Abstract
Aims/hypothesis
Inflammation is implicated in the development of type 2 diabetes and CHD, but the trigger of inflammation is unclear. Although in vitro and animal studies support a role of elevated levels of atherosclerotic lipoproteins in the activation of inflammation, plasma cholesterol cannot predict inflammatory markers in humans. Moreover, the association between inflammatory markers and other traditional risk factors of diabetes and CHD is unclear. To increase our knowledge of in vivo regulation of inflammation, we examined the association between several traditional risk factors and inflammatory markers. We hypothesised that because apolipoprotein B (ApoB) reflects atherogenic particle number, it is the primary predictor of inflammatory status.
Subjects, materials and methods
We examined the association between several traditional risk factors and plasma high-sensitivity (hs) C-reactive protein (CRP), hsTNF-α, soluble TNF receptor 1, IL-6, orosomucoid, haptoglobin and α1-antitrypsin in 77 non-diabetic overweight and obese postmenopausal women.
Results
The inflammatory markers correlated positively with total and abdominal adiposity, blood pressure, 2-h OGTT glucose, insulin resistance, triglyceride, total/HDL cholesterol, ApoB, ApoB:apolipoprotein A1 (ApoA1) ratio and Framingham CHD risk points. They correlated negatively with ApoA1, and total, LDL and HDL cholesterol. ApoB was an independent predictor of the interindividual variation in IL-6, hsCRP, orosomucoid, haptoglobin and α1-antitrypsin (R 2 range 8–40%); other risk factors were less predictive. Compared with BMI-matched control subjects, women with hyperapobetalipoproteinaemia (hyperapoB) had higher hsTNF-α, IL-6, hsCRP and orosomucoid (increase 17–104%).
Conclusions/interpretation
ApoB is the primary predictor of inflammatory markers in postmenopausal overweight and obese women. Given elevated levels of inflammatory markers in hyperapoB women, we hypothesise that hyperapoB women may have an increased risk of developing both CHD and diabetes.
Similar content being viewed by others
Introduction
Inflammation is believed to be the ‘common soil’ leading to the development of type 2 diabetes and CHD [1]. Elevated plasma concentrations of many inflammatory markers are independent predictors of an increased risk of these diseases. For example, several prospective epidemiological studies (follow-up periods ranging from 3 to 20 years) have shown that the high-sensitivity (hs) C-reactive protein (CRP) level in plasma is an independent predictor of future CHD events and type 2 diabetes in an apparently healthy population [1, 2]. Accordingly, hsCRP plasma concentrations of <1, 1–3 and >3 mg/l have been defined to represent low, moderate and high risk of CHD, respectively [1]. Similarly, elevated levels of other inflammatory markers, such as IL-6 and inflammation-sensitive plasma proteins (orosomucoid, haptoglobin, and α1-antitrypsin), were shown to be independent predictors of type 2 diabetes and CHD [3–7].
The trigger of arterial inflammation is still unclear, but it is believed that elevated levels of atherogenic lipoproteins (VLDL, LDL and lipoprotein(a) [Lp(a)]) play a major role. Several in vitro and animal studies have demonstrated that elevated atherogenic lipoproteins lead to: (1) an increase in the adhesion of monocytes and T lymphocytes to the endothelial surface; (2) an increase in the migration of monocytes and T lymphocytes into subendothelial spaces; and (3) provision of the lipid core for the transformation of monocytes/macrophages into foam cells, which secrete several plasma inflammatory markers [8, 9].
However, this association of atherogenic lipoproteins with inflammatory markers is not well supported by in vivo human studies. Large clinical studies that examined the associations between plasma lipids and hsCRP, the most used inflammatory marker in terms of clinical utility, concluded that there is little, if any, association between plasma triglycerides (TG), total, LDL or HDL cholesterol and hsCRP [10–14]. Accordingly it was concluded that it is not possible to predict plasma CRP from knowledge of plasma lipids [1]. These studies have, however, rarely included in their evaluation apolipoprotein B (ApoB), a component of all atherogenic lipoprotein and a well-established lipid-related CHD risk factor [11, 15]. Moreover, despite the fact that many traditional risk factors of type 2 diabetes and CHD have been well described, clinical data examining the relationship between traditional type 2 diabetes and CHD risk factors and inflammatory markers are scarce. We believe that examining the interaction between traditional risk factors and markers of inflammation may shed light on the in vivo trigger of inflammation and the pathophysiology of CHD and type 2 diabetes and may help target the pharmacological prevention or treatment of these diseases.
Therefore, our aim was to examine the association of several well-established traditional metabolic and CHD risk factors with markers of inflammation in a cohort of postmenopausal overweight and obese women, a high-risk population for the development of type 2 diabetes and CHD [16]. The inflammatory markers assessed were hsCRP, hsTNF-α and soluble TNF-α receptor 1 (sTNF-α-R1), IL-6, orosomucoid, haptoglobin, and α1-antitrypsin. Our hypothesis was that, as elevated plasma ApoB concentrations are a reflection of an elevated number of atherogenic lipoproteins [17], plasma ApoB would be the primary predictor of inflammatory markers in this population.
Subjects, materials and methods
Subjects
Subjects were recruited by newspaper advertisement in the University of Montreal area (Montreal, Canada). All subjects gave written, informed consent before the study started and the study was approved by the Research Ethics Board at the University of Montreal. The data presented in this paper represent baseline characteristics of obese women recruited to our laboratory for a weight loss study.
Women volunteers were included in the study if they (1) were obese with BMI ≥27 kg/m2, (2) had biological confirmation of the menopausal status (plasma follicle-stimulating hormone ≥30 U/l, (3) were non-smokers, (4) had low to moderate alcohol consumption (<2 drinks/day), and (5) had <2 h per week of structured exercise.
The exclusion criteria were: (1) diabetes (fasting glucose >7.1 mmol/l or 2-h plasma glucose of >11.1 mmol/l after a 75-g OGTT), (2) untreated thyroid disease, (3) chronic liver or renal disease, (4) asthma requiring therapy with steroids, (5) cardiovascular or peripheral vascular disease, (6) previous 3-month use of hormone replacement therapy, oestrogen, narcoleptics, steroids, or lipid-lowering or antihypertensive agents, (7) dyslipidaemia or hypertension requiring immediate medical intervention (total cholesterol >8 mmol/l), systolic BP >160 mmHg or diastolic BP >100 mmHg), (8) history of alcohol or drug abuse, (9) abnormal blood laboratory values (haematocrit <32 or >48%; creatinine >130 μmol/l), (10) use of drugs or medications to stimulate weight loss, psychoactive drugs and adrenergic agonists by any route, (11) body weight fluctuation ±2 kg in the last 3 months, (12) known history of inflammatory disease as well as cancer. One month before the study, weight stability was verified by monitoring body weight for each subject on a weekly basis.
Blood samples
Fasting plasma cholesterol, HDL cholesterol, TG and glucose were analysed on the Cobas Integra 400 (Roche Diagnostic, Montreal, QC, Canada). LDL cholesterol was calculated according to the Friedewald equation [18]. Framingham total risk points were calculated based on age, total and HDL cholesterol, smoking status (non-smokers), and systolic BP [19]. Fasting insulin levels were determined by radioimmunoassay specific for human insulin (Linco, St-Charles, MO, USA). Lipoprotein(a), ApoA1 and ApoB, hsCRP, orosomucoid, haptoglobin and α1-antitrypsin were assessed by immunonephelometry on an Immage analyser (Beckman-Coulter, Villepinte, France). Four women with hsCRP concentrations >10 mg/l were excluded from the analysis in this study because such an elevated hsCRP suggests a major infection or trauma [20]. An ELISA was used to measure hsTNF-α, sTNF-α-R1 and IL-6, using commercial kits (Quantikine, Minneapolis, MN, USA).
Insulin sensitivity
Insulin sensitivity was evaluated in the fasting state using two techniques: (1) a clinically based technique through calculation of fasting homeostasis model assessment (HOMA) according to the formula of Matthews et al. [21], supported by (2) a research-based technique which measures glucose disposal rate during a hyperinsulinaemic−euglycaemic clamp test [22, 23]. Glucose disposal rate (M clamp) was calculated as the average glucose infusion rate during the last 30 min of the clamp and is expressed as mg·kg−1·min−1 [23].
Body composition and blood pressure
Body weight was measured using an electronic scale (Balances Industrielles, Montreal, QC, Canada) and standing height by a wall stadiometer (Perspective Enterprises, Portage, MI, USA). Sitting BP was determined after subjects had rested quietly for 10 min, using a Dinamap automatic machine (Welch Allyn, San Diego, CA, USA). Fat mass was evaluated by dual energy X-ray absorptiometry (General Electric Lunar Corporation version 6.10.019, Madison, WI, USA).
Abdominal obesity was evaluated with two techniques: (1) a clinically based technique through measurement of waist circumference, supported by (2) a research-based technique by computed tomography scan (General Electric Medical Systems, Milwaukee, WI, USA) which measures abdominal visceral and subcutaneous adipose tissue (VAT and SAT) at the L4–L5 vertebral disc as described previously [23].
Statistical analysis
Data are presented as mean±standard deviation. Pearson correlation was used to examine the association between measured parametric values while stepwise forward linear regression was used to examine independent predictors of inflammatory markers. As the tests for equal variance and/or normality of residues failed in the regression analysis models used to examine the prediction of hsTNF-α, IL-6, hsCRP and orosomucoid, these inflammatory markers were log-transformed (base 10) then entered in the correlation and regression analysis as shown in the last three tables. A Benjamini correction was applied to the correlation p values to control for a false discovery rate resulting from multiple testing [24]. Unpaired one-tailed t-test was used to examine the differences between women with normal (normoapoB) and high plasma ApoB. Analysis of covariance (univariate general linear model) was used to adjust the means of hsTNF-α for HDL cholesterol in women with normoapoB and hyperapoB. Statistical analysis was performed using SigmaStat (Jandel, San Rafael, CA, USA) and S-Plus Professional 2000 release 3 (MathSoft, Cambridge, MA, USA) with significance set at p<0.05.
Results
Subject characteristics
This analysis included 77 non-diabetic overweight and obese postmenopausal women with no previous history of CHD. The traditional risk factors for type 2 diabetes and CHD, as well as the inflammatory markers measured in this population, are presented in Table 1 and Table 2, respectively. Sixty-five percent of our subjects had total plasma cholesterol >5.2 mmol/l, 33% had high TG (>1.7 mmol/l), 16% had low HDL cholesterol (<1.3 mmol/l), 22% had high ApoB (>1.2 g/l) (all reference values adapted from [19]). Moreover, 73% of the women had abdominal obesity (waist circumference >88 cm), 11% had hypertension (BP >130/85 mmHg), 6% had high insulin (>25 μU/ml), 14% had impaired glucose tolerance (plasma glucose ≥7.8 mmol/l 2 h after OGTT) and one woman had impaired fasting plasma glucose (6.1–7.0 mmol/l) [19, 25].
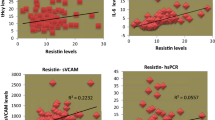
Pearson correlation of inflammatory markers with all traditional risk factors is presented in Table 3 and Fig. 1 (specifically for ApoB). In general, the inflammatory markers correlated positively with total and abdominal adiposity, BP, 2-h OGTT glucose, insulin resistance, triglyceride, total/HDL cholesterol, ApoB, ApoB: ApoA1 ratio and Framingham CHD risk points, and negatively with ApoA1 and total, LDL and HDL cholesterol. Moreover, the inflammatory markers were highly correlated with each other. Log10[IL-6] correlated with log10[hsTNF-α] (R=0.33), sTNF-α-R1 (R=0.24), log10[hsCRP] (R=0.39), log10[orosomucoid] (R=0.25) and haptoglobin (R=0.25). Log10[CRP] also correlated with sTNF-α-R1 (R=0.23), log10[orosomucoid] (R=0.63) and haptoglobin (R=0.50), while log10[orosomucoid] also correlated with haptoglobin (R=0.53). The α1-antitrypsin did not correlate with any inflammatory marker measured.
Pearson correlation between ApoB and the inflammatory markers hsTNF-α (p=0.138, NS) and sTNF-α-R1 (p=0.330, NS) (a), IL-6 (p=0.079, NS) (b), hsCRP (p=0.004) (c), orosomucoid (p<0.001) (d), haptoglobin (p=0.005) (e) and α1-antitrypsin (p=0.017) (f) as measured in overweight and obese postmenopausal women. The significant regression equations are as follows: Log10[CRP]=−0.07+(0.44 × apoB) (c), Log10[orosomucoid]=−0.31+(0.21 × apoB) (d), haptoglobin=0.55+(0.75 × apoB) (e), and α1-anti-trypsin=0.90+(0.30 × apoB) (f)
Prediction of inflammatory markers by clinically available risk factors of type 2 diabetes and CHD
Forward stepwise regression analysis was used to account for the concomitant contribution of multiple risk factors to the concentrations of inflammatory markers (Table 4). For practical relevance, clinical risk factors were used in this model as independent variables and were BMI, waist circumference, systolic BP, fasting glucose, 2-h OGTT glucose, LDL cholesterol, HDL cholesterol, TG, ApoB and ApoA1 (model 1). Moreover, to normalise the prediction of inflammatory markers, whether adipose-tissue secreted or not, all inflammatory markers were adjusted for BMI (clinical measure of obesity). Plasma ApoB predicted five out of seven of the inflammatory markers measured (Table 4). Other traditional risk factors were less predictive of inflammatory status.
Other regression models were also examined but with little change in the prediction power of ApoB reported in model 1. Replacing glucose by HOMA in model 1 resulted in ApoB then HOMA being predictors of log10[hsCRP] (total R 2=0.41) and log10[orosomucoid] (total R 2=0.34), and HOMA being the only predictor of α1-antitrypsin with no other changes. In another model, replacing LDL and HDL cholesterol by total/HDL cholesterol in model 1 eliminated the prediction power of LDL and HDL cholesterol of sTNFα-R1, log10[hsCRP], log10[orosomucoid] and α1-antitrypsin, with no other changes in the power or order of the ApoB or other remaining predictors (data not shown).
Prediction of inflammatory markers by type 2 and CHD risk factors measured by research-based techniques
The independent variables used in this regression model were fat mass (instead of BMI), VAT (instead of waist circumference), M clamp (instead of glucose or HOMA), 2-h OGTT glucose, systolic BP, LDL cholesterol, HDL cholesterol, TG, ApoB and ApoA1 (model 2). As in model 1, to account for the degree of adiposity in this model, all inflammatory markers were corrected for fat mass. As presented in Table 5, the use of more elaborate research techniques for the measurement of body composition and insulin sensitivity confirmed the results obtained with clinically based techniques (Table 4), in that ApoB was the primary predictor of inflammatory markers.
Inflammatory markers in hyperapoB women
As serum ApoB was the primary predictor of inflammatory markers, we examined whether hyperapoB women (n=17, ApoB >1.20 g/l [19]) have higher inflammatory markers than women with normal ApoB (controls). As adiposity is highly correlated to many inflammatory markers, each hyperapoB woman was BMI-matched with a normoapoB woman to eliminate the confounding effect of the level of obesity. We matched the two groups by BMI and not fat mass for clinical practicality only.
By design, BMI was equivalent in both groups (30.7±2.87 vs 30.6±2.87 kg/m2 in hyperapoB vs normoapoB women, respectively). Matching for BMI also resulted in equivalent fat mass in both groups (34.8±5.1 vs 36.3±6.9 kg in hyperapoB vs normoapoB women, respectively). Other anthropometric indices measured, BP—plasma insulin, glucose, HOMA, 2-h OGTT glucose, ApoA1 and Lp(a)— were also found to not differ between the two groups (data not shown). HyperapoB women had significantly lower HDL cholesterol (1.35±0.19 vs 1.67±0.34 mmol/l, p=0.001), lower insulin sensitivity based on the clamp technique (5.54±1.38 vs 6.48±1.61 mg glucose·kg−1·min−1, p=0.04), higher total cholesterol (6.36±0.75 vs 5.40±0.68 mmol/l, p<0.001), higher LDL cholesterol (4.04±0.68 vs 3.05±0.62 mmol/l, p<0.001) and higher TG (2.11±0.81 vs 1.54±0.71 mmol/l, p=0.018).
As shown in Fig. 2, hyperapoB women had 104% higher plasma hsTNF-α (hyperapoB: 1.08±0.99 vs normoapoB: 0.53±0.26 pg/ml, p=0.038), 40% higher IL-6 (hyperapoB: 1.01±0.60 vs normoapoB: 0.72±0.34 pg/ml, p=0.046), 61% higher hsCRP (hyperapoB: 3.69±2.05 vs normoapoB: 2.29±1.34 mg/l, p=0.012) and 17% higher orosomucoid (hyperapoB: 0.95±0.18 vs normoapoB: 0.81±0.24 g/l, p=0.014). Soluble TNF-R1, haptoglobin and α1-antitrypsin were not significantly different between the two groups. As hsTNF-α does not correlate with ApoB, we examined whether reduced hsTNF-α in hyperapoB women was driven by reduced HDL cholesterol (which correlates negatively with hsTNF-α). Indeed, correcting mean hsTNF-α for HDL cholesterol eliminated the difference between hyperapoB and normoapoB women in hsTNF-α.
Discussion
Novel data in this study demonstrate that, in postmenopausal overweight and obese women: (1) plasma ApoB was an independent predictor of the interindividual variation of IL-6, hsCRP, orosomucoid, haptoglobin and α1-antitrypsin; (2) several traditional risk factors for type 2 diabetes and CHD (particularly insulin resistance and hypertension) are positively associated with the plasma concentrations of inflammatory markers and may augment the activation of inflammatory processes by ApoB-related atherogenic lipoproteins; and (3) compared with women with normal ApoB and equivalent weight, fat distribution and BP, hyperapoB women have significantly higher plasma hsTNF-α, IL-6, hsCRP and orosomucoid.
Earlier studies in humans demonstrated that plasma CRP could not be predicted from total or LDL cholesterol [1]. Moreover, reduction in plasma CRP by hypocholesterolaemic statins was demonstrated in a large clinical trial to be independent of the reduction in plasma LDL cholesterol [10]. In agreement with this, plasma CRP in our study was not predicted by total or LDL cholesterol, nor did hypercholesterolaemic women have elevated CRP or any other inflammatory marker (data not shown). Moreover, in the present study, plasma LDL cholesterol correlated negatively with TNF-α-R1, hsCRP and α1-antitrypsin. Thus the human data do not agree with the large body of evidence in animal and cell culture studies that support the role of elevated levels of atherogenic lipoproteins in the development of arterial inflammation [8].
We believe that, although atherogenic lipoproteins may indeed trigger inflammation in humans, the tools used previously to assess this association in vivo are not accurate. Measurement of plasma cholesterol (most of which is on LDL) or TG (most of which is on VLDL) may not reflect the association of atherogenic lipoproteins with inflammatory markers because their plasma concentration is a poor measure of atherogenic particle number and size as well as of lipid composition [26]. As an inverse relationship exists between particle size and ability to cross the arterial endothelial barrier into the intimae [15], small dense LDL particles are more atherogenic than large buoyant ones [15] and may also be more pro-inflammatory. Measurement of total or LDL cholesterol may therefore underestimate particle number, particularly in obese subjects in whom small dense particles are frequent [27]. This may also explain the counterintuitive negative association between LDL cholesterol and inflammatory markers in our study.
In the present study we provide evidence that the prediction of inflammatory markers is indeed possible in humans if another component of the atherogenic lipoproteins is considered, which is ApoB. ApoB reflects the number of atherogenic particles, as LDL, VLDL and Lp(a) have only one ApoB per particle, and is closely correlated with the levels of small dense LDL particles [15]. Therefore, we hypothesised that ApoB may be a predictor of inflammation. As hypothesised, ApoB was the primary predictor of most inflammatory markers examined irrespective of the level of adiposity or the prediction model used. The prediction of hsCRP by ApoB is in line with the Insulin Resistance Arteriosclerosis Study (n=1522) [28], in which, compared with LDL and HDL cholesterol, ApoB was also more strongly associated with plasma CRP. However, our study adds a new finding to this previous one [28] in that, not only was ApoB predictive of CRP, but it also predicted IL-6, orosomucoid, haptoglobin, and α1-antitrypsin, an association that has never been reported before. Moreover, plasma ApoB was superior not only to total or LDL cholesterol but also to many other traditional risk factors of type 2 diabetes and CHD including hypertension, 2-h OGTT plasma glucose, insulin resistance (by HOMA score and the gold standard hyperinsulinaemic clamp), TG, ApoAI, Lp(a), and Framingham CHD risk score. It should be noted, however, that our data contradict the finding by Schlitt et al. [11] that ApoB did not correlate with CRP but correlated negatively (though very weakly) with IL-6. That study, however, examined CHD and diabetic patients, 74% of whom were men, who were also on medical treatment for dyslipidaemia and hypertension [11]. The large difference in the phenotype of the populations examined and possible drug interference may explain the discrepancy between our results and those of Schlitt et al.
Another plausible mechanism by which plasma ApoB may predict inflammation is its ability to reflect the efficiency of fatty acid storage in adipose tissue [29]. Several types of fatty acids, like omega-6 [30], trans [31, 32] and saturated fatty acids [32], have been shown to induce endothelial dysfunction and increased plasma concentration of CRP, IL-8, TNF-α, TNF-α-R2, adhesion molecules and IL-6. The inflammatory marker IL-6 is documented to induce systemic enhancement of inflammation and the production of hepatic acute-phase reactants like CRP, orosomucoid, haptoglobin and α1-antitrypsin [1, 33]. Therefore, ineffective adipose-tissue fatty acid storage may increase NEFA flux to endothelial cells, increasing the production of inflammatory markers (like TNF-α and IL-6), and to liver, increasing the production of ApoB-related atherogenic lipoproteins and hepatic acute-phase reactants (being induced by IL-6). In the present study, the correlation of ApoB with hepatic secreted inflammatory markers (hsCRP, orosomucoid, haptoglobin and α1-antitrypsin) rather than hsTNF-α and sTNF-α-R1, and the intercorrelation of inflammatory markers to each other, especially to IL-6, further support this hypothesis. An alternative mechanism, at least for the association of ApoB with CRP, is that ApoB may circulate associated with CRP and may facilitate its entry into the subendothelial space. CRP was found to co-localise with ApoB in the extracellular space in the atherosclerotic lesion, and because macrophages do not have the mRNA for CRP, it was suggested that CRP found in the atherosclerotic lesion was derived from the circulation [34].
The novel finding in this study that hyperapoB women had elevated levels of TNF-α, IL-6, CRP and orosomucoid may have clinical relevance for the primary prevention of type 2 diabetes. The inflammatory markers elevated in hyperapoB women have been consistently linked to the development of insulin resistance and type 2 diabetes mellitus in numerous in vitro and large clinical studies. For example, TNF-α decreases insulin secretion from pancreatic cells [35] and insulin signalling in pancreatic cells, white adipose tissue, liver and muscle [35, 36]. Plasma IL-6 [7], CRP [7, 37, 38] and orosomucoid [6] were predictors of the incidence of type 2 diabetes in several large prospective studies (ranging from 1,047 to 27,000 subjects and with up to 7-year follow-up), independently of other correlates of plasma glucose and insulin sensitivity [6, 7, 37, 38]. Thus, as these inflammatory markers are elevated in hyperapoB women, we hypothesise that hyperapoB women may be at a greater risk of developing, not only CHD, but type 2 diabetes. HyperapoB and small dense atherogenic particles are common abnormalities in type 2 diabetes [39]. HyperapoB may not be a consequence of the metabolic abnormalities associated with type 2 diabetes but rather a mediator of this disease through its activation of the inflammatory process.
It is important to point out, however, that the association between ApoB and IL-6, an adipose tissue-released inflammatory marker [40], is only evident after accounting for the degree of adiposity. This was demonstrated either by adjustment for BMI/fat mass in the regression models or by matching hyperapoB and normoapoB women by BMI. Thus the contribution of the level of adiposity to circulating IL-6 levels may mask the association between IL-6 and lipoproteins or other CHD risk factors and should be kept in mind in future studies examining this inflammatory marker.
Certain limitations to the present study should be kept in mind. As is the case in cross-sectional studies, the correlative nature of this analysis does not necessarily reflect causality, but allows the generation of hypotheses regarding plausible mechanisms and the impact of our findings. Moreover, the inclusion criteria of the study excluded frequent confounding factors like smoking and more severe hypertensive and dyslipidaemic individuals. Future prospective studies should be employed to investigate the hypotheses presented in this study and to allow their extrapolation to the general population.
In conclusion, novel findings in this study suggest that plasma ApoB is an independent predictor of many inflammatory markers in postmenopausal overweight and obese women. Moreover, women with hyperapoB in this population had elevated levels of hsTNF-α, IL-6, hsCRP and orosomucoid compared with women with normal ApoB, independent of body weight, fat distribution, BP and many indices of insulin sensitivity. As elevated levels of these inflammatory markers are independent risk factors for the development of type 2 diabetes, we hypothesise that hyperapoB women may be at a greater risk than normoapoB women of developing not only CHD, but type 2 diabetes as well.
Abbreviations
- ApoA1:
-
apolipoprotein A1
- ApoB:
-
apolipoprotein B
- CRP:
-
C-reactive protein
- HOMA-IR:
-
homeostatic model assessment of insulin resistance
- hs:
-
high-sensitivity
- hyperapoB:
-
hyperapobetalipoproteinaemia
- Lp(a):
-
lipoprotein (a)
- M clamp :
-
glucose disposal rate during the clamp
- normoapoB:
-
normal plasma ApoB
- SAT:
-
subcutaneous adipose tissue
- sTNF-α-R1:
-
soluble TNF-α receptor 1
- TG:
-
triglyceride
- VAT:
-
visceral adipose tissue
References
Bassuk SS, Rifai N, Ridker PM (2004) High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol 29:439–493
Freeman DJ, Norrie J, Caslake MJ et al (2002) C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 51:1596–1600
Vasan RS, Sullivan LM, Roubenoff R et al (2003) Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 107:1486–1491
Engstrom G, Stavenow L, Hedblad B et al (2003) Inflammation-sensitive plasma proteins, diabetes, and mortality and incidence of myocardial infarction and stroke: a population-based study. Diabetes 52:442–447
Engstrom G, Hedblad B, Stavenow L et al (2004) Incidence of obesity-associated cardiovascular disease is related to inflammation-sensitive plasma proteins: a population-based cohort study. Arterioscler Thromb Vasc Biol 24:1498–1502
Schmidt MI, Duncan BB, Sharrett AR et al (1999) Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 353:1649–1652
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Fan J, Watanabe T (2003) Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb 10:63–71
Kofler S, Nickel T, Weis M (2005) Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (London) 108:205–213
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR (2002) Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347:1557–1565
Schlitt A, Blankenberg S, Bickel C et al (2005) Prognostic value of lipoproteins and their relation to inflammatory markers among patients with coronary artery disease. Int J Cardiol 102:477–485
Albert MA, Glynn RJ, Ridker PM (2003) Plasma concentration of C-reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation 108:161–165
Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM (2002) Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol 22:1668–1673
Albert MA, Danielson E, Rifai N, Ridker PM, PRINCE investigators (2001) Effect of statin therapy on C-reactive protein levels: the Pravastatin Inflammation/CRP Evaluation (PRINCE): a randomized trial and cohort study. JAMA 286:64–70
Carmena R, Duriez P, Fruchart JC (2004) Atherogenic lipoprotein particles in atherosclerosis. Circulation 109(Suppl):III2–III7
Gorodeski GI (2002) Update on cardiovascular disease in post-menopausal women. Best Practice. Res Clin Obstet Gynaecol 16:329–355
Sniderman AD, Scantlebury T, Cianflone K (2001) Hypertriglyceridemia hyperapoB: the unappreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus. Ann Intern Med 135:447–459
Schectman G, Patsches M, Sasse EA (1996) Variability in cholesterol measurements: comparison of calculated and direct LDL cholesterol determinations. Clin Chem 42:732–737
Genest J, Frohlich J, Fodor G, McPherson R (2003) Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: summary of the 2003 update. Can Med Assoc J 169:921–924
Jialal I, Devaraj S, Venugopal SK (2004) C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension 44:6–11
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223
Karelis AD, Faraj M, Bastard JP et al (2005) The metabolically healthy, but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 90:4145–4150
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc 57:289–300
Pagana KD, Pagana TJ (1997) Diagnostic and laboratory test reference. Mosby-Year Book, St Louis
Ginsberg HN (1998) Lipoprotein physiology. Endocrinol Metab Clin North Am 27:503–519
Van Gaal L, Zhang A, Steijaert MM, De Leeuw IH (1995) Human obesity: from lipid abnormalities to lipid oxidation. Int J Obesity Rel Metab Dis 19:S21–S26
Williams K, Sniderman AD, Sattar N, D’Agostino R Jr, Wagenknecht LE, Haffner SM (2003) Comparison of the associations of apolipoprotein B and low-density lipoprotein cholesterol with other cardiovascular risk factors in the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 108:2312–2316
Sniderman AD, Cianflone K, Frayn K (1997) The pathogenetic role of impaired fatty acid trapping by adipocytes in generating the pleiotropic features of hyperapoB. Diabetologia 40:S152–S154
Young V, Toborek M, Yang F, McClain C, Hennig B (1998) Effect of linoleic acid on endothelial cell inflammatory mediators. Metabolism 47:566–572
Lopez-Garcia E, Schulze MB, Meigs JB et al (2005) Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 135:562–566
Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN (2002) Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res 43:445–452
Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V (1999) Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 148:209–214
Sun H, Koike T, Ichikawa T et al (2005) C-reactive protein in atherosclerotic lesions: its origin and pathophysiological significance. Am J Pathol 167:1139–1148
Tsiotra PC, Tsiogos C, Partis SA (2001) TNFalpha and leptin inhibit basal and glucose-stimulated insulin secretion and gene transcription in the HIT-T15 pancreatic cells. Int J Obesity Rel Metab Dis 25:1018–1026
Grossi SG (2001) Treatment of periodontal disease and control of diabetes: an assessment of the evidence and need for future research. Ann Periodontol 6:138–145
Barzilay JI, Abraham L, Heckbert SR et al (2001) The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes 50:2384–2389
Festa A, D’Agostino R Jr, Tracy RP, Haffner SM (2002) Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes 51:1131–1137
Harano Y, Kageyama A, Nakao K et al (1996) Quantitative and qualitative derangement of apolipoprotein B-containing lipoproteins as a risk factor for diabetic macroangiopathy in nonobese NIDDM subjects. Diabetes 45:S31–S34
Fried SK, Bunkin DA, Greenberg AS (1998) Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83:847–850
Acknowledgments
This work is part of the Montreal–Ottawa New Emerging Team (MONET) and is supported by grants from the Canadian Institute of Health Research (CIHR) # OHN—63279 as well as grant # MOP-62976. M. Faraj is supported by a postdoctoral fellowship from CIHR and R. Rabasa-Lhoret is supported by a fellowship from the Fonds de Recherche en Santé du Québec (FRSQ). We thank Beckman-Coulter, France for the kind gift of antibodies for all protein measurements and S. Fellahi for her participation in the inflammatory markers analysis. We also thank M. Chagnon (Statistical Department, University of Montréal) for his advice on the statistical analysis of the data.
Duality of interest
The authors declare that they have no duality of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faraj, M., Messier, L., Bastard, J.P. et al. Apolipoprotein B: a predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia 49, 1637–1646 (2006). https://doi.org/10.1007/s00125-006-0259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0259-7