Abstract
Aims/hypothesis
The electrons of the glycolysis-derived reduced form of NADH are transferred to mitochondria through the NADH shuttle system. There are two NADH shuttles: the glycerol phosphate and malate-aspartate shuttle. Mice with a targeted disruption of mitochondrial glycerol-3-phosphate dehydrogenase, a rate-limiting enzyme of the glycerol phosphate shuttle, are not diabetic and have normal islet glucose-induced secretion. In this study, we analyzed if environmental factors, such as a high carbohydrate diet could contribute to the development of Type 2 diabetes mellitus in mice with a specific defective genetic background.
Methods
The mice were fed with a high carbohydrate diet for 1 and 6 months, and several biochemical parameters were analysed. The mitochondrial respiratory activity was assayed by polarography; and the islet function was studied by islet perifusion and pancreas perfusion.
Results
The high carbohydrate diet induced hyperglycaemia, hyperinsulinaemia, and islet hyperplasia in the wild-type and heterozygote mice. Activity of the respiratory chain complex I also increased in these mice. In contrast, these effects were not observed in the null mice fed with the diet; in addition, these null mice had an increased insulin sensitivity compared to wild-type mice.
Conclusion/interpretation
The phenotype of the mice with an impairment of NADH shuttles does not worsen when fed a high carbohydrate diet; moreover, the diet does not compromise islet function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the beta cell, mitochondria exert a pivotal role in the regulation of glucose-induced insulin secretion, and mitochondrial activation is required for normal signal transduction [1]. There are at least two NADH shuttles: the glycerol-phosphate shuttle [2] and the malate-aspartate shuttle [3]. Both are essential contributors to redox traffic between the cytosolic and mitochondrial compartments. The glycerol-phosphate shuttle is thought to be a direct link in the metabolic pathway between glycolysis and mitochondrial oxidation, and has been reported to be most active in tissues that rapidly oxidize glucose, such as testis and pancreatic beta cells [4].
Mitochondrial glycerol-3-phosphate dehydrogenase (mGPDH) is a rate-limiting enzyme for the glycerol phosphate shuttle [5]. Activity of this enzyme has been reported to be low in a variety of animal models of diabetes such as neo-natal streptozotocin [6, 7], Goto-Kakizaki [8, 9] and zucker diabetic fatty rats [10]. Moreover, similar results have been obtained from human islets from Type 2 diabetic patients [11]. Therefore, several authors have suggested that mGPDH could play an important role in the pathogenesis of Type 2 diabetes [11, 12, 13]. To determine the role of the glycerol-3-phosphate shuttle in glucose-induced insulin secretion, mice with a targeted disruption of the mGPDH gene were generated [14, 15]. mGPDH –/– knockout mice are not diabetic, only when the activities of both shuttles are halted by the use of aminooxyacetate, to block the malate-aspartate shuttle, are insulin secretion, glucose metabolism, and calcium oscillations severely impaired [16, 17].
Type 2 diabetes is a multifunctional disease, in which both genetic and environmental factors are involved [18, 19]. There are reports of previously non-diabetic knockout mice becoming diabetic when challenged with an environmental factor such as diet [20, 21]. Using mGPDH knockout mice, we studied the effects of a high carbohydrate diet; analyzing whether a combination of an environmental factor such as a high carbohydrate diet and a genetic defect could contribute to the development of Type 2 diabetes.
Material and methods
Mice
The mGPDH –/– C57BL/6J mice were a gift from Dr. K. Eto from the Department of Metabolic Diseases, University of Tokyo (Tokyo, Japan). Principles of laboratory animal care were followed (EU and local government guidelines) and protocols were approved by the Animal Research Committee of the University of Barcelona. The mice were maintained at a constant temperature of 22°C with a fixed artificial light cycle (12-h light/dark cycle). Mice were given either a normal diet (Panlab A04 with 59% carbohydrates, 17% protein, and 3% fat; and 317 Kcal/100 g) or a high carbohydrate diet (ICN High Carbohydrate Diet with 70% carbohydrates, 19.5% protein, and 3% fat; and 397 Kcal/100 g). At 2 months of age male mice were fed for either 1 or 6 months, with free access to both food and fluid. During the experiment food and fluid intake, body-weight gain, and glycaemia were monitored. At the end of the experiment, the mice were killed and plasma insulin, cholesterol, triglyceride and NEFA values were measured. Plasma triglycerides and cholesterol were measured spectrophotometrically by standard techniques (Bayer Diagnostics, Germany). Free fatty acids were measured by enzymatic colorimetric assay (Roche Diagnostics, Switzerland).
Assessment of glucose and insulin tolerance test
The intraperitoneal glucose tolerance test was carried out in male mice fasted for 16 h, and glucose (150 mg/kg body weight) was administered intraperitoneally after anaesthesia with sodium pentobarbital (60 mg/kg). Blood was sampled from the tail vein before, and at 15, 30, 60, 120 and 180 min after glucose administration. Blood glucose concentrations were measured using a Glucometer Elite (Química Farmaceutica Bayer, Barcelona, Spain). Insulin plasma was also measured by ELISA method (Mercodia, Uppsala, Sweden). In the insulin tolerance test, human insulin (0.75 U/kg) was injected intraperitoneally in anaesthetized adult mice after fasting for 16 h. Blood samples were taken from the tail vein.
Pancreatic islet isolation and insulin secretion
Islets from mGPDH –/–, mGPDH –/+ and mGPDH +/+ were isolated by a modification of the method described previously [22]. In brief, 2 ml of Hanks' solution containing collagenase (Roche Diagnostics, Switzerland) was injected into the duct; the swollen pancreas was removed and incubated at 37°C for 6 min, islets were collected manually. Insulin secretion was measured from islets incubated with 95% air/5% CO2 at 37°C using a temperature-controlled multi-chamber perifusion system [23]. Groups of 50 islets were placed in four parallel perifusion chambers. The perifusion system used two computer-controlled peristaltic pumps to allow the perfusate glucose concentration to increase progressively from 2.8 to 16.7 mmol/l over 60 min. Insulin concentrations were measured in the effluent perfusate every min.
In situ pancreatic perfusions
Mice were anaesthetized and prepared for pancreas perfusion as described elsewhere [24]. The pancreas was perfused at 37°C and maintained at 1.5 ml/min. The pancreatic effluent of the first 30 min of perfusion with basal glucose (2.8 mmol/l) was not collected. Following this equilibration period, the effluent was collected in 1-min intervals from a catheter placed in the portal vein. The insulin content of each interval was determined by ELISA.
Mitochondrial activity of pancreatic islet cells
Pancreatic islets were isolated from male mice by the collagenase technique [22], the islets were hand picked and 30 to 50 islets were used per experiment. Oxygen utilization was measured polarographically with a Clark oxygen electrode in a micro-water-jacketed cell at 37°C (Hansatech Instruments, Norfolk, UK) [25]. We measured the cell respiration in digitonin-permeabilized islets using pyruvate (10 mmol/l) as metabolic substrate to measure the activity of the complex I, succinate (10 mmol/l) for complex II, and glycerol-3-phosphate (20 mmol/l) for complex III.
Morphometric analysis of the islets
Serial sections were cut from each pancreas block and immunostained for insulin using the indirect peroxidase technique, anti-serum to insulin (Dako, Glöstrup, Denmark) was used at a dilution of 1/10. The islet surface was calculated after o-toluidine staining. Morphometry was carried out using a manual optical picture image analyzer (Model MOP-01, Olympus, Tokyo, Japan) on a projected image of the histological sections of the pancreas [26].
Apoptosis detection
Apoptosis was detected on paraffin-embedded pancreatic tissue by TUNEL method using the ApoAlert DNA Fragmentation Assay Kit (Clontech Laboratories, Palo Alto, Calif., USA). After apoptosis procedure, samples were immunostained for insulin with guinea pig anti-insulin antibody (1/200 dilution, ICN Pharmaceuticals, Costa Mesa, Calif., USA).
Statistical analysis
All statistical procedures were done using SSPS statistical software. Data analysis was carried out using the Kruskal-Wallis 1-way ANOVA. When interactions were significant, differences between individual group means were analyzed by Student's t test. A p value of less than 0.05 was considered statistically significant.
Results
Physical and plasma measurements
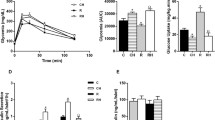
The mice were fed with a high carbohydrate diet, and, after 1 month of treatment, wild-type and heterozygous mice showed mild hyperglycaemia. However, in mGPDH –/– mice, the high carbohydrate diet did not increase the glycaemia; they remained normoglycaemic, as did the mice fed with the standard diet (Table 1). Moreover, incidence of diabetic values (>11 mmol/l) was higher in mGPDH +/+ (20%) and mGPDH +/– (11%) than in mGPDH –/– (0%) (Fig. 1A).
Glycaemia and intraperitoneal glucose tolerance test of mGPDH +/+, mGPDH +/– and mGPDH –/– mice fed with a high carbohydrate diet for 1 month. (A) Blood glucose concentrations of wild-type (n=15), mGPDH +/– (n=18) and mGPDH –/– (n=18) mice. Each value is represented as an empty circle. The mean for each group is drawn as a bar with the SEM. *, p<0.05 for mGPDH–/– mice compared to the other two groups. (B) Glucose tolerance test was done in the mGPDH –/– mice as described. Results are expressed as mean blood glucose concentration ± SEM from at least 16 mice of each genotype
Body weight gain was similar in the mice fed with the high carbohydrate diet and those fed the standard diet, with the notable exception of the mGPDH –/– mice, which grew by around 60% when fed with the high carbohydrate diet. This diet also induced hyperinsulinaemia in the wild-type and heterozygous mice, whereas in mGPDH –/– the insulin values were similar to those of the mice fed with the standard diet. It is worth noting that despite the differences in food intake, all the mice on the high carbohydrate diet and the standard one had similar caloric intake (Table 1).
The high carbohydrate diet had no significant effect on NEFA blood concentrations in wild type and heterozygous mice. However in mGPDH –/– mice the decrease was pronounced. The mGPDH –/– mice had higher cholesterol and triglyceride concentrations when fed with the standard diet (Table 1). The high carbohydrate diet induced a decrease in triglycerides and an increase in cholesterol in all the mice without differences between the three genotypes. T3 values were also increased in all the mice fed with the high carbohydrate diet; there were no differences with regard to genotype (Table 1).
Despite the differences observed in glucose and insulin values of mGPDH –/– mice fed with the high carbohydrate diet, the glucose tolerance test revealed no significant differences between the three groups (Fig. 1B). The test also did not detect differences in insulin values (data not shown).
Mitochondrial respiratory chain activity
Since the glycerol-phosphate shuttle is linked to the mitochondrial respiratory chain, we analyzed the oxygen consumption of the mitochondrial respiratory chain induced by various substrates. Oxidation of pyruvate (electrons entering complex I) was higher in the wild-type mice fed with the high carbohydrate diet; however, in mGPDH –/– mice the high carbohydrate diet did not induce an increase in oxygen consumption (Fig. 2). There were no differences in the oxidation of succinate (electrons entering complex II) between the two diets, or the different genotypes. Finally, oxygen consumption of mitochondria with glycerol-3-phosphate (electrons entering complex III) was decreased in mGPDH –/– mice regardless of diet (Fig. 2).
Respiratory activity of mGPDH –/– islets. (A) Complex (I) Substrate pyruvate; (B) Complex (II) Substrate: Succinate; (C) Complex (III) Substrate: Glycerol 3-phosphate. The respiratory activity of the three complexes was measured from islets of mGPDH +/+, mGPDH +/– and mGPDH –/– mice fed with a high carbohydrate diet for 1 month. Results are expressed as BoxPlot, with the median as the horizontal line inside the box, from six independent experiments for each genotype. The p values between different significant groups are plotted in the graph
Islet function
In order to improve our understanding of islet function, we carried out islet perifusion experiments. An increase in insulin secretion was observed in the islets from wild-type mice fed with the high carbohydrate diet when glucose was augmented from 2.8 mmol/l to 16.7 mmol/l (Fig. 3). When glucose was decreased back to 2.8 mmol/l, the insulin secretion fell to basal values. However, in mGPDH –/– mice, no increase in insulin secretion was observed. In these mice, insulin release remained at baseline values throughout the experiment at different glucose concentrations (Fig. 3). Similar results were obtained in the in situ perfused pancreas preparation. The mGPDH –/– mice had a lower secretion rate compared to wild-type mice when glucose was shifted from 2.8 to 16.7 mmol/l, despite an increase in the secretory response (Fig. 4).
Study of glucose-induced insulin secretion in mGPDH –/– islets. Insulin released from islet in perifusion obtained from mGPDH +/+ and mGPDH –/– mice fed with high carbohydrate diet. Glucose concentration was raised from 2.8 to 16.7 mmol/l at 5 min, and then decreased to 2.8 mmol/l at 35 min. Data are from six independent experiments with islets from each genotype
Insulin secretion from in situ pancreatic perfusion. Insulin released from perfused pancreas of wild type and mGPDH –/– mice fed with high carbohydrate diet. Glucose concentration was raised from 2.8 to 16.7 mmol/l at 16 min, and then decreased to 2.8 mmol/l at 40 min. Results are from two independent experiments
Long-term high carbohydrate diet
After analyzing the results obtained from the 1-month period on the high carbohydrate diet, we investigated the effects of the diet over a longer period (6 months). The blood glucose concentrations increased in the mGPDH +/+ and mGPDH +/– mice fed with the high carbohydrate diet. After 3 months of diet, the values tended to decrease, reaching values similar to the ones at the beginning of the experiment. However, mGPDH –/– mice had lower glucose concentrations than the mGPDH +/+ and mGPDH +/– mice throughout the 6-month period (Fig. 5A).
Glycaemia and body weight evolution of mGPDH –/– mice fed with a carbohydrate diet for 6 months. (A) Evolution of blood glucose concentrations during the diet. (B) Body weight evolution of the mice. Results are expressed as means ± SEM from at least six mice of each genotype. *p<0.05; **p<0.01; ***p<0.001 for mGPDH–/– mice compared to the other two groups. # p<0.05 for wild-type, mGPDH+/– mice compared to the value at time zero
The body weight of the wild-type and heterozygous mice increased during the 6 months on the high carbohydrate diet; in contrast, the body weight of mGPDH –/– mice showed a smaller increase during the 6 months of diet (Fig 5B). The mice fed with standard diet showed a similar tendency, the mGPDH –/– mice being the ones with lower body weights after 6 months with standard diet (38.7±3.2 for mGPDH +/+, 39.3±2.7 for mGPDH +/–, and 29.9±0.8 for mGPDH –/–). The mice fed with the standard diet had a higher body weight increase than the ones fed with a high carbohydrate diet.
The results of the glucose tolerance test were similar to those obtained after 1 month. No differences were observed in the blood glucose and insulin concentrations between the different genotypes during the test (data not shown). In order to assess the insulin resistance of the mice, we carried out an insulin tolerance test. In the wild-type mice, the administration of insulin decreased the glucose concentrations, as expected (Fig. 6). Surprisingly, mGPDH –/– mice had a lower fasting glycaemia, and the administration of the insulin produced relatively severe hypoglycaemia leading to death in some cases. The heterozygous mice presented an intermediate pattern.
Insulin tolerance test of mGPDH –/– mice fed with a carbohydrate diet for 6 months. Results are expressed as mean blood glucose concentration ± SEM from at least five mice of each genotype. **p<0.01 for mGPDH –/– mice compared to the other two groups. The mGPDH –/– mice had very low glycaemic values and they did not survive the test
The morphometric analysis of the pancreas of the mice showed that the high carbohydrate diet induced a hyperplasia of the islets in all three genotypes, while the pancreas weight did not change (Table 2, Fig. 7). However, the percentage of beta cells in the pancreas was lower in the mGPDH –/– mice. Moreover, no significant differences between the three genotypes were observed in the apoptosis rate (Fig. 7), nor in glycogen and lipid content in the liver. There were no variations in the epididymal adipose tissue weight.
Discussion
Pancreatic islet beta cells are poised to generate metabolic messengers in the mitochondria that link glucose metabolism to insulin exocytosis. The mGPDH is the rate-limiting enzyme of the glycerol phosphate shuttle which, together with the malate-aspartate shuttle, permits the reoxidation of cytosolic NADH. mGPDH is particularly abundant in pancreatic islets [2] and, in contrast to lactate dehydrogenase, much more so in beta cells than in non-beta cells [27]. Mice with a targeted disruption of mGPDH have recently been generated and found to be apparently normal. In this paper, using mGPDH knockout mice, we first hypothesized that an environmental factor, such as a high carbohydrate diet, would compromise the mitochondrial metabolism and, consequently, insulin secretion, thus rendering these mice diabetic. Surprisingly, these mice did not become diabetic, but showed lower blood glucose and insulin concentrations compared to the wild-type mice.
The results obtained help to further characterize mGPDH mice. Although several previous reports have described mGPDH –/– mice as normal [14, 15, 16, 17], our results showed that the blood concentrations of triglycerides and cholesterol in mGPDH –/– mice are increased. These results could be due to the absence of mGPDH activity in the liver, but not in the pancreas. Tough liver has lower concentrations of mGPDH compared to beta cell; in mGPDH –/– mice, lower concentrations of liver ATP and glycerol phosphate has been found recently [15]. Therefore, the lack of mGPDH –could modify the NADH shuttle in the liver altering lipid metabolism. In mGPDH–/– mice, the NEFA concentrations remain unchanged [15], which is consistent with our findings.
The results clearly show that the diet induced hyperglycaemia and hyperinsulinaemia in wild-type and heterozygous mice. The high carbohydrate diet decreased blood triglycerides in these mice, while NEFA remained unchanged; this could be due, at least partially, to the higher protein content of the high carbohydrate diet, since the fat content is the same. Moreover, some studies have also shown that high carbohydrate diets could decrease triglyceride concentrations [28]. The increased respiratory activity of mitochondrial respiratory chain with complex I substrate observed in the mGPDH +/+ and mGPDH +/– mice probably results from an increased glycolytic rate, since electron transport activity can adapt to the energy status of the cell [29]. This increased flow through the mitochondrial respiratory chain could increase the ATP/ADP ratio leading to higher insulin secretion, as found in the mice. Moreover, the mice have beta-cell hyperplasia in the pancreas. Therefore, all these changes seem to represent an adaptation mechanism to the high carbohydrate intake.
In the mGPDH –/– mice, the high carbohydrate diet did not induce the same changes observed in the mGPDH +/+ and mGPDH +/– mice. The mGPDH –/– mice had lower glycaemia and insulinaemia than the wild-type and heterozygous mice, and a lower beta-cell percentage in the pancreas, which is not due to an increased apoptotic rate. The mGPDH –/– mice fed with the high carbohydrate diet had a higher sensitivity to insulin, due, at least partially, to the lower NEFA concentrations. The glucose-induced insulin secretion was also lower in perifused islets and perfused pancreas. It is difficult to reconcile the normal insulin response in the whole animal during the glucose tolerance test and the impaired glucose induced insulin secretion. When we study the response of an isolated organ or some cells, regulatory factors are lost as the model is simplified. For example, the isolated islets show a biphasic secretion while the perfused pancreas only shows a monophasic secretion; while these data are well known, the explanation remains unknown [30]. For some reason, in the high carbohydrate-fed mGPDH –/– mice, the insulin response to glucose seems normal in whole mice, perhaps due to a diminished insulin clearance from the liver; notwithstanding, the isolated islets and the pancreas show an impaired response.
The lower body weight showed by the mGPDH –/– mice was already reported in the papers describing the mGPDH knockouts [14, 15], and our data agree with the percentage reported in those papers. Moreover, the high carbohydrate diet induces an increase in the thermogenesis, and the mice show a lower weight gain compared to standard diet [31], similar to what we observed in our experiments. Although it has been reported that, with standard diet, the thermogenesis was not altered in the mGPDH –/– mice [15], we cannot exclude that the lack of mGPDH alters the thermogenesis induced by the high carbohydrate diet, and enhances the body weight differences. The contribution of the brown adipose tissue or other peripheral tissues was not analyzed. It has been reported that mGPDH –/– mice fed with a high fat diet show smaller epididymal fat pads [15]; however, in our experiments, no difference was observed. These variations are probably due to the different composition of the diets; therefore only when the accumulation of lipids is greatly stimulated, i.e. high fat diets, the lack of mGPDH results phenotipically in smaller fat accumulations.
In conclusion, the high carbohydrate diet-induced hyperglycaemia and hyperinsulinaemia in the wild-type and mGPDH +/– mice. However, the mGPDH –/– fed with the high carbohydrate diet had a lower value of glycaemia and insulinaemia; and a decreased respiratory activity. Similar results were obtained when the mGPDH –/– mice were fed with a high fat diet (10%), showing lower glucose concentrations compared to wild-type mice [15]. Moreover, these mice had increased insulin sensitivity. Therefore, the lack of mGPDH acts as a rate-limiting step for glucose metabolism in the high carbohydrate fed mGPDH –/– mice. Moreover, the high carbohydrate diet does not worsen the phenotype of the animal with a partial impairment of NADH shuttle in the mitochondria.
Abbreviations
- mGPDH:
-
mitochondrial glycerol-3-phosphate dehydrogenase
References
Maechler P, Wollheim CB (2001) Mitochondrial functioning in normal and diabetic beta cells. Nature 414:807–812
MacDonald MJ (1981) High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J Biol Chem 256:8287–8290
MacDonald MJ (1982) Evidence for the malate aspartate shuttle in pancreatic islets. Arch Biochem Biophys 213:643–649
MacDonald MJ, Brown LJ (1996) Calcium activation of mitochondrial glycerol phosphate dehydrogenase restudied. Arch Biochem Biophys 326:79–84
Ishihara H, Nakazaki M, Kanegae Y et al. (1996) Effect of mitochondrial and/or cytosolic glycerol 3-phosphate dehydrogenase overexpression on glucose-stimulated insulin secretion from MIN6 and HIT cells. Diabetes 45:1238–1244
Giroix MH, Rasschaert J, Bailbe D et al. (1991) Impairment of the glycerol phosphate shuttle in islets from rats with diabetes induced by neonatal streptozotocin. Diabetes 40:227–232
Giroix MH, Baetens D, Rasschaert J et al. (1992) Enzymic and metabolic anomalies in islets of diabetic rats: relationship to beta cell mass. Endocrinology 130:2634–2640
Anak O, Giroix MH, Sener A, Malaisse WJ (1993) FAD-glycerophosphate dehydrogenase activity in splenocytes of rats with acquired or inherited diabetes mellitus. Med Sci Res 21:565–566
Giroix MH, Sener A, Portha B, Malaisse WJ (1993) Preferential alteration of oxidative relative to total glycolysis in pancreatic islets of two model of inherited and acquired type 2 (non insulin-dependent) diabetes mellitus. Diabetologia 36: 305–309
MacDonald MJ, Tang J, Polonsky KS (1996) Low mGDH and pyruvate carboxylase in pancreatic islets of Zucker Diabetic Fatty rats. Diabetes 45:1626–1630
Fernàndez-Alvarez J, Conget I, Rasschaert J, Sener A, Gomis R, Malaisse WJ (1994) Enzymatic, metabolic and secretory patterns in human islets of type 2 (non-insulin-dependent) diabetic patients. Diabetologia 37:177–181
Malaisse WJ (1994) Physiology and pathology of islet metabolism. Diabetes Metab 20: 95–98
Brown LJ, Stoffel M, Moran SM et al. (1996) Structural organization and mapping of the human mitochondrial glycerol phosphate dehydrogenase-encoding gene and pseudogene. Gene 172:309–312
Eto K, Tsubamoto Y, Terauchi Y et al. (1999) Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283:981–985
Brown LJ, Koza RA, Everett C et al. (2002) Normal thyroid thermogenesis but reduced viability and adiposity in mice lacking the mitochondrial glycerol phosphate dehydrogenase. J Biol Chem 277:32892–32898
Eto K, Suga S, Wakui M et al. (1999) NADH shuttle system regulates KATP channel-dependent pathway and steps distal to cytosolic Ca2+ concentration elevation in glucose-induced insulin secretion. J Biol Chem 274:25386–25392
Ravier MA, Eto K, Jonkers FC, Nenquin M, Kadowaki T, Henquin JC (2000) The oscillatory behavior of pancreatic islets from mice with mitochondrial glycerol-3-phosphate dehydrogenase knockout. J Biol Chem 275:1587–1593
Taylor SI (1999) Deconstructing type 2 diabetes. Cell 97:9–12
Saltiel AR (2001) New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104:517–529
Miyawaki K, Yamada Y, Yano H et al. (1999) Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 96:14843–14847
Seino S, Iwanaga T, Nagadhima K, Miki T (2000) Diverse roles of KATP channels learned from Kir6.2 genetically engineered mice. Diabetes 49:311–318
Lacy PE, Kostianovsky M (1967) Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39
Malaisse-Lagae F, Malaisse WJ (1984) Insulin release by pancreatic islets. In: Larner J, Pohl SL (eds) Methods in diabetes research. Wiley, New York, pp 147–152
Maechler P, Gjinovci A, Wollheim CB (2000) Implication of glutamate in the kinetics of insulin secretion in rat and mouse perfused pancreas. Diabetes 51(Suppl 1):S99–S102
Conget I, Barrientos A, Manzanares JM et al. (1997) Respiratory chain activity and mitochondrial DNA content of nonpurified and purified pancreatic islets cells. Metabolism 46:984–987
Clark A, Wells CA, Buley ID et al. (1988) Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 9:151–159
Sekine N, Cirulli V, Regazzi R et al. (1994) Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem 269:4895–4902
Riccardi G, Rivellese AA (2000) Dietary treatment of the metabolic syndrome—the optimal diet. Br J Nutr 83:S143–S148
Civelek VN, Deeney JT, Shalosky NJ et al. (1996) Regulation of pancreatic beta-cell mitochondrial metabolism: influence of Ca2+, substrate and ADP. Biochem J 318:615–621
Nesher R, Cerasi E (2002) Modeling phasic insulin release. Immediate and time-dependent effects of glucose. Diabetes 51:S53–S59
Rothwell NJ, Stock MJ, Warwick BP (1983) The effects of high fat and high carbohydrate cafeteria diets on diet-induced thermogenesis in rat. Int J Obes 7:263–270
Acknowledgements
We would like to thank C.B. Wollheim and T. Kadowaki for their help and advice in designing the experiments and preparing the manuscript. This work was supported by grants SAF 97/0023 from Ministerio de Ciencia y Tecnología (Spain), Red de Centros C03/08, RGDM G03/212 and FIS 96/0164 from Ministerio de Sanidad y Consumo (Spain), and SGR 2001/00378 from Generalitat de Catalunya (Spain). A.B. was a postdoctoral fellow from Ministerio de Educacion (Spain). P.M. is a fellow from the Dr. Max Cloëtta Foundation (Zurich). We thank all the members of the Laboratory of Experimental Diabetes for the invaluable discussions and support, and M. Maudsley for assistance in preparing the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Barberà and M. Gudayol have contributed equally to this paper
Rights and permissions
About this article
Cite this article
Barberà, A., Gudayol, M., Eto, K. et al. A high carbohydrate diet does not induce hyperglycaemia in a mitochondrial glycerol-3-phosphate dehydrogenase-deficient mouse. Diabetologia 46, 1394–1401 (2003). https://doi.org/10.1007/s00125-003-1206-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1206-5