normal glucose tolerance reduced muscle expression of PGC-1 and multiple mitochondrial genes to levels observed in diabetic individuals [134], providing evidence of ‘mitochondrial lipotoxicity’. Treatment of type 2 diabetic patients with pioglitazone upregulated PGC-1α (also known as PPARGC1A) and PGC-1β (also known as PPARGC1b) mRNA expression in association with increased expression of pyruvate dehydrogenase alpha 1 (PHDA1) and other mitochondrial oxidative phosphorylation genes [175] (Fig. 10), ameliorating ‘mitochondrial lipotoxicity’.
PGC-1α and PGC-1β expression in muscle of (a, b) individuals with normal-glucose-tolerance and without family history (FH−) of diabetes, insulin-resistant normal-glucose-tolerant offspring of two type 2 diabetic parents (FH+) and participants with type 2 diabetes mellitus (T2DM). c A strong linear relationship exists between PGC-1α mRNA and PDHA1 mRNA, and the expression of other mitochondrial genes (not shown) involved in oxidative phosphorylation. White diamonds, FH−; black triangles, type 2 diabetes mellitus; hatched squares, FH+. d, e Pioglitazone (PIO) treatment of type 2 diabetic patients significantly increased the expression of PGC-1α and PGC-1β mRNA in muscle. *p < 0.05 for FH + vs FH−; **p < 0.01 for FH+ and T2DM vs FH−, and (d, e) *p < 0.05 for PIO vs Basal. Figure adapted with permission from Patti et al. [182] and Coletta et al. [175]
Pioglitazone also ameliorates lipotoxicity via effects mediated through adiponectin and adiponectin receptor (Fig. 11). Hypoadiponectinaemia is characteristic of type 2 diabetes. Thiazolidinediones increase plasma adiponectin levels to or above those in non-diabetic patients [131, 173]. Adiponectin is a potent insulin sensitiser in muscle and liver [184] and exerts multiple anti-atherogenic effects [185, 186]. In type 2 diabetic patients and insulin-resistant offspring with normal glucose tolerance, adiponectin receptor 1 and 2 expression is reduced [187]. Pioglitazone treatment increases expression of muscle adiponectin receptors 1 and 2 and AMP kinase [188]. Similar effects have been demonstrated in cultured human myocytes and do not require PPARγ stimulation [188]. Pioglitazone also upregulated acetyl-CoA carboxylase and carnitine palmitoyl transferase expression/activity, leading to the postulate (Fig. 11) that pioglitazone increases plasma adiponectin levels and expression of adiponectin receptors 1 and 2, stimulating AMP kinase and acetyl-CoA carboxylase activity, with resultant inhibition of malonyl CoA. This augments carnitine palmityol transferase I activity and enhances NEFA flux into mitochondria, where they undergo beta oxidation. The result is a decrease in intramyocellular fatty acyl CoA levels, leading to improved muscle insulin signalling/insulin sensitivity.
Amelioration of lipotoxicity by action of pioglitazone in increasing the plasma adiponectin concentration and levels of adiponectin receptors (ADIPOR) 1 and 2. See text for a detailed discussion. ACC, acetyl CoA carboxylase; CPT-1, carnitine palmitoyl transferase-1; CREB, cAMP response element binding; DAG, diacylglycerol; MEF2C, myocyte enhancer factor 2C. Figure adapted with permission from Civitarese et al. [187] and Coletta et al. [175]
Thiazolidinediones and fat topography: clinical implications
Thiazolidinediones redistribute body fat by reducing visceral and increasing subcutaneous fat [189, 190] (Fig. 8). They also reduce muscle fatty acyl CoA, leading to improved insulin sensitivity and glycaemic control [126, 127, 140, 141, 172, 173, 176]. Thiazolidinediones also reduce liver fat and are effective in treating non-alcoholic steatohepatitis [139, 191]. In a randomised, placebo controlled study of 55 participants with type 2 diabetes/impaired glucose tolerance and biopsy-proven non-alcoholic steatohepatitis, pioglitazone reduced liver fat by 54% (p < 0.001), normalised alanine aminotransferase and aspartate aminotransferase, and reduced hepatic inflammation, ballooning necrosis and fibrosis [139].
Thiazolidinediones preserve beta cell function in type 2 diabetic patients [192]. Eight double-blind, placebo/active comparator-controlled studies demonstrate that thiazolidinediones have a durable effect in reducing HbA1c (reviewed by DeFronzo [1]) because of a beta cell protective effect. Five studies in participants with impaired glucose tolerance document that thiazolidinediones markedly reduced conversion to type 2 diabetes, primarily by improving beta cell function (reviewed by DeFronzo [1]). Using the gold standard insulin secretion/insulin resistance (disposition) index, both pioglitazone and rosiglitazone have been shown to improve beta cell function in drug naive and sulfonylurea-treated type 2 diabetic patients [192]. Thiazolidinediones augment beta cell function by multiple mechanisms, including direct effects (increased GLUT2, glucokinase, Pdx) exerted through PPARγ [193], and by reducing intracellular fatty acyl CoA levels [194–196].
Myocardial lipid content is increased in type 2 diabetic individuals [197, 198] and contributes to diastolic dysfunction [198, 199]. The myocardium in type 2 diabetes is markedly insulin-resistant [61, 198]. The insulin resistance cannot be explained by coronary artery disease [199] and correlates with increased myocardial fat [198, 200]. Rosiglitazone similarly improved myocardial and skeletal muscle insulin resistance [62]. Although myocardial fat content was not measured in these studies, it is reasonable to hypothesise that thiazolidinediones exert their myocardial insulin sensitising effect, in part by reducing myocardial fat content.
Coronary and carotid arterial disease represent the major cause of mortality in patients with type 2 diabetes [4]. Elevated plasma LDL-cholesterol and triacylglycerol, and reduced HDL-cholesterol all contribute to accelerated atherogenesis in type 2 diabetes. Quantitative lipid analysis of atherosclerotic plaques reveals large amounts of NEFA and fatty acid metabolites [35–37], which can stimulate inflammatory pathways involved in atherogenesis. Pioglitazone reduces plasma triacylglycerol, increases HDL-cholesterol, converts small dense LDL particles to larger more buoyant ones and decreases apolipoprotein B-100 without altering plasma LDL-cholesterol [201, 202]. Thiazolidinediones also markedly reduce plasma NEFA [127, 139, 172], which would be expected to reduce fatty acyl CoAs and other toxic fatty acid metabolites within arterial smooth muscle cells, thus inhibiting atherogenesis. Thiazolidinediones also inhibit release of proinflammatory, prothrombotic, atherogenic adipocytokines from fat tissue [139, 141, 172, 203] and redistribute fat from visceral (atherosclerotic equivalent) to subcutaneous depots [189, 190]. Thus, thiazolidinediones ameliorate all components of the ‘lipotoxicity syndrome’. Therefore, one could expect thiazolidinediones to reduce cardiovascular events in type 2 diabetes.
The Prospective Pioglitazone Clinical Trial in Macrovascular Events [204] examined the effect of pioglitazone on cardiovascular outcomes in type 2 diabetes patients with a previous cardiovascular event. Although the primary composite endpoint did not reach statistical significance, a beneficial trend (10% decrease) was observed; the second principal endpoint (time to death, myocardial infarction, stroke) was significantly reduced. Analysis of all double-blind placebo-controlled pioglitazone trials in the USA demonstrated a significant reduction in cardiovascular events [205]. Consistent with these trials, pioglitazone decreased carotid intima medial thickness [206] and coronary atherosclerotic plaque volume [207].
Weight gain and thiazolidinedione paradox
Thiazolidinedione treatment is associated with modest weight gain (1–3 kg) that occurs within the first 12 months of treatment. Paradoxically, the greater the weight gain, the greater the reduction in HbA1c and the greater the improvements in insulin sensitivity and beta cell function [173, 189, 192]. This paradox is explained as follows. Thiazolidinediones mobilise fat from muscle, liver and beta cells leading to improved insulin sensitivity and beta cell function. PPARγ receptors are abundant in hypothalamic appetite regulation centres [208]. Thus, thiazolidinediones simultaneously evoke two effects: (1) PPARγ activation in muscle/liver/adipocytes/beta cells; and (2) stimulation of appetite. The former improves glucose homeostasis, while the latter promotes weight gain. Consequently, increased body weight is a strong predictor of improved glycaemic control (HbA1c) in type 2 diabetes mellitus [204].
Summary
Insulin resistance in the PI-3 kinase pathway with normal insulin sensitivity in the MAP kinase pathway plays an important role in accelerated atherogenesis in patients with type 2 diabetes mellitus. Lipotoxicity is a major cause of insulin resistance and impaired beta cell function in type 2 diabetes mellitus and plays a central role in the accelerated CVD. Interventions that mobilise fat out of tissues (restriction of energy intake, thiazolidinediones, glucagon-like peptide-1 analogues) are likely to produce a durable reduction in glycaemia and reduce atherosclerotic cardiovascular events.
Abbreviations
- ACCORD:
-
Action to Control Cardiovascular Risk in Diabetes
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation
- CVD:
-
Cardiovascular disease
- ERK:
-
Extracellular regulated kinase
- HOMA-IR:
-
HOMA of insulin resistance
- IkB:
-
Inhibitor kβ
- MAP:
-
Mitogen-activated protein
- NF-kB:
-
Nuclear factor kβ
- PDGF:
-
Platelet-derived growth factor
- PGC-1:
-
PPARγ coactivator-1
- PI:
-
Phosphoinositol
- PKC:
-
Protein kinase C
- PPAR:
-
Peroxisome proliferator-activated receptor
- VADT:
-
Veterans Administration Diabetes Trial
- VSMC:
-
Vascular smooth muscle cells
References
DeFronzo RA (2009) From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795
Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Stratton LM, Adler AJ, Neil HA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412
Morrish NJ, Wang SL, Stevens LK et al (2001) Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44(Suppl 2):S14–S21
Haffner SM, Lehto S, Ronnemaa T et al (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234
Giorda CB, Avogaro A, Maggini M et al (2008) Recurrence of cardiovascular events in patients with type 2 diabetes. Diabetes Care 31:2154–2159
Gerstein HC, Miller ME, Byington RP et al (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2345–2359
Patel A, MacMahon S, Chalmers J et al (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med 358:2560–2725
Duckworth W, Abraira C, Mortiz T et al (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360:129–139
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Holman RR, Paul SK, Bethel MA et al (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359:1577–1589
Nathan DM, Cleary PA, Backlund JY et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type I diabetes. N Engl J Med 22:2643–53
Henry RR, Gumbiner B, Ditzler T et al (1993) Intensive conventional insulin therapy for type II diabetes. Metabolic effects during a 6-month outpatient trial. Diabetes Care 16:21–31
Holman RR, Thorne KI, Farmer AJ et al (2007) Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 357:1716–1730
Calle EE, Thun MJ, Petrelli JM et al (1999) Body-mass index and mortality in a prospective cohort of U.S. adults. New Engl J Med 341:1097–1105
Allison DB, Fontaine KR, Manson JE et al (1999) Annual deaths attributable to obesity in the United States. JAMA 282:1530–1538
Del Prato S, Leonetti F, Simonson DC et al (1994) Effect of sustained physiologic hyperinsulinemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 37:1025–1035
Iozzo P, Pratipanawatr T, Pijl H et al (2001) Physiological hyperinsulinemia impairs insulin-stimulated glycogen synthase activity and glycogen synthesis. Am J Physiol 280:E712–E719
Kashyap SR, DeFronzo RA (2007) The insulin resistance syndrome: physiologic considerations. Diab Vasc Dis Res 4:13–19
DeFronzo RA, Ferrannini E (1991) Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and ASCVD. Diabetes Care Reviews 14:173–194
DeFronzo RA (2006) Is insulin resistance atherogenic? Possible mechanisms. Atherosclerosis 7:11–15
Koopmans SJ, Kushwaha RS, DeFronzo RA (1999) Chronic physiologic hyperinsulinemia impairs suppression of plasma free fatty acids and increases de novo lipogenesis in conscious normal rats. Metabolism 48:330–337
Tobey TA, Greenfield M, Kraemer F et al (1981) Relationship between insulin resistance, insulin secretion, very low density lipoprotein kinetics and plasma triglyceride levels in normotriglyceridemic man. Metabolism 30:165–171
Azzout-Marniche D, Becard D, Guichard C et al (2000) Insulin effects on sterol regulatory-element-binding protein-1c (SREBP-1c) transcriptional activity in rat hepatocytes. Biochem J 350:389–393
Stout TW (1971) The effect of insulin on the incorporation of (1-C) sodium acetate into the lipids of the rat aorta. Diabetologia 7:367–372
King GL, Goodman D, Buzney S et al (1985) Receptors and growth promoting effects of insulin and insulin like growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest 75:1028–1036
Coletta D, Balas B, Chavez AO et al (2008) Effect of acute physiological hyperinsulinemia on gene expression in human skeletal muscle in vivo. Am J Physiol Endo Metab 294:E910–E917
Nakao J, Ito H, Kanayasu T et al (1985) Stimulatory effect of insulin on aortic smooth muscle cell migration induced by 12-L-hydroxy-5, 8, 10, 14-eicosatetraenoic acid and its modulation by elevated extracellular glucose levels. Diabetes 34:185–191
Pfeifle B, Ditschuneit H (1981) Effect of insulin on the growth of cultured arterial smooth muscle cells. Diabetologia 20:155–158
Cruz AB, Amatuzio DS, Grande F et al (1961) Effect of intra-arterial insulin on tissue cholesterol and fatty acids in alloxan-diabetic dogs. Circ Res 9:39–43
Duff GL, McMillan GC (1949) The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. J Exp Med 89:611–630
Stamler J, Pick R, Katz LN (1960) Effect of insulin in the induction and regression of atherosclerosis in the chick. Circ Res 8:572–526
Meehan WP, Buchanan TA, Hsueh W (1994) Chronic insulin administration elevates blood pressure in rats. Hypertension 23:1012–1017
Wang L, Sapuri-Butin AR, Aung HH et al (2008) Triglyceride-rich lipoprotein lipolysis increases aggregation of endothelial membrane microdomains and produces reactive oxygen species. Am J Physiol Heart Circ Physiol 295:H237–H244
Felton CV, Crook D, Davies MJ et al (1997) Relation of plaque lipid composition and morphology to the stability of human aortic plaques. Arterio Throm Vasc Biol 17:1337–1345
Felton CV, Crook D, Davies MJ et al (1994) Dietary polyunsaturated fatty acids and composition of human aortic plaques. Lancet 344:1195–1196
Stachowska E, Doxegowska B, Chlubek D et al (2004) Dietary trans fatty acids and composition of human atheromatous plaques. Eur J Nutr 43:313–318
Third report of the National Cholesterol Education Program (NCEP) (2002) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421
Miranda PJ, DeFronzo RA, Califf RM et al (2005) The metabolic syndrome: evaluation of pathologic and therapeutic outcomes. Am Heart J 149:20–45
Reaven GM (1988) Banting lecture. Role of insulin resistance in human disease. Diabetes 37:1594–1607
DeFronzo RA (1988) Lilly lecture. The triumvirate: beta cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687
DeFronzo RA, Gunnarsson R, Bjorkman O et al (1985) Effects of insulin on peripheral and splanchnic glucose metabolism in non-insulin-dependent (type II) diabetes mellitus. J Clin Invest 76:149–155
Bonadonna R, Groop L, Kraemer N et al (1990) Obesity and insulin resistance in man: a dose response study. Metabolism 39:452–459
Bajaj M, DeFronzo RA (2003) Metabolic and molecular basis of insulin resistance. J Nucl Cardiol 10:311–323
Shulman GI, Rothman Dl, Jue T et al (1990) Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322:223–238
Chen IY-D, Jeng C-Y, Hollenbeck CB et al (1988) Relationship between plasma glucose and insulin concentration, glucose production, and glucose disposal in normal subjects and patients with non-insulin-dependent diabetes. J Clin Invest 82:21–25
Reaven G (2003) Insulin resistance, hypertension, and coronary heart disease. J Clin Hypertens 5:269–274
Sironi AM, Pingitore A, Ghione S et al (2008) Early hypertension is associated with reduced regional cardiac function, insulin resistance, epicardial, and visceral fat. Hypertension 51:282–288
Ferrannini E, Buzzigoli G, Bonadonna R et al (1987) Insulin resistance in essential hypertension. New Eng J Med 317:350–357
Solini A, DeFronzo RA (1992) Insulin resistance, hypertension, and cellular ion transport systems. Acta Diabetologica 29:196–200
DeFronzo RA (1997) Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med 50:191–197
Rana JS, Visser ME, Arsenault BJ et al. (2010) Metabolic dyslipidemia and risk of future coronary heart disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Int J Cardiol doi:10.1016/j.ijcard2009.03.123
Stamler J, Vaccaro O, Neaton JD et al (1993) Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16:434–444
Jeppesen J, Hollenbeck CB, Zhou MY et al (1995) Relation between insulin resistance, hyperinsulinemia, postheparin plasma lipoprotein lipase activity, and postprandial lipemia. Arteriosclerosis Thromb Vasc Biol 15:320–324
Sheu WH, Shieh SM, Fuh MM et al (1993) Insulin resistance, glucose intolerance, and hyperinsulinemia. Hypertriglyceridemia vs hypercholesterolemia. Arteriosc Throb 13:367–370
Galvan AQ, Santoro D, Natali A et al (1993) Insulin sensitivity in familial hypercholesterolemia. Metabolism 42:1359–1364
Kudolo GB, Bressler P, DeFronzo RA (1997) Plasma PAF acetylhydrolase in non-insulin dependent diabetes mellitus and obesity: effect of hyperinsulinemia and lovastatin treatment. J Lipid Mediat Cell Signal 17:97–113
Howard BV, Robbins DC, Sievers ML et al (2000) LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arterioscler Thromb Vasc Biol 20:830–835
Bressler P, Bailey S, Matsuda M et al (1996) Insulin resistance and coronary artery disease. Diabetologia 39:1345–1350
Paternostro G, Camici PG, Lammerstma AA et al (1996) Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest 98:2094–2099
Iozzo P, Chareonthaitawee P, Dutka D et al (2002) Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes 51:3020–3024
Lautamaki R, Juhani Airaksinen KE, Seppanen M et al (2005) Rosiglitazone improves myocardial glucose uptake in patients with type 2 diabetes and coronary artery disease. Diabetes 54:2787–2794
Abdul-Ghani M, Tripathy D, DeFronzo RA (2006) Contribution of beta cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29:1130–1139
Abdul-Ghani M, Jenkinson C, Richardson D et al (2006) Insulin secretion and insulin action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study (VAGES). Diabetes 55:1430–1435
Gulli G, Ferrannini E, Stern M et al (1992) The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 41:1575–1586
Kashyap SR, DeFronzo RA (2007) The insulin resistance syndrome: physiological considerations. Diabetes Vasc Dis Res 4:13–19
Hanley AJ, Williams K, Stern MP et al (2002) Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 25:1177–1184
Isomaa B, Almgren P, Tuomi T et al (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24:683–689
Bonora E, Kiechl S, Willeit J et al (2007) Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck Study. Diabetes Care 30:318–324
Bonora E, Formentini G, Calcaterra F et al (2002) HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care 25:1135–1141
Howard G, O’Leary DH, Zaccaro D et al (1996) Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 93:1809–1817
Bedblad B, Nilsson P, Janzon L et al (2002) Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet Med 17:299–307
Ferrannini E, Balkau B, Coppack SW et al (2007) Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab 92:2885–2892
D’Agostino RB Sr, Grundy S, Sullivan LM et al (2001) Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 286:180–187
Wilson PWF, D’Agostino RB, Levy D et al (1998) Prediction of coronary heart disease using risk factors categories. Circ 97:1837–1847
Goldsen SH, Folsom AR, Coresh J et al (2002) Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the atherosclerosis risk in communities study. Diabetes 51:3069–3076
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signaling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96
DeFronzo RA (1997) Pathogenesis of type 2 diabetes mellitus: metabolic and molecular implications of identifying diabetes genes. Diabetes Rev 5:177–269
White MF, Livingston JN, Backer JM et al (1992) Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell 54:641–649
Sun XJ, Miralpeix M, Myers MG Jr et al (1992) Expression and function of IRS-1 in insulin signal transmission. J Biol Chem 267:22662–22672
Ruderman N, Kapeller R, White MF et al (1990) Activation of phosphatidylinositol-3-kinase by insulin. Proc Natl Acad Sci USA 87:1411–1415
Brady MJ, Nairin AC, Saltiel AR (1997) The regulation of glycogen synthase by protein phosphatase 1 in 3T3-I 1 adipocytes. J Biol Chem 272:29698–29703
Dent P, Lavoinne A, Nakielny S et al (1990) The molecular mechanisms by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature 348:302–308
Osawa H, Sutherland C, Robey R et al (1996) Analysis of the signaling pathway involved in the regulation of hexokinase II gene transcription by insulin. J Biol Chem 272:16690–16694
Cross D, Alessi DR, Vandenhead JR et al (1994) The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem 303:21–26
Steinberg HO, Brechtel G, Johnson A et al (1994) Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. J Clin Invest 94:1172–1179
Zeng G, Nystrom FH, Ravichandran LV et al (2000) Roles of insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101:1539–1545
Montagnani M, Chen H, Barr VA et al (2001) Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276:30392–30398
Brunner H, Cockcroft JR, Deanfield J et al (2005) Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens 23:233–246
Naruse K, Shimizu K, Muramatsu M et al (1994) Long-term inhibition of NO synthesis promotes atherosclerosis in the hypercholesterolemic rabbit thoracic aorta. PGH2 does not contribute to impaired endothelium-dependent relaxation. Arterioscler Thromb 14:746–752
Tokudome T, Horio T, Yoshihara F et al (2004) Direct effects of high glucose and insulin on protein synthesis in cultured cardiac myocytes and DNA and collagen synthesis in cardiac fibroblasts. Metabolism 53:710–715
Sasaoka T, Ishiki M, Sawa T et al (1996) Comparison of the insulin and insulin-like growth factor 1 mitogenic intracellular signaling pathways. Endocrinology 137:4427–4434
Wang L, Goalstone ML, Draznin B (2004) Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes 53:2735–2740
Cusi K, Maezono K, Osman A et al (2000) Insulin resistance differentially affects the PI 3-kinase and MAP kinase-mediated signaling in human muscle. J Clin Invest 105:311–320
Jiang ZY, Lin YW, Clemont A et al (1999) Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104:447–457
Lazer DF, Wiese RJ, Brady MJ et al (1995) Mitogen-activated protein kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem 270:20801–20807
Yuan M, Konstantopoulos N, Lee J et al (2001) Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293:1673–1677
Hirosumi J, Tuncman G, Chang L et al (2002) A central role for JNK in obesity and insulin resistance. Nature 420:333–336
Lonroth P, Digirolamo M, Krotkiewski M et al (1983) Insulin binding and responsiveness in fat cells from patients with reduced glucose tolerance and type II diabetes. Diabetes 32:748–754
Caro JF, Sinha MK, Raju SM et al (1987) Insulin receptor kinase in human skeletal muscle from obese subjects with and without non-insulin dependent diabetes. J Clin Invest 79:1330–1337
Nyomba BL, Ossowski VM, Bogardus C et al (1990) Insulin-sensitive tyrosine kinase relationship with in vivo insulin action in humans. Am J Physiol 258:E964–E974
Freidenberg GR, Reichart D, Olefsky JM et al (1988) Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin dependent diabetes mellitus. Effect of weight loss. J Clin Invest 82:1398–1406
Pratipanawatr W, Pratipanawatr T, Cusi K et al (2001) Skeletal muscle insulin resistance in normoglycemic subjects with a strong family history of type 2 diabetes is associated with decreased insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation. Diabetes 50:2572–2578
Pendergrass M, Bertoldo A, Bonadonna R et al (2007) Muscle glucose transport and phosphorylation in type 2 diabetic, obese non-diabetic, and genetically predisposed individuals. Am J Physiol Endocrinol Metab 292:E92–E100
Pendergrass M, Koval J, Vogt C et al (1998) Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes 47:387–394
Kashyap SR, Roman LJ, McLain J et al (2005) Insulin resistance is associated with impaired nitric oxide synthase (NOS) activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metab 90:1100–1105
Kashyap S, Lara A, Zhang R et al (2008) Insulin reduces plasma arginase activity in type 2 diabetes patients. Diabetes Care 31:134–139
Baron AD (1994) Hemodynamic actions of insulin. Am J Physiol 267:E187–E202
Cabellero AE, Arora S, Saouaf R et al (1999) Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 48:1856–1862
Cerosismo E, DeFronzo RA (2006) Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diab Metab Res Rev 22:423–436
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Hsueh WA, Lyon CJ, Quiñones MJ (2004) Insulin resistance and the endothelium. Am J Med 117:109–117
Behrendt D, Ganz P (2002) Endothelial function. From vascular biology to clinical applications. Am J Cardiol 21:40L–48L
Draznin B (2006) Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes 55:2392–2397
Aguirre V, Werner Ed, Giraud J et al (2002) Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277:1531–1537
Liu YF, Herschkovitz A, Boura-Halfon S et al (2004) Serine phosphorylation proximal to its phosphotyrosine binding domain inhibits insulin receptor substrate 1 function and promotes insulin resistance. Mol Cell Biol 24:9668–9681
Sasaoka T, Rose DW, Jhun BH et al (1994) Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem 269:13689–13694
Hsueh WA, Law RE (1999) Insulin signaling in the arterial wall. Am J Cardiol 84:21J–24J
Lettner JW, Kline T, Carel K et al (1997) Hyperinsulinemia potentiates activation of p21 Ras by growth factors. Endocrinol 138:2211–2214
Golovchenko I, Goalstone ML, Watson P et al (2000) Hyperinsulinemia enhances transcriptional activity of nuclear factor-kB induced by angiotensin II, hyperglycemia, and advanced glycosylation end products in vascular smooth muscle cells. Circ Res 87:746–762
Hajra L, Evans AI, Chen M et al (2000) The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. PNAS 97:9052–9057
de Winther MP, Kanters E, Kraal G et al (2005) Nuclear factor kappaB signaling in atherogenesis. Art Throm Vasc Biol 25:904–914
Gao Z, Hwang D, Bataille F et al (2002) Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277:48115–48121
Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP (1997) Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. J Clin Invest 100:2158–2169
Uusitupa MI, Niskanen LK, Siitonen O et al (1993) Ten-year cardiovascular mortality in relation to risk factors and abnormalities in lipoprotein composition in type 2 (non-insulin-dependent) diabetic and non-diabetic subjects. Diabetologia 36:1175–1184
Miyazaki Y, He H, Mandarino LJ et al (2003) Rosiglitazone improves downstream insulin-receptor signaling in type 2 diabetic patients. Diabetes 52:1943–1950
Bajaj M, Baig R, Suraamornkul S et al. (2010) Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab doi:10.1210/jc.2009-0911
Vinik AI, Stansberry KB, Barlow PM (2003) Rosiglitazone treatment increased nitric oxide production in human peripheral skin: a controlled clinical trial in patients with type 2 diabetes mellitus. J Diab Comp 17:279–285
Natali A, Baldeweg S, Toschi E et al (2004) Vascular effects of improving metabolic control with metformin or rosiglitazone in type 2 diabetes. Diabetes Care 27:1349–1357
Pistrosch F, Passauer J, Fischer S et al (2004) In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care 27:484–490
Bays H, Mandarino L, DeFronzo RA (2004) Role of the adipocytes, FFA, and ectopic fat in the pathogenesis of type 2 diabetes mellitus: PPAR agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 89:463–478
Unger RH (2003) Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab 14:398–403
Kashyap S, Belfort R, Berria R et al (2004) Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab 287:E537–E546
Richardson DK, Kashyap S, Bajaj M et al (2005) Lipid infusion induces an inflammatory/fibrotic response and decreases expression of nuclear encoded mitochondrial genes in human skeletal muscle. J Biol Chem 280:10290–10297
Dresner A, Laurent D, Marcucci M et al (1999) Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259
Kashyap S, Belfort R, Gastaldelli A et al (2003) A sustained increase in plasma free fatty acids impairs insulin secretion in non-diabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52:2461–2474
Belfort R, Mandarino L, Kashyap S et al (2005) Dose response effect of elevated plasma FFA on insulin signaling. Diabetes 54:1640–1648
Mayerson AB, Hundal RS, Dufour S et al (2002) The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51:797–802
Belfort R, Harrison SA, Brown K et al (2006) A placebo controlled trial of pioglitazone in patients with non-alcoholic steatohepatitis. NEJM 355:2297–2307
Miyazaki Y, Mahankali A, Matsuda M et al (2002) Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 87:2784–2791
Bajaj M, Suraamornkul S, Pratipanawatr T et al (2003) Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes mellitus. Diabetes 52:1364–1370
Griffin ME, Marcucci MJ, Cline GW et al (1999) Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48:1270–1274
Bajaj M, Suraamornkul S, Romanelli A et al (2005) Effect of sustained reduction in plasma free fatty acid concentration on intramuscular long chain-fatty acyl-CoAs and insulin action in patients with type 2 diabetic patients. Diabetes 54:3148–3153
Kim JK, Fillmore JJ, Chen Y et al (2001) Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. PNAS 98:7522–7527
Gao Z, Zhang X, Zuberi A et al (2004) Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18:2024–2034
Morino K, Neschen S, Bliz S et al (2008) Muscle-specific IRS-1 Ser->Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes 57:2644–2651
Yu CL, Chen Y, Cline GW et al (2002) Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 2-kinase activity in muscle. J Biol Chem 277:50230–50236
Itani SI, Ruderman NB, Schmieder F et al (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and I kappa B-alpha. Diabetes 51:2005–2011
Heydrick SJ, Ruderman NB, Kurowski TG et al (1991) Enhanced stimulation of diacylglycerol and lipid synthesis by insulin in denervated muscle. Altered protein kinase C activity and possible link to insulin resistance. Diabetes 40:1707–1711
Adams JM, Pratipanawatr T, Berria R et al (2004) Ceramide content is increased in skeletal muscle from obese insulin resistant humans. Diabetes 53:25–31
Haus JM, Kashyap SR, Kasumov T et al (2009) Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58:337–343
Evans JL, Goldfine ID, Maddux BA et al (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23:599–622
Garg R, Tripathy D, Dandona P (2003) Insulin resistance as a proinflammatory state: mechanisms, mediators, and therapeutic interventions. Curr Drug Targets 4:487–492
Pickup JC, Crook MA (1998) Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41:1241–1248
Ghanim H, Garg R, Aljada A et al (2001) Suppression of nuclear factor-kappa β by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab 86:1306–1312
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor to chronic inflammatory diseases. N Engl J Med 336:1066–1071
Yamamoto Y, Gaynor RB (2004) IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci 29:72–79
De Alvaro C, Teruel T, Hernandez R et al (2004) Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem 279:17070–17078
Sriwijitkamol A, Christ-Roberts C, Berria R et al (2006) Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes 55:760–767
Sinha S, Perdomo G, Brown JF et al (2004) Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279:41294–41301
Bhatt BA, Dube JJ, Dedousis N et al (2006) Diet-induced obesity and acute hyperlipidemia reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regu Integr Comp Physiol 290:R233–R240
Reyna SM, Ghosh S, Tantiwong P et al (2008) Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57:2595–2602
Perbal B (2004) CCN proteins: multifunctional signaling regulators. Lancet 363:62–64
Cicha I, Yilmaz A, Klein M et al (2005) Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Art Throm Vas Biol 25:1008–1013
Steinberg HO, Chaker H, Leaming R et al (1996) Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97:2601–2610
Steinberg HO, Tarshoby M, Monestel R et al (1997) Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100:1230–1239
Wellen KE, Hotamisligil GS (2003) Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785–1788
Lapidus L, Bengtsson C, Larsson B et al (1984) Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. BMJ 289:1257–1261
Despre JP, Moorjani S, Lupien PJ et al (1990) Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arterioscler Thromb Vas Biol 10:497–511
Anderson JW, Kendall CW, Jenkins DJ (2003) Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 22:331–339
Triplitt C, DeFronzo RA (2006) Exenatide: first in class incretin mimetic for the treatment of type 2 diabetes mellitus. Expert Rev Endocrinol Metab 1:329–341
Miyazaki Y, Glass L, Triplitt C et al (2001) Effect of rosiglitazone on glucose and free fatty acid metabolism in type 2 diabetic patients. Diabetologia 44:2210–2219
Miyazaki Y, Mahankali A, Matsuda M et al (2001) Improved glycemic control and enhanced insulin sensitivity in liver and muscle in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 24:710–719
Gastaldelli A, Miyazaki Y, Mahankali A et al (2006) The effect of pioglitazone on the liver. Diabetes Care 29:2275–2281
Coletta DK, Sriwijitkamol A, Wajcberg E et al (2009) Pioglitazone stimulates AMPK signaling and increases the expression of genes involved in adiponectin signaling. Mitochondrial function and fat oxidation in human skeletal muscle in vivo. Diabetologia 52:723–732
Petersen KF, Befroy D, Dufour S et al (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142
Petersen KF, Dufour S, Shulman GI (2005) Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Medicine 2:879–884
Phielix E, Schrauwen-Hinderling VB, Mensink M et al (2008) Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 57:2943–2949
Abdul-Ghani MA, DeFronzo RA (2008) Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep 8:173–178
Abdul-Ghani MA, Jani R, Chavez A et al (2009) Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia 52:574–582
Abdul-Ghani MA, Muller FL, Liu Y et al (2008) Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol 295:E678–E685
Patti ME, Butte AJ, Crunkhorn S et al (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471
Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90
Yamauchi T, Kamon J, Waki H et al (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Yamauchi T, Kadowaki T (2008) Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obesity 32:S13–S18
Kubota N, Terauchi Y, Yamauchi T et al (2002) Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866
Civitarese AE, Jenkinson CP, Richardson D et al (2004) Adiponectin receptor gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without family history of type 2 diabetes. Diabetologia 47:816–820
Civitarese AE, Ukropcova B, Carling S et al (2006) Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4:74–87
Miyazaki Y, Glass L, Triplitt C et al (2002) Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 46:E1135–E1143
Kelly JE, Hans TS, Walsh K et al (1999) Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22:288–293
Aithal GP, Thomas JA, Kaye PV et al (2008) Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135:1176–1184
Gastaldelli A, Ferrannini E, Miyazaki Y et al (2007) Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Phyisol Endocrinol Metab 292:E871–E883
Moibi JA, Gupta D, Jetton TL et al (2007) Peroxisome proliferator-activated receptor-gamma regulates expression of PDX-1 and NKX6.1 in INS-1 cells. Diabetes 56:88–95
Matsui J, Terauchi Y, Kubota N et al (2004) Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gamma-deficient mice on a high-fat diet. Diabetes 53:2844–2854
Del Prato S, Marchetti P (2004) Targeting insulin resistance and beta-cell dysfunction: the role of thiazolidinediones. Diabetes Tech Thera 6:719–731
Lupi R, del Guerra S, Marselli L et al (2004) Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARgamma2 in the modulation of insulin secretion. Am J Physiol Endocrinol Metab 286:E560–E567
Sharma S, Adrogue JV, Golfman L et al (2004) Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18:1692–1700
Kankaanpaa M, Lehto HR, Parkka JP et al (2006) Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab 91:4689–4695
McGavock JM, Lingvay I, Zib I et al (2007) Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 116:1170–1175
Zib I, Jacob AN, Lingvay I et al (2007) Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med 55:230–236
Deeg MA, Buse JB, Goldberg RB et al (2007) Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30:2458–2464
Chiquette E, Ramirez G, DeFronzo R (2004) A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med 164:2097–2104
Miyazaki M, de Filippis E, Bajaj M et al (2005) Predictors of improved glycaemic control with rosiglitazone therapy in type 2 diabetic patients: a practical approach for the primary care physician. Br J Diabetes Vasc Dis 5:28–35
Dormandy JA, Charbonnel B, Eckland DJ et al (2005) Proactive investigators: secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitazone Clinical Trial in macroVascular Events): a randomized controlled trial. Lancet 266:1279–1289
Lincoff AM, Wolski K, Nicholls SJ et al (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298:1180–1188
Mazzone T, Meyer PM, Feinstein SB et al (2006) Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2: a randomized trial. JAMA 296:2572–2581
Nissen SE, Nicholls SJ, Wolski K et al (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the Periscope randomized controlled trial. JAMA 299:1561–1573
Sarruf DA, Yu F, Nguyen HT et al (2008) Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 150:707–712
Acknowledgement
Duality of interest
R. A. DeFronzo is a speaker for Amylin-Lilly and Takeda, has received grants from Amylin, Lilly, Takeda and Bristol Myers Squibb, and is an Advisory Board member and/or consultant for Amylin, Takeda, Boehringer-Ingelheim, ISIS, Novartis and Roche.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
DeFronzo, R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53, 1270–1287 (2010). https://doi.org/10.1007/s00125-010-1684-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-010-1684-1