Abstract
Aims/hypothesis
The aim of this study was to compare the magnitude of the effect of diabetes and stroke at baseline and during follow-up on risk of stroke mortality.
Materials and methods
Study cohorts included 25,155 Finnish men and 26,423 women aged 25–74 years. Data on diabetes and stroke history at baseline, their incidence during follow-up, and stroke death were obtained from national registers.
Results
During a mean follow-up of 18.9 years, 838 stroke deaths were recorded. In the baseline study, hazard ratios (HRs) for stroke mortality were 5.26 for men with prior diabetes only, 4.76 for men with prior stroke only, and 13.4 for men with both prior diabetes and stroke compared with men without diabetes and stroke at baseline and during follow-up. In women, the corresponding hazard ratios were 7.29, 5.27 and 5.52, respectively. When only diabetes and stroke status during the follow-up were considered, the hazard ratios for stroke mortality were 1.41 for men and 1.56 for women with incident diabetes only, 5.62 for men and 5.58 for women with incident stroke only, and 5.59 for men and 4.48 for women with both incident diabetes and stroke compared with men and women without diabetes and stroke at baseline and during follow-up.
Conclusions/interpretation
Diabetes and stroke, present either at baseline or during follow-up, markedly increase the risk of stroke death. Prior stroke at baseline carries a similar risk of stroke mortality as prior diabetes. There is a greater risk of stroke mortality associated with incident stroke during follow-up than with incident diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes is one of the fastest growing public health problems worldwide as a result of increasing obesity and a sedentary lifestyle. The number of diabetic patients in the world has been estimated to at least double over the next 30 years [1]. Cardiovascular disease (CVD) accounts for more than 75% of total mortality among patients with type 2 diabetes [2–4]. Epidemiological studies have indicated that the risk of CVD mortality is two to four times higher in patients with type 2 diabetes than in those without diabetes [5]. Recent studies have investigated whether a history of myocardial infarction has the same risk on coronary death as a history of type 2 diabetes [6–13]. Our previous study reported that, among men, those who experienced myocardial infarction at baseline or during follow-up were at greater risk of coronary mortality than those with prior or incident diabetes; in women, prior myocardial infarction at baseline was associated with a lower risk of coronary mortality than prior diabetes, but incident myocardial infarction during follow-up was associated with a greater risk than incident diabetes [13]. Several prospective studies have assessed the independent effect of type 2 diabetes on stroke risk, with inconsistent results. Some [14–22], but not all studies [23, 24], have identified type 2 diabetes as an independent risk factor for stroke. Moreover, it is not fully understood whether the risk of stroke death associated with incident diabetes is similar to that associated with incident stroke. The aim of this study was to compare the magnitude of the effect of diabetes and stroke at baseline and during follow-up on the risk of stroke mortality.
Subjects and methods
Subjects
Six independent cross-sectional population surveys were carried out in Kuopio and North Karelia provinces in eastern Finland in 1972, 1977, 1982, 1987, 1992 and 1997 [25]. The survey areas were expanded to Turku-Loimaa region in southwestern Finland in 1982, the Helsinki capital area in 1992, and the northern province of Oulu in 1997. In 1972 and 1977, a randomly selected sample making up 6.6% of the population born between 1913 and 1947 was drawn. Since 1982, the sample has been stratified by area, sex and age (10-year groups) according to the World Health Organization Monitoring Trends and Determinants of Cardiovascular Disease (MONICA) protocol [26]. In six surveys, the subjects included were 25–64 years of age, and, in the 1997 survey, subjects aged 65–74 years were also included. Subjects were recruited by six independent surveys, and subjects who participated in more than one survey were included in the first survey cohort only. The total sample size of the six surveys was 53,166. The participation rate varied by year from 74 to 88% [25]. After excluding 113 subjects with type 1 diabetes at baseline or during follow-up, 1,204 subjects with incomplete data on any required variable, and 271 patients who died within 28 days of the diagnosis date of incident diabetes or stroke during follow-up, the present analyses included 25,155 men and 26,423 women. The participants gave informed consent (verbal 1972–1992 and signed 1997). These surveys were conducted according to the ethics rules of the National Public Health Institute and the investigations were carried out in accordance with the Declaration of Helsinki.
Measurements
A self-administered questionnaire was mailed to the participants. This included questions about smoking, socioeconomic factors, alcohol consumption, physical activity and medical history. Based on the responses, the participants were classified as never, ex- and current-smokers. Years of education were divided into birth cohort-specific tertiles. Since questions on alcohol consumption were different between the first two surveys (1972 and 1977) and the latter surveys, the participants were categorised as either abstainers or alcohol users. Physical activity included occupational, commuting and leisure-time physical activity, and results were merged and regrouped into three categories: low, moderate and high [27–34]. At the study site, specially trained research nurses measured blood pressure, height and weight using a standardised protocol [26]. Blood pressure was measured using a standard mercury manometer after 5 min of rest. Height was measured without shoes. Weight was measured with light clothing. BMI was calculated as weight in kilograms divided by the square of height in metres. After blood pressure measurement, a venous blood specimen was drawn. Total cholesterol concentration was determined using the Lieberman Burchard method in 1972 and 1977 [35], and an enzymatic method (CHOD-PAP, Boehringer Mannheim, Mannheim, Germany) in 1982 onwards. Because the enzymatic method gave 2.4% lower values than the Lieberman Burchard method, 1972 and 1977 values were corrected by this percentage. All samples were analysed in the same laboratory at the National Public Health Institute.
Assessment of diabetes and stroke at baseline and during follow-up
Assessment of diabetes and stroke status was based on self-reporting and on the data of two nationwide registers. The National Hospital Discharge Register data included information on hospital discharge diagnosis from 1968 through the end of 2002. The validity of the diagnosis of stroke in Finland from this register is good [36, 37]. Data on diabetes medication were obtained from the National Social Insurance Institution’s register on special reimbursement for glucose-lowering drugs from 1964 through the end of 2002. Glucose-lowering drugs prescribed by a physician are free of charge in Finland and are subject to approval of a physician who reviews each case history. The physician confirms the diagnosis of diabetes by applying the World Health Organization criteria [38, 39]. All patients receiving free medication (either oral glucose-lowering agents or insulin) are entered into a register maintained by the National Social Insurance Institution.
Subjects who reported having diabetes on the questionnaire, or who had had a hospital discharge diagnosis of diabetes, or the approval for free-of-charge medication for diabetes before the baseline survey were classified as having prior diabetes at baseline. The subjects with prior stroke at baseline were those who reported having stroke on the questionnaire, or had a hospital discharge diagnosis of stroke before the baseline survey.
Subjects who had the first hospital discharge diagnosis with diabetes, or the approval for free-of-charge medication for diabetes after the baseline survey were classified as having incident diabetes during follow-up. Subjects with incident stroke during follow-up comprised those who had a hospital discharge with a diagnosis of stroke after the baseline survey. The patients who died within 28 days of the diagnosis date of incident diabetes and stroke during follow-up were excluded from the analysis, thus eliminating the deaths directly due to stroke.
Prospective follow-up
The study cohorts were followed until the end of 2003 through computerised register linkage using the personal identification number assigned for every resident in Finland. Mortality data were obtained from Statistics Finland. The International Classification of Diseases (ICD), Eighth, Ninth and Tenth Revisions, were used for coding the causes of death; ICD codes 430–438 and I60–I66 were classified as stroke deaths. During a mean follow-up of 18.9 years, 838 stroke deaths were recorded.
Statistics
SPSS for Windows 13.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Cox proportional hazard models were used to estimate the hazard ratios for stroke mortality associated with diabetes and stroke status. Subjects who did not have a history of stroke or prevalent diabetes at baseline and who did not develop either of the two conditions during follow-up were used as the reference group. We carried out two analyses: the baseline cohort analysis included the reference group and the patients with prior diabetes or stroke at baseline, and the follow-up cohort analysis included the reference group and the patients with incident (diagnosed after the baseline survey) diabetes or stroke during follow-up. The analyses were first carried out adjusting for age and study year, and were further adjusted for BMI, systolic blood pressure, total cholesterol, education, alcohol drinking, physical activity and smoking at baseline. A likelihood ratio test for interaction was carried out to test whether the effect of disease status on stroke mortality differed between men and women. The risk of stroke mortality associated with diabetes was compared with that associated with stroke, both at baseline and during the follow-up, using Cox regression. Age and person-years of follow-up were also calculated at the date of new diagnosis of diabetes or stroke during follow-up. A p value of less than 0.05 was considered statistically significant and all p values are two-sided.
Results
Baseline cohort study
Baseline characteristics and multivariate-adjusted (age, study year, BMI, systolic blood pressure, cholesterol, education, alcohol drinking, physical activity and smoking) hazard ratios for stroke mortality according to history of diabetes and stroke at baseline are presented in Table 1. Compared with men without diabetes and stroke at baseline and during follow-up, the multivariate-adjusted hazard ratios for stroke mortality were 5.26 (95% CI: 3.59–7.71) for men with prior diabetes only, 4.76 (95% CI: 3.02–7.49) for men with prior stroke only, and 13.4 (95% CI: 5.99–30.1) for men with both prior diabetes and stroke at baseline. In women, the corresponding HRs were 7.29 (95% CI: 4.93–10.8), 5.27 (95% CI: 3.21–8.64) and 5.52 (95% CI: 1.32–23.0), respectively. The number of women with both prior diabetes and stroke at baseline was only 23, two of whom died due to stroke, and therefore the risk estimate in this category was unreliable.
Patients with incident diabetes and/or stroke during follow-up
When diabetes and stroke status during follow-up were included in the analyses, the multivariate-adjusted hazard ratios for stroke mortality were 1.41 (95% CI: 0.96–2.09) for men and 1.56 (95% CI: 1.12–2.16) for women with incident diabetes only, 5.62 (95% CI: 4.46–7.09) for men and 5.58 (95% CI: 4.34–7.19) for women with incident stroke only, and 5.59 (95% CI: 3.55–8.81) for men and 4.48 (95% CI: 2.92–6.90) for women with both incident diabetes and stroke compared with men and women without diabetes and stroke at baseline and during the follow-up (Table 2). Sex and disease status did not interact with each other to have a significant effect on stroke mortality, either at baseline or at follow-up.
Comparison of stroke mortality between diabetes and stroke at baseline and during follow-up
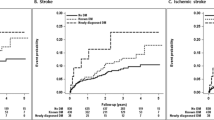
When stroke mortality in persons with prior diabetes only was compared with that in persons with a history of stroke only at baseline, men and women with prior stroke showed almost the same risk of stroke mortality as men (hazard ratio [HR]=0.97, 95% CI: 0.57–1.67) and women (HR=0.75, 95% CI: 0.42–1.33) with prior diabetes, after adjustment for all confounding factors (Fig. 1). When stroke mortality was compared between persons who developed diabetes during follow-up and those who experienced stroke only, men and women with incident stroke had significantly higher multivariate-adjusted hazard ratios of stroke mortality (HR=4.07, 95% CI: 2.67–6.21 in men; HR=3.53, 95% CI: 2.45–5.09 in women) than men and women with incident diabetes (Fig. 2). When age and person-years of follow-up were calculated at the date of diagnosis of incident diabetes or incident stroke during follow-up, the multivariate-adjusted hazard ratios for stroke mortality were 4.03 (95% CI: 2.65–6.14) for men and 4.06 (95% CI: 2.80–5.88) for women with incident stroke compared with men and women with incident diabetes.
Hazard ratios for stroke mortality in diabetic subjects without a history of stroke as compared with non-diabetic subjects with a history of stroke at baseline. Adjusted for age, study year, BMI, systolic blood pressure, total cholesterol, education, alcohol drinking, physical activity and smoking. Blue bars, prior diabetes; red bars, prior stroke
Hazard ratios for stroke mortality in diabetic subjects without stroke as compared with non-diabetic subjects with stroke during follow-up. Adjusted for age, study year, BMI, systolic blood pressure, total cholesterol, education, alcohol drinking, physical activity and smoking. Blue bars, incident diabetes; red bars, incident stroke
Discussion
In both sexes, a history of diabetes and stroke at baseline and incident diabetes and stroke during follow-up markedly increased the risk of stroke mortality. Subjects with a history of stroke at baseline were at the same risk of stroke mortality as those with a history of diabetes at baseline. When disease status during follow-up was considered, subjects with incident stroke had a higher risk of stroke mortality than those with incident diabetes.
Coronary heart disease has been identified as the leading cause of death among patients with type 2 diabetes [2]. In recent years, several studies have compared the magnitude of the risk of a history of type 2 diabetes and myocardial infarction on subsequent coronary mortality [6–13]. A Finnish prospective study found that the risk of coronary death among diabetic subjects without prior myocardial infarction was similar to that in non-diabetic subjects with prior myocardial infarction [6], but this finding has been challenged by several later studies [8–13]. The Health Professionals’ Follow-up Study and the Atherosclerosis Risk in Communities Study reported that the magnitude for coronary or cardiovascular mortality was lesser for diabetes than that associated with prior myocardial infarction [7, 10]. The analyses of data from the Finland Cardiovascular Risk (FINRISK) study revealed that, among men, the risk of coronary mortality is greater in those with myocardial infarction at baseline or during follow-up than in those with diabetes. In women, however, prior myocardial infarction at baseline confers a lower risk of coronary mortality than prior diabetes does, but incident myocardial infarction during follow-up confers a greater risk than incident diabetes does [13].
Several studies have recently assessed the relationship between type 2 diabetes and the risk of stroke. Type 2 diabetes has been shown to be an independent risk factor for stroke morbidity [15, 18–22] and mortality [14, 16, 17, 22, 40]. The Renfrew and Paisley Study found that type 2 diabetes was a predictor of stroke incidence among women but not among men [41]. The Dubbo Study in the Australian elderly failed to confirm prediction of incident ischemic stroke by diabetes [24]. Moreover, several studies have found that subjects with impaired fasting glucose levels have an increased risk of stroke [3, 20, 42, 43].
However, whether the risk of stroke death associated with a history of stroke is similar to that associated with a history of type 2 diabetes is not fully understood. The Women’s Pooling Project, which included nine prospective epidemiological studies, was the only study to compare the magnitude of the risk of stroke mortality in subjects with a history of diabetes at baseline with that in subjects with a history of stroke [40], using the same design as our baseline cohort study. The results indicated that the risk of fatal stroke in diabetic subjects without stroke was similar to that in non-diabetic subjects with a history of stroke. However, this observational study analysed a single baseline measurement. In the present study, prior stroke at baseline was associated with almost the same risk of stroke mortality as prior diabetes at baseline, whereas in the follow-up study, incident stoke was associated with a higher risk of stroke mortality than incident diabetes. The results were almost similar in men and women. The magnitude of the effect of diabetes and stroke on stroke risk probably depends on the duration of the disease, and this time factor may operate differently for diabetes than for stroke. Among the patients who have survived an acute stroke, the risk of subsequent stroke event is highest immediately after the attack and the risk stabilizes over time, whereas among diabetic subjects, the stroke risk increases with the duration of the disease [15]. As in the baseline cohort analyses of our study, most previous studies have not paid attention to the time of stroke event or duration of diabetes in their data analyses.
The definition of type 2 diabetes is different between these previous studies and our study. In most observational prospective studies, diabetes status is usually recorded only once at baseline [14, 15, 17–21, 24, 40, 41]. Subjects who develop diabetes later are considered non-diabetic, or an unexposed reference group. This misclassification causes an underestimation of the diabetes-related risk of stroke. In our study, patients with newly diagnosed diabetes during follow-up had a 40–60% higher risk of stroke death, and patients with both diabetes and stroke newly diagnosed during follow-up had a 4.5–5.6 fold risk of stroke death compared with subjects who were free of diabetes and stroke both at baseline and during the follow-up.
Our finding may have some important implications for the minimising stroke risk in diabetic patients, because a history of diabetes has the same stroke risk as a history of prior stroke. Better management and prevention of diabetes are needed. The UKPDS demonstrated the importance of maintaining good glycaemic control for prevention of the development and progression of complications among patients with type 2 diabetes [44]. Clinical trials have shown that pharmacological treatment of hypertension [45–47] and cholesterol reduction with a statin therapy [42, 43, 48–50] are efficient ways of preventing stroke and other cardiovascular events in patients with diabetes or impaired fasting glucose.
Our study has several strengths and limitations. We have the unique possibility of stratifying for both baseline and follow-up status of diabetes and stroke. There are a large number of participants from a homogeneous population. The mean follow-up was sufficiently long to ascertain a large number of stroke endpoint events. We excluded the subjects with type 1 diabetes from the analysis, and, due to computerized data linkage of national mortality data, endpoint data collection was practically complete. A limitation of our study was that we did not carry out either a fasting glucose test or a glucose tolerance test at baseline or at follow-up. We would therefore have failed to identify cases of asymptomatic or diet-treated diabetes, especially among those patients who were diagnosed with stroke at baseline or during follow-up, although the clinical diagnosis of diabetes in the hospital discharge register included a proportion of those not using drugs, reducing this potential under-diagnosis. Thus, we cannot completely exclude the bias due to the above limitation. However, the total prevalence of type 2 diabetes at baseline and during follow-up in the present study was 8.1%, which is almost the same as the prevalence of this Finnish population according to a survey with an oral glucose tolerance test [51]. Another limitation of our study was that we did not have data on the severity of the diabetes or stroke, glycaemic control, or the type of drug treatment used for diabetes, stroke and other chronic diseases.
In conclusion, we have compared the independent effects of diabetes and stroke at baseline and during follow-up on the risk of stroke mortality. Diabetes and stroke, either present at baseline or during follow-up, markedly increase the risk of stroke death. Prior stroke at baseline has the same risk on stroke mortality as prior diabetes. There is a greater risk of stroke mortality associated with incident stroke during follow-up than with incident diabetes.
Abbreviations
- CVD:
-
cardiovascular disease
- HR:
-
hazard ratio
- MONICA:
-
Monitoring Trends and Determinants of Cardiovascular Disease
References
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Muller WA (1998) Diabetes mellitus—long time survival. J Insur Med 30:17–27
DECODE Study Group (2001) Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 161:397–405
DECODE Study Group (2003) Gender difference in all-cause and cardiovascular mortality related to hyperglycaemia and newly-diagnosed diabetes. Diabetologia 46:608–617
Laakso M (2001) Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med 249:225–235
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234
Hu FB, Stampfer MJ, Solomon CG et al (2001) The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med 161:1717–1723
Lotufo PA, Gaziano JM, Chae CU et al (2001) Diabetes and all-cause and coronary heart disease mortality among US male physicians. Arch Intern Med 161:242–247
Natarajan S, Liao Y, Cao G, Lipsitz SR, McGee DL (2003) Sex differences in risk for coronary heart disease mortality associated with diabetes and established coronary heart disease. Arch Intern Med 163:1735–1740
Lee CD, Folsom AR, Pankow JS, Brancati FL (2004) Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation 109:855–860
Vaccaro O, Eberly LE, Neaton JD et al (2004) Impact of diabetes and previous myocardial infarction on long-term survival: 25-year mortality follow-up of primary screenees of the Multiple Risk Factor Intervention Trial. Arch Intern Med 164:1438–1443
Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J (2005) Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia 48:856–861
Hu G, Jousilahti P, Qiao Q, Peltonen M, Katoh S, Tuomilehto J (2005) The gender-specific impact of diabetes and myocardial infarction at baseline and during follow-up on mortality from all causes and coronary heart disease. J Am Coll Cardiol 45:1413–1418
Stamler J, Vaccaro O, Neaton JD, Wentworth D (1993) Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16:434–444
Lehto S, Ronnemaa T, Pyorala K, Laakso M (1996) Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 27:63–68
Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E (1996) Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle-aged Finnish population. Stroke 27:210–215
Tanne D, Yaari S, Goldbourt U (1998) Risk profile and prediction of long-term ischemic stroke mortality: a 21-year follow-up in the Israeli Ischemic Heart Disease (IIHD) Project. Circulation 98:1365–1371
Folsom AR, Rasmussen ML, Chambless LE et al (1999) Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care 22:1077–1083
Iso H, Imano H, Kitamura A et al (2004) Type 2 diabetes and risk of non-embolic ischaemic stroke in Japanese men and women. Diabetologia 47:2137–2144
Lawes CM, Parag V, Bennett DA et al (2004) Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care 27:2836–2842
Kissela BM, Khoury J, Kleindorfer D et al (2005) Epidemiology of ischemic stroke in patients with diabetes: the Greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 28:355–359
Hu G, Sarti C, Jousilahti P et al (2005) The impact of history of hypertension and type 2 diabetes at baseline on the incidence of stroke and stroke mortality. Stroke 36:2538–2543
Haheim LL, Holme I, Hjermann I, Leren P (1993) Risk factors of stroke incidence and mortality. A 12-year follow-up of the Oslo Study. Stroke 24:1484–1489
Simons LA, McCallum J, Friedlander Y, Simons J (1998) Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke 29:1341–1346
Vartiainen E, Jousilahti P, Alfthan G, Sundvall J, Pietinen P, Puska P (2000) Cardiovascular risk factor changes in Finland, 1972–1997. Int J Epidemiol 29:49–56
WHO MONICA Project Principal Investigators (1988) The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol 41:105–114
Hu G, Qiao Q, Silventoinen K et al (2003) Occupational, commuting, and leisure-time physical activity in relation to risk for type 2 diabetes in middle-aged Finnish men and women. Diabetologia 46:322–329
Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P (2004) Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension 43:25–30
Hu G, Lindstrom J, Valle TT et al (2004) Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Arch Intern Med 164:892–896
Hu G, Eriksson J, Barengo NC et al (2004) Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation 110:666–673
Hu G, Tuomilehto J, Silventoinen K, Barengo N, Jousilahti P (2004) Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J 25:2212–2219
Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J (2005) Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke 36:1994–1999
Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J (2005) Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care 28:799–805
Hu G, Tuomilehto J, Silventoinen K, Barengo NC, Peltonen M, Jousilahti P (2005) The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47,212 middle-aged Finnish men and women. Int J Obes (Lond) 29:894–902
Jousilahti P, Vartiainen E, Pekkanen J, Tuomilehto J, Sundvall J, Puska P (1998) Serum cholesterol distribution and coronary heart disease risk: observations and predictions among middle-aged population in eastern Finland. Circulation 97:1087–1094
Leppälä JM, Virtamo J, Heinonen OP (1999) Validation of stroke diagnosis in the National Hospital Discharge Register and the Register of Causes of Death in Finland. Eur J Epidemiol 15:155–160
Mahonen M, Salomaa V, Keskimaki I et al (2000) The feasibility of combining data from routine Hospital Discharge and Causes-of-Death Registers for epidemiological studies on stroke. Eur J Epidemiol 16:815–817
WHO Study Group on Diabetes Mellitus (1985) Diabetes Mellitus: Report of a WHO Study Group. WHO Technical Report Series No 727. World Health Organization, Geneva
WHO Consultation (1999) Definition, diagnosis and classification of diabetes mellitus and its complications: Part 1. Diagnosis and classification of diabetes mellitus. Report of a WHO Consultation 99.2. World Health Organisation, Geneva
Ho JE, Paultre F, Mosca L (2003) Is diabetes mellitus a cardiovascular disease risk equivalent for fatal stroke in women? Data from the women’s pooling project. Stroke 34:2812–2816
Hart CL, Hole DJ, Smith GD (2000) Comparison of risk factors for stroke incidence and stroke mortality in 20 years of follow-up in men and women in the Renfrew/Paisley Study in Scotland. Stroke 31:1893–1896
Goldberg RB, Mellies MJ, Sacks FM et al (1998) Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 98:2513–2519
Keech A, Colquhoun D, Best J et al (2003) Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care 26:2713–2721
Stratton IM, Adler AI, Neil HAW et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective Observational Study. BMJ 321:405–412
Curb JD, Pressel SL, Cutler JA et al (1996) Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 276:1886–1892
UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317:703–713
Tuomilehto J, Rastenyte D, Birkenhager WH et al (1999) Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N Engl J Med 340:677–684
Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G (1997) Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 20:614–620
Heart Protection Study Collaborative Group (2003) MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361:2005–2016
Colhoun HM, Betteridge DJ, Durrington PN et al (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364:685–696
Valle T, Tuomilehto J, Eriksson J (1997) Epidemiology of NIDDM in Europids. In: Alberti KGMM, Zimmet P, DeFronzo RA, Keen H (eds) International Textbook of Diabetes Mellitus, 2nd edn. Wiley, London, pp 125–142
Acknowledgements
This study was supported by grants from the Finnish Academy (grant numbers 46558, 204274, and 205657), the Ministry of Education, and the Finnish Foundation for Cardiovascular Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, G., Jousilahti, P., Sarti, C. et al. The effect of diabetes and stroke at baseline and during follow-up on stroke mortality. Diabetologia 49, 2309–2316 (2006). https://doi.org/10.1007/s00125-006-0378-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0378-1