Abstract
Aims/hypothesis
Type 1 diabetes mellitus is a multifactorial autoimmune disease characterised by selective destruction of beta cells in the islets of Langerhans. We have previously shown that IL-1β modulates beta cell function, causes beta cell death and induces expression changes in 82 out of 1815 protein spots detected by two-dimensional gel electrophoresis (2-DGE) in diabetes-prone bio-breeding (BB-DP) rat islets in vitro. The aim of this study was to describe the relevance of these proteins in the development of diabetes in vivo.
Methods
Syngeneic neonatal islets (n=200) were transplanted under the kidney capsule of 30-day-old BB-DP and control rats, removed to different time points after transplantation or at the onset of diabetes, and metabolically labelled with S35-methionine for 2-DGE. The 82 proteins were re-localised and followed. In addition, transplants were examined for expression of IL-1β mRNA by in situ hybridisation.
Results
All 82 proteins could be re-localised in all syngeneic transplants from BB-DP and control rats. A total of 60 of the 82 proteins were changed during development of diabetes. Of the 82 proteins, 32 were changed in expression at the onset of diabetes compared to non-diabetic BB-DP rats, and 25 of these were changed as by IL-1β in vitro. Highest expression of IL-1β mRNA was found at the onset of diabetes.
Conclusions/interpretation
IL-1β-induced protein expression changes in islets in vitro also occur in vivo and change in a complex pattern during the development of diabetes in the BB-DP rat. No single protein seems to be responsible for the development of diabetes, but rather the cumulative numbers of changes seem to interfere with the intracellular stability of the beta cell.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pro-inflammatory cytokine IL-1β is thought to play a role during destruction of beta cells in Type 1 diabetes mellitus, a multifactorial polygenetic autoimmune disease characterised by mononuclear cell infiltration in the islets of Langerhans (insulitis) and selective destruction of the insulin-producing beta cells [1, 2, 3]. It is generally accepted that the destruction of the beta cells results from interactions between various environmental factors and immune mechanisms in genetically susceptible individuals [3].
Beta cells are more sensitive to IL-1β [4], nitric oxide (NO) donors (e.g. 3-morpholinosydnonimine [SIN-1] [5] and S-nitrosoglutathione [GSNO] [6]) and streptozocin [7] than other cell types. IL-1β can be released within the islets in sufficient quantities to modulate and inhibit beta cell function in vitro [8], and beta cells can be stimulated to produce IL-1β themselves [9]. IL-1β can influence important cellular functions, such as reducing DNA content, decreasing protein synthesis and intracellular energy production, and inducing beta cell apoptosis and necrosis. During the beta cell destructive process in vivo, IL-1β, TNF-α and IFN-γ are released in the islets, resulting, for example, in production of free radicals (such as NO, super oxide [O2 −] and hydroxyl [OH−]) [3]. Free radicals are normally scavenged by protective proteins (e.g. catalase, haeme oxygenases and manganese superoxide dismutase) [10, 11, 12].
The first events in the destructive process are not yet described in detail. Many IL-1β effects are mediated through induction of the inducible NO syntase (iNOS) and its product, NO [4, 13]. However, NO-independent mechanisms also occur [14, 15, 16]. We hypothesise that the beta cell, when exposed to IL-1β, initiates a protective response in competition with a series of deleterious events, and that in beta cells the deleterious events prevail [3]. In support of this, overexpression in islets and beta cell lines of scavengers of reactive oxygen species and NO, such as catalase, glutathione peroxidase and manganese superoxide dismutase, reduces the deleterious effects of cytokines on beta cells [17, 18, 19, 20].
The diabetes-prone bio-breeding (BB-DP) rat spontaneously develops a diabetic syndrome with several characteristics in common with human Type 1 diabetes [21]. Originally, the BB-DP rat strain was bred from an outbred Wistar rat colony, and subsequent inbreeding of the BB-DP rat fixed the diabetes trait with the class II MHC haplotype RT1u similar to that of Wistar Furth (WF) rats [22]. Strain-dependent variations in beta cell sensitivity to IL-1β have been demonstrated in vitro and in vivo [23, 24], with islets from Brown Norway rats being less sensitive to IL-1β than those of WF rats, Lewis-Scripps and BB-DP, as well as diabetes-resistant BB (BB-DR) rats. BB-DP rat islets produce lower protective stress responses (heat shock protein 70 [HSP70]) than BB-DR rat islets, which may predispose BB-DP rat beta cells for destruction [25, 26]. The relative resistance to IL-1β-induced inhibition of beta cell function in vitro and in vivo in Brown Norway rat islets was associated with lower levels of expression of iNOS compared with that in Wistar Kyoto and Lewis-Scripps rat islets [24].
Proteome analysis, in the sense of two-dimensional gel electrophoresis (2-DGE), mass spectrometry and bioinformatics, applied to BB-DP and WF rat islets exposed to IL-1β has provided a detailed picture of beta cell destruction at the protein level in vitro [14, 15, 16, 27, 28].
We have previously demonstrated by proteome analysis that IL-1β-induced reproducible and statistically significant (Student’s t test, p<0.01) changes in the expression of 82 out of 1815 protein spots in BB-DP rat islets in vitro represent proteins involved in (i) energy transduction and redox potentials; (ii) glycolysis and Krebs cycle; (iii) protein, DNA and RNA synthesis, chaperoning and protein folding; (iv) signal transduction, regulation, differentiation and apoptosis; and (v) cellular defence [27, 28].
In this study we describe the possible relevance of the proteins in the spots identified in vitro for the development of diabetes in vivo in syngeneically transplanted pre-diabetic BB-DP and BB-DR and WF rat islets. Our overall aim was to follow proteins, the expression of which was influenced by IL-1β in islets of Langerhans from neonatal BB-DP rats in vitro, in syngeneic transplanted islets to BB-DP, BB-DR and WF recipients. Furthermore, we wanted to follow these proteins during the development of diabetes and to investigate to what extent IL-1β is present in the transplants.
More specifically, we aimed to investigate: (i) whether IL-1β-induced protein changes in DP islets in vitro could be found in syngeneic transplants from BB-DP, BB-DR and WF rats, and whether there are changes in expression profile during the development of diabetes in the BB-DP rat; (ii) whether changes in expression profile in BB-DP transplants were similar to changes found in BB-DP islets in vitro after IL-1β exposure; (iii) whether changes in expression profile in BB-DP transplants were different from those in BB-DR/WF transplants; and (iv) whether the protein expression profiles in syngeneic transplants in the few BB-DP animals escaping diabetes was different from those seen in age-matched syngeneic BB-DR transplants.
Materials and methods
We have previously exposed isolated neonatal BB-DP rat islets to recombinant human IL-1β in vitro and examined the protein expression pattern by proteome analysis. Out of 1815 protein spots, 82 were found to change in expression [27, 28]. To examine the putative role of these proteins in vivo during the development of diabetes in the BB-DP rat, 200 neonatal islets were isolated and syngeneically transplanted under the kidney capsule of 30-day-old BB-DP, BB-DR and WF rats. The islets were then removed after 7, 12, 17 (WF rats only), 23, 37, 48 or 174 days, or at the onset of diabetes (day 48±5) [29]. The transplants were excised immediately after cervical dislocation and metabolically labelled with [S35]-methionine. The proteins were separated by 2-DGE and the protein expression profile was analysed as previously described [27, 29]. The 82 protein spots that changed in expression in BB-DP islets after IL-1β exposure in vitro were all re-identified in the transplants and their expression was examined in the excised transplants at the different time points (n=3–6 transplants for each time point). Identical procedures were carried out in syngeneic islets transplanted to BB-DR and WF rats, which served as control rats. The expression of the 82 protein spots, reported as percentage of the integrated optical density (%IOD) of the gel, in the different transplantation groups was measured in all transplants. The expression profiles were compared over time (e.g. day 7 vs day 12, day 12 vs day 23) and between BB-DP and control transplants. Furthermore, in situ hybridisation for IL-1β mRNA was performed on transplants from BB-DP and BB-DR rats on day 7, day 23 and on day 48 in BB-DR rats and at the onset of diabetes in BB-DP rats.
Animals
The BB-DP and BB-DR rats (BB/Wor/Mol-BB2) as well as the WF rats were purchased from M&B (Lille Skensved, Denmark). All animal experiments were carried out according to national and international law, and ethical standards were approved by the Danish Council for Animal Welfare under the Ministry of Justice.
Isolation and preparation of islets of Langerhans
Islets were isolated by collagenase digestion of the pancreata from 4 to 5-day-old BB-DP, BB-DR and WF rats. For BB-DP islets, this was done between 5 and 7 days prior to transplantation and exposure to IL-1β [29, 30].
IL-1β exposure of diabetes-prone bio-breeding islets
In brief, in our previous studies, batches of 150 BB-DP islets were exposed to 150 pg/ml recombinant human IL-1β for 24 h [27] and the protein expression patterns were analysed by proteome analysis [27, 28, 31].
Islet transplantation to 30-day-old rats
A total of 200 neonatal BB-DP, BB-DR or WF islets were syngeneically transplanted under the kidney capsule of 30-day-old BB-DP, BB-DR and WF rats under sterile conditions [29, 32, 33].
Retrieval and preparation of transplants for two-dimensional gel electrophoresis
The transplants were retrieved after 7, 12, 17 (WF only), 23, 37, 48 and 174 days, or at the onset of diabetes, defined as blood glucose being above 14 mmol/l on two consecutive days [29]. The kidneys with the transplanted islets (now one coherent tissue piece) were removed immediately after cervical dislocation, placed under an operation microscope and divided into two pieces, one for immunohistochemistry and the other for 2-DGE [29]. The part for 2-DGE was carefully dissected from the kidney and the kidney capsule using a pair of scissors designed for eye surgery, and washed twice in Hanks’ Balanced Salt Solution. The approximate size of the excised transplants ranged from 1 to 2 mm3. The transplants were placed in the labelling media less than 5 min after cervical dislocation of the rat, and were metabolically labelled with [35S]-methionine for 4 h in methionine-free DMEM with 10% normal human serum dialysed for amino acids. Thereafter, the labelled transplants were washed in Hanks’ Balanced Salt Solution, were snap frozen and were then stored for 2-DGE [27]. The frozen transplants were crushed in a mortar, re-suspended in DNAse I/RNAse A solution, lysed by freeze thawing twice, and freeze dried [27].
Two-dimensional gel electrophoresis and preparative gels
Analytical two-dimensional gels (2-DG) were produced from the [35S]-methionine-labelled part of the transplants. Preparative 2-DGs were produced from a pool of approximately 200 000 neonatal WF rat islets, with 20 000 islets for each gel, to increase the amount of protein for protein identification. For localisation of the spots, radioactively labelled tracer islets were mixed with the non-labelled islets and visualised by non-equilibrium pH gradient electrophoresis (NEPHGE; pH range 6.5–10.5) and isoelectric focusing (IEF; pH range 3.5–7.0) gels. The procedure for 2-DGE has been described in detail previously [27, 28, 34, 35].
Computer analysis of two-dimensional gels and statistics
Computer analysis was performed using the BioImage program 2D-Analyzer (version 6.1). All analytical gels were matched, and the same spots on different gels assigned the same match number. Spots were quantitated, and %IODs obtained for all spots. The data were then transferred to Microsoft Excel for comparison of corresponding time points and for comparisons within each strain. Changes in expression were considered significant at a p value of less than 0.05 (Student’s t test).
Protein identification
Protein spots that changed in expression after IL-1β exposure were cut out of preparative gels and identified by matrix-assisted laser desorption/ionisation mass spectrometry (MALDI-MS) as previously described [16, 28].
Protein information
Information about the identified proteins and about putative biological functions was found in the ExPASy Molecular Biology Database at Swiss-Prot [36] and at the National Centre for Biotechnology Information (NCBI) [37].
In situ hybridisation for IL-1β mRNA
Full-length rat IL-1β was cloned into pBluescript KS+, from which a 310 bp BamH1/Sac1 fragment was cut and reinserted in the same vector. Linearisation with BamH1 or Sac1, transcription with T7 (T3) polymerase with [35S]-UTP and mild alkaline hydrolysis for 1 h at 60 °C produced the radio-labelled antisense and the sense riboprobe respectively. BB-DP and BB-DR transplant cryostat sections from day 7, 23 or 48 after transplantation or from the day of onset of diabetes were postfixed overnight in 4% paraformaldehyde. They were then rinsed and acetylated, and in situ hybridisation with the radioactive riboprobes was carried out as previously described [38]. Kidney sections with the transplant site for islets were examined under dark-field light to score the number of clusters of grains over the islet transplant area. The clusters were then expressed as clusters per mm2 of islet transplant area. The observers were blinded to the origins of the sections.
Results
In total, 2591 different protein spots were detected and quantified in all transplants. Among these, all 82 protein spots that changed expression in neonatal BB-DP islets in vitro after IL-1β exposure could be localised and followed in all syngeneic transplants from BB-DP, BB-DR and WF rats (Fig. 1). It was possible to follow the expression of each single protein spot during the development of diabetes in the syngeneic BB-DP transplants, as well as in the BB-DR and WF transplants and in the transplanted BB-DP rats escaping diabetes. The BB-DR and the WF rats were pooled to serve as one group of controls for the following reasons: (i) neither rat type develops diabetes; (ii) the two have similar transplantation antigens (genetic background) and only scarce infiltration with inflammatory cells during ageing [29]; and (iii) there are few differences in expression of the IL-1β-induced proteins between the two.
Images of two-dimensional gels of neonatal islets of Langerhans in diabetes-prone bio-breeding rats (BB-DP) (a, b) and in syngeneic BB-DP islet transplants (c, d). The gels shown are representative of three to six independent experiments, and the marked proteins represent proteins significantly changing in expression after 24 h of exposure to IL-1β in vitro with and without number identities. Isoelectric focusing (IEF) gel (pH 3.5–7) is on the right-hand side and non-equilibrium pH gradient electrophoresis (NEPHGE) gel (pH 6.5–10.5) is on the left-hand side. All 82 protein spots found in vitro were found in all transplants. The numbers correspond to the protein numbers in Tables 1 and 2
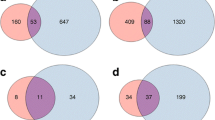
As the 82 in vitro IL-1β-changed proteins were followed through consecutive time points (day 7 to day 12, day 12 to 23 etc) in BB-DP and BB-DR/WF, 72 of the 82 protein spots significantly changed at one or more time points (Fig. 2). Of these 72 proteins, 28 changed over time (from day 7 to day 174 after transplantation) in both groups, 32 changed in expression in BB-DP transplants only, and as such were “specific” for the development of diabetes, and 12 changed specifically in the BB-DR/WF transplants (Fig. 2 and Table 1). Of the 82 proteins, 22 (twelve only in BB-DR/WF and ten not changed in any group) were not affected at any time point during the development of diabetes (Fig. 2 and Table 1). In the control rats (BB-DR/WF), 42 of the 82 proteins were unaffected over time (day 7 to day 12, day 12 to 17/23 etc). Examples of proteins changing expression in the BB-DP or BB-DR/WF transplants over time or between the two groups are shown in Figure 3.
Number of proteins among the 82 proteins changed in vitro, found to be changed in diabetes-prone bio-breeding rat islet (BB-DP) and diabetes-resistant bio-breeding/Wistar Furth rat islet (BB-DR/WF) transplants over time (e.g. from day 7 to day 12, from day 12 to day 23). The number of proteins that do not change in expression in vivo is given in brackets. Of the 82 proteins, 60 in the BB-DP and 40 in the BB-DR/WF transplants change in expression at one or more time points. Most changes are seen in the BB-DP transplants, and ten of the 82 proteins do not change in expression in vivo at all
Examples of protein expression profiles for diabetes-prone bio-breeding rat islet (BB-DP) and diabetes-resistant bio-breeding/Wistar Furth islet (BB-DR/WF) transplants. Shown are the average percentages of optical density (%IOD; n=3–6) and standard deviations for all time points analysed in both groups. Significant differences (p<0.05) in expression over time or between the two groups are indicated by *. Changes over time are written with the profiles (e.g. DP12 vs DP23) and changes between groups are marked with * only. DM, at the onset of diabetes mellitus; DP, BB-DP; DRWF, BB-DR/WF; I, isoelectric focusing gel; N, non-equilibrium pH gradient electrophoresis gel; squares, BB-DR/WF; triangles, BB-DP
Changes in expression (Table 1) were observed in 32 of the 82 proteins at the onset of diabetes compared with non-diabetic BB-DP rats on day 48 after transplantation (average time of onset is day 48±5). Of these 32 proteins, the expression of the majority (25) was changed in the same direction, as previously demonstrated in islets exposed to IL-1β in vitro [28]. Comparing transplants from the day of onset of diabetes in the BB-DP rats with age-matched BB-DR/WF rats (day 48), 19 of the 82 proteins were changed at the onset of diabetes and 16 of these were changed in the same direction as they were by IL-1β in vitro (Table 2). Comparing these 32 (BB-DP 48 vs diabetes [Table 1]) and 19 (diabetes vs BB-DR/WF day 48 [Table 2]) proteins, 14 were common to both groups (N: 370, 1247, 279, 207 and I: 660, 6347, 293, 266, 1139, 418, 6363, 2410, 8311 and 712). Except for two proteins (N: 1247 and I: 712), these proteins changed their expression in the same way as seen previously when induced by IL-1β in vitro. Removing these 14 “common proteins” from the 32 proteins leaves only 18 proteins that changed and were specific for the changes seen from day 48 in BB-DP compared with at the onset of diabetes (N: 268, 1355, 272, 317, 381, 284, 509, 212, 68 and I: 706, 75, 237, 62, 217, 1096, 8264, 683, 838).
Comparing transplants from BB-DP rats escaping diabetes (174 days after transplantation) and BB-DR rats 174 days after transplantation, only five of the 82 protein spots differed in expression (N: 377 and I: 248, 1202, 6585 and 712) (Table 2). These five proteins are galectin-3 (N 377, involved in cell differentiation and apoptosis), 25-Dx (I 1202, membrane-associated progesterone receptor component with high homology to the IL-6 receptor), adenosine phosphoribosyltransferase/UMP-CMP kinase (I 248, both involved in purine/pyrimidine synthesis) and two unidentified proteins (I 6585 and I 712) (Tables 1 and 2).
The presence of mRNA for IL-1β in the BB-DP and BB-DR transplants was assessed by in situ hybridisation on day 7, 23 and 48 and at the onset of diabetes (Fig. 4), and demonstrated that mRNA of IL-1β is present early on in the development of diabetes. A significant difference in IL-1β mRNA at the onset of diabetes compared with on day 48 in BB-DR was observed (p=0.00052), whereas no significant differences were observed on day 7 and 23 (p=0.45 and 0.78 respectively).
In situ hybridisation for IL-1β in transplants. IL-1β mRNA expression in diabetes-prone bio-breeding rat islet (BB-DP) and diabetes-resistant bio-breeding rat islet (BB-DR) transplants on day 7, 23 and 48 after transplantation or at the onset of diabetes. IL-1β expression is expressed as clusters of grains of mRNA for IL-1β per mm2 transplant in BB-DP (n=6) and BB-DR (n=3). Error bars indicate standard deviation. IL-1β mRNA was present at all time points examined, and significantly higher expression of IL-1β mRNA is observed in BB-DP transplants at the onset of diabetes than in BB-DR transplants. *** p=0.00052; DM, at the onset of diabetes mellitus; black, BB-DP; white, BB-DR
Among the previously unidentified proteins, we have identified proteins from six spots (Table 1), owing to the constantly increasing number of proteins in the databases used, and to improvement of the search programs. With these six new identifications, the total number of spots with identified proteins goes up to 57 out of 82 (69% identification rate).
Discussion
IL-1β induces time- and dose-dependent changes in beta cells, affecting function, biosynthesis and gene transcription, and induces cell death through different pathways, e.g. apoptosis and necrosis [4, 39, 40, 41]. We have previously demonstrated by proteome analysis that IL-1β significantly influences the expression of 82 protein spots in BB-DP islets [27]. Of these 82, we have initially identified the proteins in 51 spots, corresponding to 45 different proteins [28]. Among the previously unidentified proteins we have subsequently identified proteins in six additional spots after new searches in the constantly growing databases.
In the present study we were able to identify all of the 82 protein spots with changes in expression in BB-DP rat islets in vitro after exposure to IL-1β in syngeneically transplanted BB-DP, BB-DR and WF rat islets. These 82 proteins were followed in the transplants throughout the development of diabetes and in transplants from BB-DP rats escaping diabetes, and followed to corresponding time points in syngeneic BB-DR/WF transplants. Furthermore, we have shown the presence of mRNA for IL-1β in syngeneic BB-DP islet transplants early on in the pre-diabetic period, suggesting that IL-1β is present when minimal cellular infiltration is seen in the transplants [29]. There was no significant difference between BB-DP and BB-DR in IL-1β expression prior to development of diabetes. Combined with the number of expression changes in the transplants 7 days after transplantation, this suggests a role of IL-1β in influencing the protein expression pattern and in the development of diabetes. The cellular origin of mRNA for IL-1β in the transplants remains unknown. Macrophages, either resident or invading, could be a source for the IL-1β. IL-1β can be released in islets in quantities sufficient to influence beta cell function from macrophages or from beta cells themselves, which have been shown to be able to produce IL-1β [8, 9]. The production of IL-1β in beta cells may be induced by elevated glucose concentration in vitro in the absence of an autoimmune process [42]. In vivo, this effect may accelerate and perpetuate the destruction of beta cells when an autoimmune destruction is ongoing and blood glucose level is rising. The transplanted rats all had normal blood glucose values until immediately prior to and at the onset of diabetes [29]. Taken together, these observations suggest that IL-1β is present early on in the pre-diabetic period and may participate in the initiation of diabetes in the BB-DP rat through induction of changes in the protein expression profile that are different from the changes in BB-DR transplants. These protein changes may be involved in changing the state of the beta cell from one of dynamic stability into one of dynamic instability, thereby leading to destruction [43].
The 18 proteins specific for the changes seen on day 48 in BB-DP compared with at the onset of diabetes (N: 268, 1355, 272, 317, 381, 284, 509, 212, 68 and I: 706, 75, 237, 62, 217, 1096, 8264, 683, 838) represent proteins and functional entities from most functional groups influenced by IL-1β in vitro [28]. Furthermore, most of these proteins are changed as by IL-1β in vitro, further suggesting that islet destruction in vitro as well as in vivo involves the same pathways and proteins. The complexity of the effects of the changes described here substantiates the idea that development of Type 1 diabetes is the result of a collective, dynamic instability, rather than of a single factor [43].
Not all of the changes that were previously observed in vitro were seen in the present BB-DP and BB-DR/WF transplant experiments. Ten of the 82 protein spots did not change in expression, suggesting no major role for these proteins in vivo, and leaving 72 proteins with a potential role in diabetes development. The highest number of changes is found when comparing the BB-DP transplants from day 48 (non-diabetic) with those from the day of onset of diabetes (day 48±5 days after transplantation). Most of these (25 out of 32) are changed, as seen previously, by IL-1β exposure in vitro. This correlates well with the time point with the highest expression of mRNA for IL-1β. Of the 32 proteins, 18 are “diabetes specific” (seen only in BB-DP) and most of them change expression levels as seen in islets exposed to IL-1β in vitro. This supports the relevance of data obtained in vitro from IL-1β-exposed BB-DP islets, and further suggests that the effects of IL-1β on protein expression are relevant and important during diabetes development. It also suggests that IL-1β may play a role in the pathogenesis of diabetes [3, 4].
During destruction of the islets in the transplant, the transplants are infiltrated with inflammatory cells [29]. Proteins from the inflammatory cells and fibrous tissue are isolated from the transplant together with islet proteins, and can potentially “dilute” the protein’s profile from the islets. In the study of the IL-1β-exposed islets, p values below 0.01 were considered statistically significant, which in this study corresponds to changes that are approximately two-fold or greater [27]. This may, however, result in an underestimation of the number of changes in response to IL-1β in vitro and thereby also in vivo.
Furthermore, it is important to remember that a plethora of cytokines, including IL-1β, are involved during beta cell destruction in vivo [44], and that IL-1β exposure of islets is a simplified model for beta cell destruction. The BB-DP transplantation model reflects the spontaneous development of diabetes, with its known and unknown influencing factors and their interactions [21]. Using other cytokines or combinations of cytokines and exposure time in vitro may give another protein expression profile, as seen in mRNA expression studies of sorted beta cells and beta cell lines [45, 46, 47]. However, we build on data from previous studies by our group showing that the chosen concentration of IL-1β has substantial impact on rat beta cell function and viability [4, 24, 48, 49, 50, 51].
Throughout life, the beta cell mass changes dependently upon function and demand [52], and re-organisation and re-vascularisation take place in transplanted islets [53]. Since beta cell mass changes during life [54], a number of proteins are expected to change in expression throughout life as part of normal processes. In rats that do not develop diabetes (BB-DR/WF transplants), proteins that change expression over time may in part originate from the normal processes of ageing and beta cell turnover. Hence, such proteins may be of minor interest for the disease process, and are putatively removed from the list of proteins of particular interest for the development of diabetes in our model system. On the other hand, this study does not allow us to give importance to small and statistically non-significant changes in protein expression levels and to decide whether many minor changes together are what produce beta cell destruction and diabetes.
Fewer changes in protein expression are seen in the BB-DR/WF transplants over time than in the BB-DP transplants, suggesting genetically influenced differences in IL-1β sensitivity. Other studies have shown strain-dependent differences in beta cell function using the same concentration of IL-1β and genetically influenced differences in IL-1β sensitivity [24, 55]. Proteins that only changed in the BB-DP rats as well as proteins only expressed in rats not developing diabetes are therefore of particular interest for the development of diabetes and for protection against the development of diabetes respectively.
As expected, in BB-DP rats escaping diabetes (day 174 after transplantation) and in age-matched BB-DR rats, few proteins (five) are expressed differently. This is the lowest number of proteins in all the comparisons between BB-DP and BB-DR/WF transplants, suggesting that BB-DP rats escaping diabetes are more similar to BB-DR rats regarding IL-1β-induced proteins. Interestingly, these proteins are galectin-3, 25-Dx, adenosine phosphoribosyltransferase/UMP-CMP kinase (two proteins identified in the same spot) and two unidentified proteins. Four of these (galectin-3, 25-Dx, adenosine phosphoribosyltransferase/UMP-CMP kinase and one unidentified protein) changed in expression, as detected previously by IL-1β exposure in vitro.
Galectin-3, an inhibitor of apoptosis [56, 57, 58], was up-regulated in three spots in the IL-1β-exposed BB-DP islets in vitro [28] and was found to be less expressed in BB-DR transplants than in BB-DP transplants escaping diabetes. This may suggest a lower need for the inhibition of apoptosis in BB-DR transplants.
We found 25-Dx, a receptor for progesterone, to be down-regulated after IL-1β exposure of BB-DP islets and up-regulated in BB-DR transplants compared with BB-DP transplants. The protein 25-Dx has 71% homology with the transmembrane domain of the precursor for the IL-6 receptor [59]. IL-6 is a pro-inflammatory cytokine also involved in autoimmune diseases through regulation of the immune response [60], which suggests that there is a difference in regulation of the immune response between BB-DP and BB-DR rats.
Adenosine phosphoribosyltransferase/UMP-CMP kinase are both involved in purine/pyrimidine synthesis. DNA/RNA synthesis was down-regulated in BB-DP islets and in BB-DP rats escaping diabetes compared with that in BB-DR rats, suggesting a down-regulation of DNA and RNA synthesis in BB-DP rats as a result of, for example, less repair or regeneration.
Of the newly identified proteins, three seem to be of particular interest: Rho GDP dissociation inhibitor alfa (I 266), peroxiredoxin 2 (I 242) and pyridoxal kinase (I 418). Rho GDP dissociation inhibitor is an endogenous inhibitor of Rho small GTPases, which are required for gene transcription through the mitogen-activated protein kinase pathway, cytokinesis, growth and cell shape changes [61, 62]. The Rho GDP dissociation inhibitor is up-regulated by IL-1β in vitro in BB-DP islets [28], and changed in BB-DP transplants but not in the BB-DR/WF transplants. This suggests a role for Rho GDP dissociation inhibitor in the development of diabetes.
Peroxiredoxin 2 is involved in reducing hydrogen peroxide levels and resistance to TNF-α-induced apoptosis [63]. It is down-regulated in IL-1β-exposed BB-DP islets, and up-regulated in BB-DP transplants from day 7 to 12 and in BB-DR/WF transplants on day 12 compared with BB-DP transplants on day 12. This is suggestive of a protective role for this scavenging protein in BB-DP transplants in the early phases of the development of diabetes. The role is lost as development proceeds.
Pyridoxal kinase is necessary for the conversion of B6-vitamins to pyridoxal-5-phosphate, an essential cofactor for numerous enzymatic reactions of intermediary metabolism [64]. The gene for pyridoxal kinase is located on chromosome 21q22.3 [65] close to the AIRE gene for autoimmune polyglandular disease type 1 [66, 67, 68]. Here, IL-1β down-regulated pyridoxal kinase in BB-DP islets in vitro as it was at the onset of diabetes in BB-DP rats, which suggests a role for pyridoxal kinase in the pathogenesis of diabetes.
The proteins that are commonly changed in both BB-DP and BB-DR/WF over time (28 proteins) may represent proteins that change during ageing in normal islet ageing, and as such are not of primary interest in the pathogenesis of diabetes.
The findings in the present study reflect the specific experimental conditions, e.g. concentration of IL-1β, exposure time and labelling interval, as well as the general culture conditions and methodological limitations. This is reflected in, for example, the expression of the iNOS protein, which is known to change in response to IL-1β, but is not tracked in this study. One possible explanation for this may be that the iNOS protein has an isoelectric point of 6.89 and a molecular weight of 130 Mr, which places the iNOS protein spot near the border of the IEF and the NEPHGE gels covering pH in the ranges of 3.5–7.0 and 6.5–10.5 respectively. The resolution and reproducibility of the gels in the border areas are inaccurate. Using other gels with different pH ranges may solve this problem.
In summary, in this study we have tracked and followed IL-1β-induced changes in protein expression in vitro in an in vivo model and related these changes to the development of diabetes in the BB-DP rat. Furthermore, we have produced a rather detailed picture of protein expression during the development of diabetes in the BB-DP rat and produced evidence to suggest that IL-1β-induced in vitro protein expression changes in islets also occur in vivo. No single protein seems to be responsible for the development of diabetes, but rather the cumulative numbers of changes seem to interfere with the stability of the beta cell, thereby pushing it towards destruction and towards the development of diabetes.
Abbreviations
- BB-DP:
-
diabetes-prone bio-breeding
- BB-DR:
-
diabetes-resistant bio-breeding
- IEF:
-
isoelectric focusing
- iNOS:
-
inducible NO syntase
- %IOD:
-
percentage of the integrated optical density
- MALDI-MS:
-
matrix-assisted laser desorption mass spectrometry
- NCBI:
-
National Centre for Biotechnology Information
- NEPHGE:
-
non-equilibrium pH gradient electrophoresis
- NO:
-
nitric oxide
- O2 − :
-
super oxide
- OH− :
-
hydroxyl
- S35 :
-
sulphur 35
- 2-DG:
-
two-dimensional gel
- 2-DGE:
-
two-dimensional gel electrophoresis
- WF:
-
Wistar Furth
References
Gepts W (1965) Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14:619–633
Junker K, Egeberg J, Kromann H, Nerup J (1977) An autopsy study of the islets of Langerhans in acute onset juvenile diabetes mellitus. Acta Pathol Microbiol Scand Sect Pathol 85:699–706
Nerup J, Mandrup-Poulsen T, Helqvist S et al. (1994) On the pathogenesis of IDDM. Diabetologia 37 [Suppl 2]:S82–S89
Mandrup-Poulsen T (1996) The role of interleukin-1 in the pathogenesis of insulin-dependent diabetes mellitus. Diabetologia 39:1005–1029
Green IC, Delaney CA, Cunningham JM, Karmiris V, Southern C (1993) Interleukin-1 beta effects on cyclic GMP and cyclic AMP in cultured rat islets of Langerhans-arginine-dependence and relationship to insulin secretion. Diabetologia 36:9–16
Eizirik DL, Delaney CA, Green MH et al. (1996) Nitric oxide donors decrease the function and survival of human pancreatic islets. Mol Cell Endocrinol 118:71–83
Bonner-Weir S, Trent DF, Honey RN, Weir GC (1981) Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes 30:64–69
Corbett JA, McDaniel ML (1995) Intraislet release of interleukin 1 inhibits beta-cell expression of inducible nitric oxide synthase. J Exp Med 181:559–568
Heitmeier MR, Arnush M, Scarim AL, Corbett JA (2001) Pancreatic beta-cell damage mediated by beta-cell production of interleukin-1—a novel mechanism for virus-induced diabetes. J Biol Chem 276:11151–11158
Asayama K, Kooy NW, Burr IM (1986) Effect of vitamin E deficiency and selenium deficiency on insulin secretory reserve and free radical scavenging systems in islets: decrease of islet manganosuperoxide dismutase. J Lab Clin Med 107:459–464
Sumoski W, Baquerizo H, Rabinovitch A (1989) Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia 32:792–796
Tiedge M, Lortz S, Munday R, Lenzen S (1998) Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes 47:1578–1585
Eizirik DL, Flodström M, Karlsen AE, Welsh N (1996) The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia 39:875–890
Andersen HU, Fey SJ, Mose Larsen P et al. (1997) Interleukin-1beta induced changes in the protein expression of rat islets: a computerized database. Electrophoresis 18:2091–2103
John N, Andersen H, Fey S et al. (2000) Cytokine or chemically-derived nitric oxide alters the expression of proteins detected by two-dimensional gel electrophoresis in neonatal rat islets of Langerhans. Diabetes 49:1819–1829
Mose Larsen P, Fey SJ, Larsen MR et al. (2001) Proteome analysis of IL-1β induced changes in protein expression in rat islets of Langerhans. Diabetes 50:1056–1063
Benhamou PY, Moriscot C, Richard MJ et al. (1998) Adenovirus-mediated catalase gene-transfer reduces oxidant stress in human, porcine and rat pancreatic-islets. Diabetologia 41:1093–1100
Lortz S, Tiedge M, Nachtwey T, Karlsen AE, Nerup J, Lenzen S (2000) Protection of insulin-producing RINm5F cells against cytokine-mediated toxicity through overexpression of antioxidant enzymes. Diabetes 49:1123–1130
Bertera S, Crawford ML, Alexander AM et al. (2003) Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes 52:387–393
Lortz S, Tiedge M (2003) Sequential inactivation of reactive oxygen species by combined overexpression of SOD isoforms and catalase in insulin-producing cells. Free Radic Biol Med 34:683–688
Bone AJ (2000) Animal models of type I diabetes. Curr Opin Oncol Endocr Metab Invest Drugs 2:192–200
Nakhooda AF, Like AA, Chappel CI, Murray FT, Marliss EB (1977) The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes 26:100–112
Andersen HU, Mandrup-Poulsen T, Egeberg J, Helqvist S, Nerup J (1989) Genetically determined differences in newborn rat islet sensitivity to interleukin-1 in vitro: no association with the diabetes prone phenotype in the BB-rat. Acta Endocrinol (Copenh) 120:92–98
Reimers JI, Andersen HU, Mauricio D et al. (1996) Strain dependent differences in sensitivity of rat beta-cells to IL-1beta in vitro and in vivo: Association with islet nitric oxide synthesis. Diabetes 45:771–778
Bellmann K, Hui L, Radons J, Burkart V, Kolb H (1997) Low stress response enhances vulnerability of islet cells in diabetes-prone BB rats. Diabetes 46:232-236
Wachlin G, Heine L, Kloting I, Dunger A, Hahn HJ, Schmidt S (2002) Stress response to pancreatic islets from diabetes prone BB rats of different age. Autoimmunity 35:389–395
Christensen UB, Larsen PM, Fey SJ et al. (2000) Islet protein expression changes during diabetes development in islet syngrafts in BB-DP rats and during rejection of BB- DP islet allografts. Autoimmunity 32:1–15
Sparre T, Christensen UB, Larsen PM et al. (2002) IL-1β induced protein changes in diabetes prone BB rat islets of Langerhans identified by proteome analysis. Diabetologia 45:1550–1561
Christensen U, Sparre T, Cooke A, Andersen H, Mandrup-Poulsen T, Nerup J (1998) Syngeneic islet transplantation in prediabetic BB-DP rats-a synchronized model for studying beta-cell destruction during the development of IDDM. Autoimmunity 28:91–107
Brunstedt J, Nielsen JH, Lernmark Å, The Hagedorn Study Group (1984) Isolation of islets from mice and rats. In: Larner J, Pohl SL (eds) Methods in diabetes research. Laboratory methods, part C, vol 1. Wiley, New York, pp 254–288
Karlsen AE, Sparre T, Nielsen K, Nerup J, Pociot F (2001) Proteome analysis—a novel approach to understand the pathogenesis of Type 1 diabetes mellitus. Disease Mark 17:205–216
Weringer EJ, Like AA (1988) Identification of T cell subsets and class I and class II antigen expression in islet grafts and pancreatic islets of diabetic BioBreeding/Worcester rats. Am J Pathol 132:292–303
Korsgren O, Jansson L, Sandler S, Andersson A (1990) Hyperglycemia-induced B cell toxicity. The fate of pancreatic islets transplanted into diabetic mice is dependent on their genetic background. J Clin Invest 86:2161–2168
O’Farrell PZ, Goodman HM, O’Farrell PH (1977) High resolution two dimentional electrophoresis of basic as well as acidic proteins. Cell 12:1133–1142
Fey SJ, Nawrocki A, Larsen MR et al. (1997) Proteome analysis of Saccharomyces cerevisiae: a methodological outline. Electrophoresis 18:1361–1372
Swiss-Prot. ExPASy Molecular Biology Server. Available from http://www.expasy.org, last accessed 17 October 2002, last updated 17 October 2002
The National Center for Biotechnology Information (NCBI). Available from http://www.ncbi.nlm.nih.gov, last accessed 17 October 2002, last updated 16 October 2002
Bokvist K, Olsen HL, Hoy M et al. (1999) Characterisation of sulphonylurea and ATP-regulated K+ channels in rat pancreatic A-cells. Pflugers Arch 438:428–436
Ankarcrona M, Dypbukt JM, Brune B, Nicotera P (1994) Interleukin-1 beta-induced nitric oxide production activates apoptosis in pancreatic RINm5F cells. Exp Cell Res 213:172–177
Kaneto H, Fujii J, Seo HG et al. (1995) Apoptotic cell-death triggered by nitric-oxide in pancreatic beta-cells. Diabetes 44:733–738
Vassiliadis S, Dragiotis V, Protopapadakis E et al. (1999) The destructive action of IL-1alpha and IL-1beta in IDDM is a multistage process: evidence and confirmation by apoptotic studies, induction of intermediates and electron microscopy. Mediators Inflamm 8:85–91
Maedler K, Sergeev P, Ris F et al. (2002) Glucose-induced beta cell production of IL-1 beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860
Freiesleben De Blasio B, Bak P, Pociot F, Karlsen AE, Nerup J (1999) Onset of type 1 diabetes: a dynamical instability. Diabetes 48:1677–1685
Mathis D, Vence L, Benoist C (2001) Beta-cell death during progression to diabetes. Nature 414:792–798
Rieneck K, Bovin LF, Josefsen K, Buschard K, Svenson M, Bendtzen K (2000) Massive parallel gene expression profiling of RINm5F pancreatic islet b-cells stimulated with interleukin-1b. APMIS 108:855–872
Cardozo AK, Kruhøffer M, Leeman R, Ørntoft T, Eizirik DL (2001) Identification of novel cytokine induced genes in pancreatic b-cells by high density oligonucleotide arrays. Diabetes 50:909–920
Kutlu B, Cardozo AK, Darville MI et al. (2003) Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes 52:2701–2719
Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH (1986) Affinity-purified human interleukin 1 is cytotoxic to isolated islets of Langerhans. Diabetologia 29:63–67
Helqvist S, Polla BS, Johannesen J, Nerup J (1991) Heat shock protein induction in rat pancreatic islets by recombinant human interleukin 1beta. Diabetologia 34:150–156
Andersen HU, Jørgensen KH, Egeberg J, Mandrup-Poulsen T, Nerup J (1994) Nicotinamide prevents interleukin-1 effects on accumulated insulin release and nitric oxide production in rat islets of Langerhans. Diabetes 43:770–777
Karlsen AE, Andersen HU, Vissing H et al. (1995) Cloning and expression of cytokine-inducible nitric oxide synthase cDNA from rat islets of Langerhans. Diabetes 44:753–758
Finegood DT, Scaglia L, Bonner-Weir S (1995) Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44:249–256
Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC (1996) Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 45:1161–1167
Bonner-Weir S (2001) Beta-Cell turnover: Its assessment and implications. Diabetes 50:S20–S24
Johannesen J, Pociot F, Karlsen AE, Mandrup-Poulsen T, Nerup J (2001) Strain-dependent difference in inducible nitric oxide synthesis (NOS) expression in rat pancreatic islets correlates with interferon regulating factor 1 (IRF-1) and heat shock protein 70 (HSP70) expression. Eur Cytokine Network 12:501–509
Lotz MM, Andrews CW, Korzelius CA et al. (1993) Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci USA 90:3466–3470
Hsu DK, Dowling CA, Jeng KCG, Chen JT, Yang RY, Liu FT (1999) Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer 81:519–526
Yang R (2003) Galectins in cell growth and apoptosis. Cell Mol Life Sci 60:267–276
Selmin O, Lucier GW, Clark GC et al. (1996) Isolation and characterization of a novel gene induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Carcinogenesis 17:2609–2615
Ishihara K, Hirano T (2002) IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev 13:357–368
Sasaki T, Takai Y (1998) The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun 245:641–645
Toksoz D, Merdek KD (2002) The Rho small GTPase: functions in health and disease. Histol Histopath 17:915–927
Rabilloud T, Heller M, Gasnier F et al. (2002) Proteomics analysis of cellular response to oxidative stress—evidence for in vivo overoxidation of peroxiredoxins at their active site. J Biol Chem 277:19396–19401
McCormick DB, Chen H (1999) Update on interconversions of vitamin B-6 with its coenzyme. J Nutr 129:325–327
Hanna MC, Turner AJ, Kirkness EF (1997) Human pyridoxal kinase. cDNA cloning, expression, and modulation by ligands of the benzodiazepine receptor. J Biol Chem 272:10756–10760
Aaltonen J, Björses P, Sandkuijl L, Perheentupa J, Peltonen L (1994) An autosomal locus causing autoimmune disease: autoimmune polyglandular disease type I assigned to chromosome 21. Nat Genet 8:83–87
Bjorses P, Aaltonen J, Vikman A et al. (1996) Genetic homogeneity of autoimmune polyglandular disease type I. Am J Hum Genet 59:879–886
Meyer G, Badenhoop K (2002) Autoimmune regulator (AIRE) gene on chromosome 21: Implications for autoimmune polyendocrinopathy-candidiasis—ectodermal dystrophy (APECED) and more common manifestations of endocrine autoimmunity. J Endocrinol Invest 25:804–811
Acknowledgements
The skilful technical assistance of Ellis Schjerning, Lotte Christensen, Andrea Lorentzen and Viola Mose Larsen is highly appreciated, as is the secretarial assistance of Nina Meier. The study was in part supported by the Danish Diabetes Association, the University of Copenhagen, the King Christian X’s Foundation, the Højmosegård Foundation, the Juvenile Diabetes Foundation International (grant no. DK-96-012 and RFA, grant no. 998005), Novo Nordisk, the Danish Biotechnological Instrument Centre, a Biotech grant from the Danish Medical Research Council (grant no. 9502027), the Sehested Hansen Foundation and the Michaelsen Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sparre, T., Bjerre Christensen, U., Gotfredsen, C.F. et al. Changes in expression of IL-1β influenced proteins in transplanted islets during development of diabetes in diabetes-prone BB rats. Diabetologia 47, 892–908 (2004). https://doi.org/10.1007/s00125-004-1382-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1382-y