Abstract
Aims/hypothesis
The aim of this study was to determine the influence of type 2 diabetes on fibrinolysis by assessing interactions between the regulatory components of fibrinolysis and the fibrin clot, using fibrinogen purified from 150 patients with type 2 diabetes and 50 matched controls.
Methods
Clot lysis rates were determined by confocal microscopy. Plasmin generation was measured using a plasmin-specific chromogenic substrate. Surface plasmon resonance was used to determine the binding interactions between fibrin, tissue-type plasminogen activator (t-PA) and Glu-plasminogen; cross-linkage of plasmin inhibitor to fibrin by factor XIII was determined using a microtitre plate assay.
Results
Lysis of diabetic clots was significantly slower than that of controls (1.35 vs 2.92 μm/min, p<0.0001) and plasmin generation was significantly reduced. The equilibrium binding affinity between both t-PA and Glu-plasminogen and fibrin was reduced in diabetic subjects: t-PA, K D=0.91±0.3 μmol/l (control subjects), 1.21±0.5 μmol/l (diabetic subjects), p=0.001; Glu-plasminogen, K D=97±19 nmol/l (control subjects), 156±66 nmol/l (diabetic subjects), p=0.001. Cross-linkage of plasmin inhibitor to fibrin by factor XIII was enhanced in diabetic subjects, with the extent of in vitro cross-linkage correlating with in vivo glycaemic control (HbA1c) (r=0.59, p=0.001).
Conclusions/interpretation
These results indicate that impairment of the fibrinolytic process in diabetic patients is mediated via a number of different mechanisms; these may be a consequence of post-translational modifications to fibrinogen molecules, resulting from their exposure to the abnormal metabolic milieu associated with diabetes.
Similar content being viewed by others
Introduction
The morbidity and mortality associated with diabetes are largely the result of the micro- and macrovascular complications that develop during the disease. Although the relationship between glycaemic control and the development of complications is close [1, 2], a multitude of pathophysiological processes are likely to be involved, including the haemostatic system [3], the potential contribution of which is supported by the observation that fibrin is deposited at sites of both micro- [4–6] and macrovascular [7] damage.
The coagulation and fibrinolytic systems are integrated and highly regulated to maintain the balance between thrombosis and clot lysis, such that vascular patency is preserved whilst providing adequate protection against blood loss. Fibrin deposition may therefore reflect a disruption of this balance, favouring coagulation and fibrin formation. Fibrin clots formed by diabetic subjects have a denser, less porous structure than those formed by healthy non-diabetic subjects [8, 9] (changes which correlate with glycaemic control). Clots from this phenotype have been shown to be more resistant to fibrinolysis, which may result from the clots’ influence over the binding, activation and activity of the fibrinolytic proteins [10–12]. This resistance could result in the enhanced deposition of fibrin within the vascular tree of diabetic subjects, promoting the development of micro/macrovascular disease [13, 14].
We used fibrinogen purified from patients with diabetes, as opposed to plasma or in vitro glycation methods used previously, to investigate whether diabetes influences the fibrinolytic process and to examine the potential mechanisms involved.
Subjects and methods
Subjects
Type 2 diabetic subjects (n=150) were recruited through the Diabetes Centre at Leeds General Infirmary. Age- and sex-matched control subjects (n=50) were recruited from the Leeds Health Authority Family Health Service Register [9]. All subjects were White and of northern European origin, and gave informed consent according to a protocol approved by the Leeds Teaching Hospitals Research Ethics Committee. Patients with microvascular complications (history of ophthalmologic intervention to treat retinopathy in the past, background/proliferative retinopathy on examination of dilated fundi at recruitment, or proteinuria/microalbuminuria on analysis of urine samples provided at recruitment) and/or macrovascular complications (presence of vascular bruits, abnormal peripheral pulses, ECG changes indicative of coronary artery disease at recruitment, or history of coronary artery disease, cerebrovascular disease or peripheral vascular disease) were excluded from the study. None of the subjects was taking oral anticoagulants or aspirin.
Blood sampling
Venous blood was taken between 08.00 and 10.00 h, following an overnight fast, into siliconised tubes containing 15 IU/ml lithium heparin for purification of fibrinogen, or EDTA for HbA1c measurement (using a Glycomat auto-analyser; Ciba Corning, Halstead, UK; reference range 4.5–6.5%). Samples for fibrinogen purification were centrifuged within 1 h (2,540 g for 20 min) at room temperature to obtain platelet-poor plasma, which was separated, frozen in liquid nitrogen, and stored at −40°C.
Fibrinogen purification
Fibrinogen was purified from plasma of subjects by IF-1 affinity chromatography, as described previously [9].
Factor XIII purification
Human factor XIII (FXIII) was purified from outdated platelet-poor plasma by ammonium sulfate precipitation and gel filtration, as previously described [15].
Modifications to fibrinogen molecules associated with poor glycaemic control
The extent of fibrinogen glycation was determined using a modified fructosamine assay of samples purified from 20 diabetic subjects and 20 matched controls [16]. Fibrinogen samples were concentrated to 8 mg/ml, then dialysed into 0.01 mol/l NaH2PO4, 0.14 mol/l NaCl, pH 7.4 for 3 h at 4°C. Each sample (100 μl) was added to 1 ml of 0.1 mol/l NaHCO3, pH 11.5, containing 0.25 mmol/l nitroblue tetrazolium, and incubated at 37°C. Absorbance at 530 nm was measured at 10 and 30 min using an SP75 UV/Vis Spectrophotometer (Sanyo, Loughborough, UK), and the proportion of the glycated fibrinogen present in each sample was expressed as a function of a sample’s capacity to reduce the chromogenic substrate nitroblue tetrazolium compared with the reducing activity of the reducing agent dimethylfructosamine at standardised concentrations.
Mass spectrometric analysis of fibrinogen chains
The mass of each fibrinogen chain (Aα-, Bβ- and γ-) was determined for fibrinogen purified from ten diabetic and ten matched control subjects. Individual fibrinogen chains were separated as previously described [17]. Samples were analysed on a quadruple time-of-flight orthogonal acceleration mass spectrometer equipped with nano-electrospray ionisation (Micromass, Manchester, UK). Samples were dissolved in 1:1 (v/v) aqueous methanol with 0.5% (w/v) formic acid. Positive ionisation was used for the sample analyses, with a capillary voltage of 900 V and a sampling cone voltage of 40 V. Nitrogen was employed as the drying gas. The microchannel plate detector was set at 2,700 V. Data were acquired over the appropriate m/z range and spectra processed using the MassLynx software (Waters, Elstree, UK ). The m/z spectra were converted into true molecular mass using maximum entropy. The spectra were calibrated using horse heart myoglobin (M r 16,950.5) as reference material; a mass accuracy of 0.01% was expected.
Fibrinolysis
Lysis rates of fibrin clots formed from fibrinogen (1 mg/ml) purified from 25 randomly selected diabetic and 25 matched control subjects, FXIII (22 μg/ml), human α-thrombin (American Diagnostica, Stamford, CT, USA) (1 U/ml) and CaCl2 (5 mmol/l) were assessed by confocal microscopy as described previously by Collet et al. [12]. Lysis buffer (10 μl), containing tissue-type plasminogen activator (t-PA) (5 μg/ml) and Glu-plasminogen (210 μg/ml) (both from Technoclone, Vienna, Austria) in 140 mmol/l NaCl, 50 mmol/l Tris, pH 7.4 (Tris-buffered saline, TBS), was applied to the lower edge of the clot. After 15 min incubation in a moist atmosphere, the clot edge was visualised using a Leica TCS SP-2 laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany). Clots were scanned at low magnification to generate images that were obtained using a standardised format; 100×100×20 μm, with ten optical sections collected at z-intervals of 2 μm. The images were analysed using the Leica image analysis software. The distance of the lysis front from a fixed point was measured at different time points. Lysis front velocity was determined at six different sites along the lysis front, and used to calculate the mean overall lysis rate in μm/min.
Cross-linkage of plasmin inhibitor to fibrin/fibrinogen
Plasmin inhibitor (PI) is cross-linked to fibrin by activated factor XIII (FXIIIa), enhancing resistance of the clot to lysis. We assessed whether PI incorporation was altered in subjects with type 2 diabetes using a method modified from that described by Schröder and Kohler [18]. Microtitre plates were coated with 80 μg/ml fibrinogen (a concentration at which it has been shown to adopt a solution-like conformation [19]). Control experiments were undertaken to ensure that no difference in the coating efficiency of fibrinogen or the generation of fibrin existed between the two groups. Plates were incubated with PI (Merck Biosciences, Nottingham, UK) (10 μg/ml), α-thrombin (1 U/ml), CaCl2 (5 mmol/l) and FXIII (22 μg/ml). The reaction was stopped after 1 and 50 min by the addition of 200 mmol/l EDTA (200 μl EDTA–100 μl reaction mixture). Plates were washed twice and incubated with goat anti-human PI peroxidase-conjugated IgG (Enzyme Research Laboratories, Lafayette, IN, USA) and developed with o-phenylenediamine dihydrochloride. Increase of absorbency at 490 nm was monitored using an MRX microplate reader (Dynex Technologies, Ashford, UK) until the fibrin standard (Merck) reached an absorbency of 0.7 to 0.8, when the reaction was stopped by the addition of 1.5 mol/l sulfuric acid and a final reading made. The quantity of PI cross-linked to the fibrin was calculated from the mean change in absorbance for each sample, expressed as a fraction of that cross-linked to the fibrin standard present on each plate over the same time interval.
As FXIII has also been shown to cross-link PI to circulating fibrinogen in vivo [20], western blot analysis was undertaken to determine the extent to which PI had been cross-linked to the fibrinogen prior to its purification. Fibrinogen was subjected to SDS-PAGE gel analysis under reducing conditions, with gels cast at a polyacrylamide concentration of 8% (bis-acrylamide ratio 1:35.5) in 1.5 mol/l Tris-HCl. Gels were immunoblotted for PI with goat anti-human PI peroxidase-conjugated IgG using standard methods with 3,3′,5,5′-tetramethylbenzidine substrate (Sigma-Aldrich, St Louis, MO, USA). Images were obtained using Kodak Image Station 2000R (Eastman Kodak, New Haven, CT, USA). Protein-band density was analysed using Kodak 1D Image Analysis Software, and results were expressed as a fraction of the density of a reference PI sample (50 μg/ml) loaded onto each gel. Cross-reactivity of the antibody with fibrinogen was tested on dot-blots with recombinant wild-type fibrinogen.
Plasminogen activation
The rate of plasmin generation by t-PA at the fibrin surface was determined using a method modified from that described by Bobbink et al. [21]. Microtitre plates were coated with 80 μg/ml fibrinogen in TBS for 40 min at room temperature then blocked with TBS containing 3% BSA and 0.01% Tween for 90 min at 37°C. Plates were washed with 50 mmol/l Tris, 110 mmol/l NaCl, 0.01% Tween, pH 7.4, then incubated with α-thrombin (1 U/ml) and CaCl2 (5 mmol/l) in 50 mmol/l Tris-HCl, 110 mmol/l NaCl, pH 7.4, for 45 min at room temperature, then washed with 1 mol/l NaCl, 50 mmol/l Tris, pH 7.4, and again with low-salt wash buffer. t-PA (50 μl; 1, 2.5, 5 and 10 nmol/l) in blocking buffer was added, and incubated for 90 min at 37°C, following which the wells were washed with TBS. Plasminogen activation was initiated by the addition of 600 nmol/l Glu-plasminogen in 50 mmol/l Tris, 110 mmol/l NaCl, pH 7.4. Plasmin activity was detected by measuring the hydrolysis of 0.8 mmol/l plasmin-specific chromogenic substrate S-2251 (Chromogenix, Epsom, UK) at 37°C over 60 min. The quantity of plasmin generated (mg/l) was determined using the change in absorbance over 60 min.
Surface plasmon resonance
The interaction of fibrin with Glu-plasminogen and t-PA was analysed in real time by surface plasmon resonance (SPR) using fibrinogen purified from 15 randomly selected diabetic subjects and 15 matched controls, using a Biacore 3,000 system (Biacore, Stevenage, UK). Fibrinogen was covalently attached to activated carboxymethyl dextran-coated biosensor chips (CM5) by amine coupling, using the manufacturer’s recommended protocol, to yield approximately 1,000 resonance units (RU). α-Thrombin (1 U/ml) in 140 mmol/l NaCl, 10 mmol/l HEPES, pH 7.4, was passed over the sensor chip surface at 2 μl/min for 45 min to convert coupled fibrinogen to fibrin, following which the sensor surface was regenerated with 1 mol/l NaCl, 10 mmol/l HEPES, 0.005% (w/v) P20, pH 7.4 (injection volume 90 μl, flow-rate 30 μl/min). The subsequent reduction in refractive index (reflecting fibrinopeptide cleavage) was identical for fibrinogen purified from diabetic and control subjects. Association/dissociation of the analytes (t-PA or Glu-plasminogen) and ligand (fibrin) was reflected by changes in refractive index.
t-PA
Binding experiments were performed at 25°C in 140 mmol/l NaCl, 10 mmol/l HEPES, 0.005% (w/v) P20, pH 7.4. t-PA (40 μl) was passed over the sensor chip surface at 10 μl/min (contact time 4 min) to achieve equilibrium, followed by a dissociation time of 3 min to allow re-equilibration between sensor surface and running buffer. The sensor surface was regenerated with 1 mol/l NaCl, 10 mmol/l HEPES, 0.005% (w/v) P20, pH 7.4 (contact time 3 min), after which it was re-equilibrated with running buffer for 5 min. The association of t-PA with fibrin was assessed over a defined concentration range (31.25–2,000 nmol/l). Association/dissociation of t-PA and fibrin, following subtraction of the reference cell signal (which was activated and blocked in the absence of ligand) and that for zero concentration of t-PA (i.e. double subtraction), was assessed using BIA evaluation kinetics software (Biacore). The analysis was repeated at least six times for each sample.
Glu-plasminogen
Binding was performed at 25°C in 140 mmol/l NaCl, 10 mmol/l HEPES, 2.5 mmol/l benzamidine, 0.005% (w/v) P20, pH 7.4. Glu-plasminogen (90 μl) was passed over the sensor surface at 30 μl/min (contact time 3 min), followed by a dissociation time of 3 min. The sensor surface was regenerated with 2 mol/l MgCl2, 10 mmol/l HEPES, pH 7.4 (contact time 3 min) after which it was re-equilibrated with running buffer for 5 min. The association of Glu-plasminogen with fibrin over a defined concentration range (31.25–1,000 nmol/l) was determined. Association/dissociation of Glu-plasminogen and fibrin was recorded and double subtraction applied (as described above). Binding analysis was repeated at least six times for each sample over the defined concentration range.
Data were fitted to a 1:1 ligand-binding model to establish the overall affinity constant (K D) for the interaction between Glu-plasminogen and t-PA with fibrin. The 1:1 (Langmuir) binding model employing kinetic analysis in BIA evaluation kinetics analysis software was used to give an overall affinity for binding of Glu-plasminogen with fibrin as equilibrium was not reached at the end of the association phase of these two proteins. The association phase of t-PA with fibrin reached equilibrium; therefore affinity analysis, rather than kinetic analysis, was employed to establish the equilibrium dissociation constant K D for this interaction. Mass transfer limitations may influence kinetic analysis (but not affinity analysis); however, no evidence of mass transfer limitation of Glu-plasminogen binding to fibrin was found.
Statistical analysis
Data were tested for conformity to the normal distribution using Kolmogorov–Smirnov analysis. Data were not normally distributed, and non-parametric tests were used. The Spearman correlation was used to investigate associations between lysis rates and cross-linkage of PI, plasmin generation and HbA1c. Differences in t-PA and Glu-plasminogen binding, and plasmin generation, were compared using the Mann–Whitney U test. Statistical significance was taken as p<0.05 and all analyses were performed with Statistics Package for Social Scientists for Windows, version 11.5 (SPSS, Chicago, IL, USA).
Results
Subject characteristics
Diabetic and control subjects were matched for age, sex and smoking habits (Table 1). Whilst significant differences existed between the two groups for a number of clinical parameters, none of these (with the exception of fasting glucose levels and HbA1c) had a significant effect on the parameters assessed.
Fructosamine assay
The fructosamine content of each fibrinogen sample (indicating the extent of glycation) was significantly correlated with the subjects’ glycaemic control (as assessed by HbA1c) (Fig. 1).
Mass spectrometric analysis of separated fibrinogen chains
Mass spectrometric analysis of the mean molecular masses of the Aα-, Bβ- and γ-chains were in agreement with the predicted values of 66,132, 54,195 and 48,385 Da, respectively [17]. Analysis of the Bβ and γ-chain masses showed that each chain had two distinct components differing in mass by ∼280–295 Da, a heterogeneity thought to result from the loss of the terminal sialic acid residue (mass 291 Da) (partial desialylation) from the oligosaccharides attached to these chains [17]. Quantification of the percentage of desialylation of the Bβ and γ-chains revealed no difference in the sialylation status of both Bβ and γ-chains between diabetic and control subjects. There was no significant difference in the mean mass of Aα-chains purified from diabetic and control subjects; however, the mean mass of the predominant isoforms of the Bβ and γ-chains was significantly greater in the diabetic subjects (Table 2). The increase could be accounted for by individual glucose molecules (molecular mass 162) and their subsequent modification by other processes, such as oxidation/reduction or dehydration. The smaller difference observed in the Aα-chain may have arisen due to the heterogeneity of the Aα-chain, which is more susceptible to proteolysis.
Lysis rates
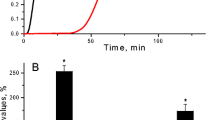
The lysis front velocity of fibrin clots formed from fibrinogen purified from diabetic subjects (n=25) was significantly slower (1.35±0.37 μm/min) than that of clots from control subjects (2.92±0.57 μm/min) (p<0.0001, n=25) (Fig. 2). There was a significant negative correlation between clot lysis rates and chronic glycaemic control (HbA1c) (r=−0.83, p<0.0001) and fasting plasma glucose (r=−0.72, p<0.0001).
Representative three-dimensional reconstruction images from control (a–c) and diabetic (d–f) subjects illustrating dynamic progression of lysis fronts observed by confocal microscopy (100×100×20 μm). Progression of the lysis front after 0 (a, d), 10 (b, e) and 20 min (c, f), respectively. Fibrin clots were formed from purified fibrinogen (1 mg/ml), FXIII (22 μg/ml), α- thrombin (1 U/ml) and CaCl2 (5 mmol/l). Lysis was induced by application of 10 μl of a solution of t-PA (5 μg/ml) and Glu-plasminogen (210 μg/ml)
Incorporation of plasmin inhibitor by FXIII cross-linkage
Cross-reactivity between the antibody to PI and fibrinogen was excluded by dot-blots using recombinant fibrinogen (Fig. 3).
Dot-blot analysis to assess non-specific binding of antibody against PI to fibrinogen. 1 PI (50 μg/ml); 2 recombinant fibrinogen (100 μg/ml); 3 fibrinogen purified from a diabetic subject (100 μg/ml); 4 fibrinogen purified from control subject (100 μg/ml). There is no evidence of cross-reactivity between the antibody generated against PI and fibrinogen; the antibody bound to the fibrinogen purified from diabetic and control subjects therefore reflects PI cross-linked to the fibrinogen molecules in vivo by FXIII
The FXIII-induced cross-linkage of PI to fibrin (assessed in the microtitre plate assay; ratio PI cross-linked to sample:PI cross-linked to standard fibrin) was significantly greater in diabetic subjects (1.14±0.02 [mean±SEM], n=150) than controls (0.95±0.01 [mean±SEM], n=50) (p<0.0001), with the extent of PI incorporation being significantly correlated with the subjects’ chronic glycaemic control (HbA1c) (r=0.587, p=0.001) (Fig. 4) and fasting plasma glucose levels (r=0.52, p=0.001). The inter- and intra-assay CVs for the assay were 4.1 and 5.7%, respectively.
Correlation between FXIII-induced cross-linking of PI to fibrin prepared from fibrinogen purified from diabetic and control subjects (assessed by microtitre plate assay) and glycaemic control (HbA1c). Cross-linking was expressed as the relative amount of PI incorporated compared with a standard purified fibrinogen. Regression analysis showed r=0.59, p<0.0001
PI may be cross-linked to fibrinogen prior to fibrin clot formation. Quantification of PI present in fibrinogen purified from all subjects at baseline by western blot analysis showed that a ∼133–134 kDa band was present for each reduced fibrinogen sample (Fig. 5a,b), which was thought to represent PI (molecular mass ∼67 kDa) cross-linked to the γ-chain of fibrinogen (molecular mass ∼66 kDa). This was confirmed by repeating the western blot analysis using a monoclonal antibody against the fibrinogen Aα-chain (Mouse anti-fibrinogen Aα290–348/349–406 IgG; Accurate Chemical and Scientific, New York, USA), which co-localised to the same position on the membrane (Fig. 5c). There was, however, no significant difference in the extent to which PI was cross-linked into the fibrinogen prior to its purification from the two groups of subjects.
Examples of western blot analysis of reduced fibrinogen purified from diabetic (a) and control (b) subjects immunoblotted with antibody against PI, and against Aα-chain of fibrinogen (c). a,b: lane 1, molecular mass (MM) marker; lane 2, PI (50 μg/ml) (non-reduced); lanes 3–6, reduced purified fibrinogen (0.5 mg/ml). c: lane 1, MM marker; lane 2, PI (50 μg/ml) (non-reduced); lanes 3 and 4, reduced purified fibrinogen (0.5 mg/ml) from diabetic subjects; lanes 5 and 6, reduced purified fibrinogen (0.5 mg/ml) from control subjects. a,b Purified non-reduced PI is shown as monomer (MM ∼67 kDa) and dimer (MM ∼134 kDa). The single band (by coincidence molecular mass also about 133–134 kDa) present in purified reduced fibrinogen samples appears to indicate PI cross-linked to Aα-chain of fibrinogen by FXIII (combined MM ~133–134 kDa). This is confirmed (c), where the Aα-chain of fibrinogen is present at ∼133–134 kDa location. Closed arrows, PI cross-linked to fibrinogen Aα-chain; open arrows, PI control
Plasmin activation
Possible mechanisms underlying the impaired lysis of purified fibrin clots formed by diabetic subjects were investigated. Generation of plasmin at the surface of fibrin produced from fibrogen purified from diabetic (n=150) and control subjects (n=50) was compared. The inter- and intra-assay CVs for the assay were 6.9 and 3.9%, respectively. The quantity of plasmin generated was significantly greater in control than diabetic subjects for each concentration of t-PA (Fig. 6), and was negatively correlated with chronic glycaemic control (HbA1c) (r=−0.46, p=0.001) and fasting plasma glucose levels (r=−0.53, p=0.001).
t-PA and Glu-plasminogen binding
We further assessed whether the impaired plasmin generation resulted from altered binding of t-PA and Glu-plasminogen to the fibrin of diabetic subjects using SPR. Preliminary experiments demonstrated that no t-PA or Glu-plasminogen bound to fibrinogen prior to its conversion to fibrin, reflecting the presence of cryptic binding sites within the fibrinogen molecule, sites that are only exposed following its conversion to fibrin. The fibrin immobilised on the sensor-chip-bound t-PA and Glu-plasminogen in a dose-dependent manner (Fig. 7). The calculated K D values for the interactions between t-PA and Glu-plasminogen and fibrin revealed significant differences in the binding of t-PA and Glu-plasminogen to fibrin from each group. The binding of both t-PA and Glu-plasminogen to fibrin in diabetic subjects (K D 1.21±0.3 μmol/l and 156±34 nmol/l, respectively, [mean±SD]) was significantly reduced compared with that in controls (K D 0.91±0.2 μmol/l and 97±10 nmol/l, p=0.001).
SPR analysis of the binding of plasminogen and tPA to fibrin. a Example of the affinity analysis of the binding of Glu-plasminogen to the immobilised fibrin surface using SPR (concentrations of Glu-plasminogen: 1,000 nmol/l, dark green line; 500 nmol/l, brown line; 250 nmol/l, dark blue line; 125 nmol/l, light blue line; 62.5 nmol/l, light green line; 31.25 nmol/l, red line). b Increasing concentrations of t-PA were flowed over the immobilised fibrin surface, and the association/dissociation was monitored in real time by monitoring the respective changes in the resonance signal (response) (results of three independent runs for each concentration are shown) (concentrations of t-PA: 2,000 nmo/l, brown line; 100 nmol/l, dark blue line; 750 nmol/l, light grey line; 500 nmol/l, purple line; 250 nmol/l, sky blue line; 125 nmol/l, dark grey line; 62.5 nmol/l, green line; 31.25 nmol/l, pink line). c Affinity analysis (response signal reached at equilibrium for each t-PA concentration against t-PA concentration) was used to calculate the affinity constant (K D) for the binding interaction
Discussion
Type 2 diabetes is associated with an impairment of the fibrinolytic process, characterised by prolongation of ex vivo clot lysis times and elevated circulating levels of both plasminogen activator inhibitor type 1 (PAI-1) and t-PA [22]. Fibrinolysis is dependent upon the generation of plasmin at the clot surface, and although it is generally held that elevated PAI-1 is responsible for the suppression of fibrinolysis in diabetes, activation of plasminogen by t-PA does in fact occur on the clot surface, largely protected from the inhibitory effects of PAI-1.
The fibrin clot is not a passive substrate: it is centrally involved in both the activation and regulation of fibrinolysis, enhancing the activation of plasminogen by t-PA by at least three orders of magnitude [23]. Fibrin polymerisation exposes t-PA and cryptic plasminogen-binding sites within the fibrinogen molecule [24]; it is the lysine residues present at these sites that are of key importance for mediating the binding interactions between these proteins, enabling the formation of a ternary complex between t-PA, plasminogen and fibrin that potentiates the generation of plasmin by t-PA. New carboxy-terminal lysine residues (additional plasminogen/t-PA binding sites) are also generated within the fibrin network by the actions of plasmin [25], mediating a positive feedback mechanism to upregulate the lytic process. This process is, however, regulated by the actions of thrombin activatable fibrinolysis inhibitor (TAFI), which following its activation by the thrombin–thrombomodulin complex cleaves these plasmin-generated carboxy-terminal lysine residues from the fibrin clot (Fig. 8).
Simplified schemata of the processes of coagulation and fibrinolysis. Thrombin (IIa) is formed from prothrombin (II) by the prothrombinase complex (factors Xa, Va, calcium and phospholipid membrane) generated from activation of the intrinsic and extrinsic coagulation pathways. Thrombin cleaves fibrinopeptides from fibrinogen to form fibrin monomers, which polymerise to form the fibrin clot, which is then stabilised by cross-links formed by FXIIIa (activated by IIa). Plasmin is generated from plasminogen by the actions of t-PA, a reaction that is enhanced by the binding of plasminogen and t-PA to binding sites on the fibrin molecules, which are exposed following its polymerisation. The activity of t-PA is inhibited by PAI-1. PI inhibits fibrinolysis by inhibiting the binding of plasminogen to fibrin, and directly inhibiting the activity of plasmin. Plasmin is also inhibited by α2-macroglobulin (α2-MG). Activated TAFI (TAFIa) inhibits the action of plasmin to upregulate fibrinolysis by cleaving the carboxy-terminal lysine residues generated within the fibrin clot by plasmin
We used a purified protein system to investigate the PAI-1-independent effects of type 2 diabetes on the fibrinolytic process. Our data indicate that clots formed from fibrinogen purified from diabetic subjects are more resistant to plasminolysis than those formed by controls, and that this results from changes in a number of key regulatory stages in the fibrinolytic process. We showed that the binding of both t-PA and plasminogen to fibrin (an event essential for fibrin to fulfil its role as a cofactor in the promotion of plasminogen activation by t-PA) is impaired in diabetic subjects, and that there is a corresponding reduction in the generation of plasmin compared with control subjects.
Regulation of the fibrinolytic process occurs at a number of stages, which include inhibition of both plasminogen activation (PAI-1) and plasmin activity (PI, α2-macroglobulin) (Fig. 8). During coagulation, PI becomes cross-linked to lys303 within the αC-domain of fibrin by FXIIIa [26], with its inhibitory effect being directly proportional to the concentration that is cross-linked [27]. Our results indicate that the FXIIIa-catalysed cross-linkage of PI to fibrin is enhanced in diabetic subjects, which would serve to increase clot resistance to plasminolysis. The mechanism which leads to this increase in cross-linkage is unclear, but may result from alterations in the structure of the fibrin(ogen) molecule that act to enhance the cross-linking reaction itself, or to promote the activation of FXIII by thrombin [28]. Western blot analyses did, however, indicate that the differences in clot lysis and plasmin generation between diabetic and control subjects were not due to differences in the quantity of PI that had become cross-linked to (and as such co-purified with) fibrinogen in vivo.
Previous studies have demonstrated that fibrin clots formed by diabetic subjects have a denser, less porous structure than those formed by healthy controls [8, 9]; whilst clot structure has been shown to influence its susceptibility to fibrinolysis [10, 12], modification to the structure/function of individual fibrin molecules comprising the clot could also influence its lytic susceptibility, because of their intimate involvement in the activation and regulation of the fibrinolytic process. The persistent hyperglycaemia that occurs in diabetes is associated with enhanced protein glycation, the extent of which is dependent upon ambient glucose levels and the biological half-life of the protein [29, 30]: analysis of the fibrinogen purified from 40 diabetic and control subjects using the fructosamine assay confirmed the occurrence of fibrinogen glycation in vivo. Poor glycaemic control is, however, associated with complex metabolic derangements in addition to hyperglycaemia, such as oxidative and carbonyl stress [31], which may also alter the structure of the fibrinogen molecule [32] as indicated by the results of the mass spectrometric analysis. The increase in the mean mass of individual Bβ- and γ-chains of fibrinogen purified from diabetic subjects compared with those purified from controls was not an exact multiple of that of individual glucose molecules, but rather a mixture of their subsequent complex modifications by oxidation/reduction and dehydration, although heterogeneity of plasma purified fibrinogen may have also contributed.
The free ɛ-amino groups of lysine residues are the predominant sites for glucose attachment within protein molecules [29], and as such their modification (through glycation and subsequent oxidation, dehydration, etc.) may (by altering their functional properties) contribute to the reduced fibrinolytic susceptibility of the fibrin clot, given their central role in the fibrinolytic process. Previous studies utilising fibrinogen glycated in vitro have demonstrated that glycation reduces the susceptibility of fibrin to plasmin degradation [33] and interferes with the specific fibrinolytic enzyme–substrate interaction [21]. In the study by Bobbink et al. [21] modification of fibrinogen lysine residues by acetylation and carbamylation resulted in the complete resistance of fibrin to plasminolysis. The qualitatively similar effects of glycation and these other manipulations suggest that chemical modification of lysine amino groups is responsible for the defects produced by hyperglycaemia. The process of in vitro glycation is, however, non-physiological, and may result in glycation at more sites per molecule or at a different profile of sites than would occur in vivo [34, 35], and as such the behaviour of fibrinogen may differ from that of fibrinogen glycated in vivo. Our study is the first to analyse the fibrinolytic process using fibrinogen purified from diabetic subjects, and has therefore addressed this issue by using fibrinogen that was glycated in vivo.
In summary, fibrinogen purified from subjects with type 2 diabetes generates a fibrin structure that is resistant to the actions of the fibrinolytic system. This is due to decreased binding of t-PA and plasminogen to fibrin, increased PI cross-linking, and diminished plasmin generation on the clot surface. This concatenation of events contrives to further intensify the prothrombotic and anti-fibrinolytic milieu characteristic of type 2 diabetes and may have a role in increasing vascular risk in this group. Future work needs to be directed at understanding the specific sites influenced by post-translational modifications that are responsible for the changes described in this study. This might allow the development of targeted disruption of these processes to ameliorate some of the deleterious effects on thrombotic risk of the metabolic changes associated with type 2 diabetes.
Abbreviations
- CVD:
-
Cardiovascular disease
- FXIII:
-
Factor XIII
- FXIIIa:
-
Activated Factor XIII
- NBT:
-
Nitroblue tetrazolium
- PAI-1:
-
Plasminogen activator inhibitor type 1
- PI:
-
Plasmin inhibitor (α2-antiplasmin)
- RU:
-
Resonance units
- SPR:
-
Surface plasmon resonance
- TAFI:
-
Thrombin activatable fibrinolysis inhibitor
- TBS:
-
Tris-buffered saline
- t-PA:
-
Tissue-type plasminogen activator
References
Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
UK Prospective Diabetes Study Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Westberg N, Michael A (1972) Immunohistopathology of diabetic glomerulosclerosis. Diabetes 21:163–174
Cunha-Vaz J (1978) Pathophysiology of diabetic retinopathy. Br J Ophthalmol 62:351–355
Timperley W, Ward J, Duckworth T, O’Malley B (1976) Clinical and histological studies in diabetic neuropathy. Diabetologia 12:237–243
Bini A, Fenoglio JJ, Sobel J, Owen J, Fejgl M, Kaplan K (1987) Immunohistochemical characterization of fibrinogen, fibrin I, and fibrin II in human thrombi and atherosclerotic lesions. Blood 69:1038–1045
Jorneskog G, Egberg N, Fagrell B et al (1996) Altered properties of the fibrin gel structure in patients with IDDM. Diabetologia 39:1519–1523
Dunn E, Ariens R, Grant P (2005) The influence of type 2 diabetes on fibrin structure and function. Diabetologia 48:1198–1206
Carr M, Alving B (1995) Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coagul Fibrinolysis 6:567–573
Gabriel D, Muga K, Boothroyd E (1992) The effect of fibrin structure on fibrinolysis. J Biol Chem 267:24259–24263
Collet J, Park D, Lesty C et al. (2000) Influence of fibrin network conformation and fibrin fibre diameter on fibrinolysis speed: dynamic and structural analysis approaches by confocal microscopy. Arterioscler Thromb Vasc Biol 20:1354–1361
Rowland F, Donovan M, Picciano P, Wilner G, Kreutzer D (1984) Fibrin-mediated vascular injury. Identification of fibrin peptides that mediate endothelial cell retraction. Am J Pathol 117:418–428
Seeger F, Blessing E, Gu L, Bomhold R, Denger S, Kreuzer J (2002) Fibrinogen induces chemotactic activity in endothelial cells. Acta Physiol Scand 176:109–115
Ariens R, Philippou H, Nagaswami C, Weisel J, Lane D, Grant P (2000) The factor XIII V34L polymorphism accelerates thrombin activation of factor XIII and affects cross-linked fibrin structure. Blood 96:988–995
Johnson R, Metcalf P, Baker J (1983) Fructosamine: a new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin Chim Acta 127:87–95
Brennan S (1997) Electrospray ionisation analysis of human fibrinogen. Thromb Haemost 78:1055–1058
Schroder V, Kohler H (2000) Effect of factor XIII Val34Leu on alpha 2-antiplasmin incorporation into fibrin. Thromb Haemost 84:1128–1130 (Letter)
Moskowitz K, Kudryk B, Coller B (1998) Fibrinogen coating density affects the conformation of immobilised fibrinogen: implications for platelet adhesion and spreading. Thromb Haemost 79:824–831
Siebenlist K, Mosesson M, Meh D, DiOrio J, Albrecht R, Olson J (2000) Coexisting dysfibrinogenaemia (gammaR275C) and factor V Leiden deficiency associated with thromboembolic disease (fibrinogen Cedar Rapids). Blood Coagul Fibrinolysis 11:293–304
Bobbink I, Tekelenburg W, Sixma J, de Boer H, Banga J, de Groot P (1997) Glycated proteins modulate tissue-plasminogen activator-catalyzed plasminogen activation. Biochem Biophys Res Commun 240:595–601
Carr M (2001) Diabetes mellitus: a hypercoagulable state. J Diabetes Complications 15:44–54
Ranby M (1982) Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta 704:461–469
Medved L, Nieuwenhuizen W (2003) Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost 89:409–419
Kline D, Reddy K (1980) Fibrinolysis. CRC, Boca Raton, Florida
Kimura S, Aoki N (1986) Cross-linking site in fibrinogen for alpha 2-plasmin inhibitor. J Biol Chem 261:15591–15595
Clemmensen I, Thorsen S, Mullertz S, Petersen L (1981) Properties of three different molecular forms of the alpha 2 plasmin inhibitor. Eur J Biochem 120:105–112
Naski M, Lorand L, Shafer J (1991) Characterization of the kinetic pathway for fibrin promotion of alpha-thrombin-catalyzed activation of plasma factor XIII. Biochemistry 30:934–941
Watkins N, Thorpe S, Baynes J (1985) Glycation of amino groups in protein. J Biol Chem 260:10629–10636
Shaklai N, Garlick R, Bunn H (1984) Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem 259:3812–3817
Baynes J, Thorpe S (1999) Role of oxidative stress in diabetic complications. A new perspective on an old paradigm. Diabetes 48:1–9
Shacter E, Williams J, Levine R (1995) Oxidative modification of fibrinogen inhibits thrombin catalyzed clot formation. Free Radic Biol Med 18:815–821
Brownlee M, Vlassara H, Cerami A (1983) Nonenzymatic glycosylation reduces the susceptibility of fibrin to degradation by plasmin. Diabetes 32:680–684
Shapiro R, McManus M, Zalut C, Bunn H (1980) Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem 255:3120–3127
Shilton B, Walton D (1991) Sites of glycation of human and horse liver alcohol dehydrogenase in vivo. J Biol Chem 266:5587–5592
Acknowledgements
We thank A. E. Ashcroft (School of Biochemistry and Microbiology, University of Leeds) for performing the mass spectroscopic analysis; K. Standeven (Academic Unit of Molecular Vascular Medicine, University of Leeds) for the expression of wild-type recombinant fibrinogen; and S. T. Lord (University of North Carolina, Chapel Hill, NC, USA) for the kind gift of CHO cells transfected with the human wild-type fibrinogen chains. E. Dunn is supported by a grant from Diabetes UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dunn, E.J., Philippou, H., Ariëns, R.A.S. et al. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia 49, 1071–1080 (2006). https://doi.org/10.1007/s00125-006-0197-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0197-4