Abstract
Key message
A new restorer of fertility gene, Rfs, of Ogura cytoplasmic male sterility (CMS) in radish encodes a pentatricopeptide repeat protein that binds to 15 nucleotides in mRNA of the CMS gene, orf138. Nucleotide substitutions in both Rfs and orf138 determine effectiveness and specificity of restoration.
Abstract
Cytoplasmic male sterility (CMS) in plants caused by the expression of abnormal mitochondrial genes results from impaired pollen production. The manifestation of CMS is suppressed by the restorer of fertility (Rf) genes in the nuclear genome. Thus, the CMS-Rf system is a suitable model for studying the direct interactions of mitochondrial and nuclear genes. At least nine haplotypes, of which Type B is ancestry, have been reported for the Ogura CMS gene, orf138, in radish (Raphanus sativus). We previously observed that Rfo encoding a pentatricopeptide repeat (PPR) protein, ORF687, which inhibits the translation of orf138 is ineffective in one haplotype (i.e., Type H). Here, we carried out map-based cloning of another Rf gene (Rfs) that cleaves the orf138 mRNA of Type H. Rfs produces a PPR protein consisting of 15 PPR motifs that binds to the mRNA, cleaving the mRNA at about 50nt downstream of the binding site. However, Rfs was ineffective for Type A because of a single nucleotide substitution in the binding site. Both Rfo and Rfs suppress orf138 expression in ancestral Type B, but they are rendered ineffective in Type H and Type A, respectively, by a single nucleotide substitution in orf138.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytoplasmic male sterility (CMS) in plants caused by a mitochondrial gene results in pollen sterility, but does not affect viability of the female organs. The final phenotypic expression of CMS, which has been observed in about 150 plant species (Chen et al. 2017), is suppressed by a restorer of fertility (Rf) gene that is present in the nuclear genome. The majority of Rf genes characterized to date encode organelle-targeted pentatricopeptide repeat (PPR) proteins (Kim et al. 2018). The CMS-Rf system is a good model for studying the interactions between mitochondrial and nuclear genes. In addition to being of considerable biological interest, CMS plays an important role in plant breeding. For example, in crop plants, F1 varieties between the parental lines generally exhibit hybrid vigor, which manifests a marked increase in yield performance; indeed, manipulating hybrid vigor in this way has been used in a wide variety of agricultural crops over an extended period. Given that CMS naturally prevents self-fertilization, it can be exploited to produce F1 seeds. Maize, rice, rapeseeds, and cabbage are a few examples in which F1 breeding based on CMS is applied commercially. Thus, CMS is highly interesting from both academic and applied perspectives.
One of the most extensively studied CMS-Rf systems is that of the Ogura CMS in radish. Following the discovery of the Ogura CMS (Ogura 1968), the causal mitochondrial gene (orf138) which is located adjacent to and is co-transcribed with atp8 was identified (Bonhomme et al. 1992). Subsequently, the Rf gene (Rfo) that encodes OFR687––a member of PPR proteins consisting of 17 PPR motifs––was cloned (Brown et al. 2003; Desloire et al. 2003; Koizuka et al. 2003). ORF687 does not affect the amount and/or size of orf138 mRNA but inhibits the production of ORF138 protein by blocking the translation (Wang et al. 2021). We observed that Rfo was not distributed widely in Japanese wild radishes (Yasumoto et al. 2008), finding another gene, Rft, that induced the cleavage at around the 103rd nucleotide in the coding sequence of orf138 mRNA (Yasumoto et al. 2009). However, Rft has not been cloned hitherto. Besides such academic approaches, the Ogura CMS is also widely used in the breeding of Brassicaceae crops (Yamagishi and Bhat 2014). Specifically, orf138 was introduced into various Brassica crops and has been used to produce F1 progeny worldwide.
Although the origin of orf138 is unclear, we previously reported that it was widely distributed in Japanese wild radishes (Yamagishi and Terachi 1996). By determining the DNA sequences of orf138 in Japanese wild radishes, cultivated radishes, and wild species of Raphanus, we found that orf138 contains six nucleotide substitutions and one insertion/deletion (Indel) mutation (Yamagishi and Terachi 2001). These sequence variations could be used to classify orf138 of wild and cultivated radishes into nine haplotypes (Type A—Type I). Among these haplotypes, Type A was originally discovered by Ogura (1968) and is used widely in practical breeding. On the other hand, the number of plants with each haplotype and the relationships of DNA variations among the haplotypes indicated that Type B is the ancestral orf138. This finding was corroborated by Giancola et al. (2007) who reported that only the Type B haplotype was found in European populations of wild radish (Raphanus raphanistrum).
We recently reported interesting relationships between the orf138 haplotypes and the effect of Rf genes in radish (Yamagishi et al. 2021a). Particularly, we found that Rfo had no effect on the Type H orf138 haplotype, even though the fertility of Type A was restored by Rfo. Further, the fertility of Type H plants was recovered by a novel Rf gene, Rfs. The action of Rfs was like that of Rft (Yasumoto et al. 2009), i.e., it induced cleavage of orf138 mRNA of Type H in the coding sequence, though the cleavage site was different from that induced by Rft. In contrast to Rfo, Rfs did not restore the fertility of Type A plants. The reason for the difference in effectiveness of Rfo was due to a single nucleotide substitution between Type A and Type H haplotypes in a 17-nucleotide region in orf138 mRNA to which ORF687 binds. The DNA mutations in the different orf138 haplotypes (Yamagishi and Terachi 2001) indicate that Type A and Type H both arose from the ancestral Type B haplotype by a single nucleotide substitution, and that the two haplotypes differ from each other by two substitutions. Based on these relationships among the DNA sequences of Type B, A, and H haplotypes and the response to the Rf genes of the corresponding orf138 sequences, we sought to clarify the interactions between the two Rf genes and the Type B orf138.
Of the two Rf genes, Rfo and Rfs, Rfo has been identified 20 years ago as mentioned above. Recently, the biological function of ORF687 encoded by Rfo was finally clarified (Wang et al. 2021). Seventeen PPR motifs of ORF687 bind to orf138 mRNA in the coding region and block its translation by stopping the progression of ribosomes along the orf138 coding sequence. On the other hand, Rfs whose function is the cleavage of orf138 mRNA (Yamagishi et al. 2021a) has not been cloned to date. Here, we identified Rfs by the map-based cloning using the cross-hybridized progeny population with the cytoplasm of Type H orf138. Based on the DNA and amino acid sequences, the function of the product of Rfs was clarified. It was found that Rfs encodes a PPR protein that contains 15 PPR motifs and binds with the coding region of orf138 mRNA, and that the effect of Rfs is diminished to Type A orf138 by a single nucleotide substitution in the binding site.
The results showed that two Rf genes with distinct functions suppress the expression of the ancestral type of a mitochondrial CMS gene in radish. Rfo and Rfs produce PPR proteins that bind to the coding sequences of orf138 mRNA in the different regions. While the ORF687 of Rfo suppresses the translation, the product of Rfs induces the cleavage of the transcript. However, it was shown, for the first time, that a single nucleotide substitution from the ancestral type of orf138 renders Rfo or Rfs ineffective, respectively.
Materials and methods
Plant materials
We previously observed that a population of Japanese wild radish collected in Zanpazaki (hereafter, Znp) was comprised of plants with the Type B orf138 haplotype (Yamagishi and Terachi 2001). We, therefore, used a Znp radish plant (Znp-8) as the female parent, which we then crossed with a radish cultivar ‘Uchiki-Gensuke (UG)’ that is known to be a maintainer of the Ogura CMS. Self-fertilization of an F1 plant was used to produce F2 progeny that contained male sterile plants.
One of the male sterile plants of the F2 progeny (F2 (Znp-8 × UG) No. 1) (Table 1) was cross-hybridized with a plant belonging to the population of ‘MR × (BK × SK)’ used in our previous study (Yamagishi et al. 2021a) to produce a total of 32 progeny plants (Table 2). The pollen parent was heterozygous for the Rfo gene and lacked another Rf gene (Rft), which cleaves orf138 mRNA, as described in Yamagishi et al. (2021a) (Table 1).
For the mapping of Rfs, we used the population (‘MR x (BK x SK)’) that was studied in our previous paper (Yamagishi et al. 2021a) with the increased number of plants (n = 128). The characteristics of the parents are shown in Table 1. Briefly, all the plants in the population had Type H orf138 and heterozygous genotype (Rforfo) for the Rfo locus. Rfo is ineffective to restore the fertility of Type H as previously found (Yamagishi et al. 2021a). Meanwhile, because ‘SK’ had the genotype of RfsRfs, and ‘MR’ and ‘BK’ had the genotype of rfsrfs (Yamagishi et al. 2021a), the population was expected to be segregated into fertile and sterile plants due to the segregation into Rfsrfs and rfsrfs at the Rfs locus. After identifying Rfs locus, we determined the DNA sequences of ‘SK’ having RfsRfs genotype as indicated in Yamagishi et al. (2021a). After sowing the seeds, the plants were grown in a glasshouse at a temperature of 20 °C–30 °C.
Phenotypic observations of CMS
Upon flowering, the pollen viability of each plant was assessed several times. Briefly, the plant was judged to be male fertile if pollen adhered to the finger after touching the anthers. Conversely, plants that did not produce any pollen in which pollen adherence was not observed were determined to be male sterile.
Isolation and analysis of DNA
Total DNA was isolated from young leaves of parent and progeny plants using a DNeasy Plant Mini Kit (QIAGEN, California, USA). The isolated DNA was then used to examine the Rfo genotype, the presence of Rft, and the haplotype of orf138. For the Rfo genotypes, the method of Yasumoto et al. (2008) was used for PCR–RFLP analysis with the restriction enzyme (SspI) to investigate whether the plant DNA contained the ORF687 (Rfo) sequence or had the rforfo genotype. The presence of Rft was investigated using the Sequence-Tagged Site (STS) marker that discriminates between Rft and rft as described in Yasumoto et al. (2009). Further, PCR products containing the entire coding and flanking regions of orf138 were obtained from the female parent and two plants in the progeny population. The PCR products were purified and sequenced to identify the orf138 haplotypes as described in Yamagishi et al. (2021a).
Investigation of orf138 mRNA
Total RNA was isolated from approximately 0.1 g of flower buds collected from each of the progeny plants using an RNeasy Plant Mini Kit (QIAGEN). Using the method of Yasumoto et al. (2009), northern blot analysis of the orf138 mRNA with the probe covering the entire coding region of orf138 was carried out. To detect the precise cleavage site in the orf138 mRNA of the sterile plants, circular RT-PCR was conducted using the methods described in Perrin et al. (2004) and Giancola et al. (2007).
Map-based cloning of Rfs
The DNA of the population of ‘MR x (BK x SK)’ was used to establish genetic maps according to the methods of Shirasawa and Kitashiba (2017) and Shirasawa et al. (2020). Briefly, the isolated DNA was used for double digested RAD-Seq(ddRAD-Seq) library construction with the restriction enzymes of Pst I and Hps I. The library was subjected to sequencing analysis by DNBSEQ-G400RS with a paired-end mode of 100 bases. The obtained sequences were mapped on the reference genome sequences of ‘Okute-Sakurajima’ and the linkage analysis was conducted. By observing the number of recombinant plants between phenotypic pollen fertility and DNA markers (single nucleotide polymorphism (SNP) or Indel), we determined the chromosome on which Rfs was located and the region where DNA markers and Rfs showed complete linkage. In the region, we searched for the PPR genes using the genetic map of ‘Okute-Sakurajima’ (RSAskr_r1.0) shown in Plant GARDEN (https://plantgarden.jp/ja/index) provided by Kazusa DNA Research Institute. After picking up PPR genes within the mapped region, we examined PPR codes of their products and predicted their possible binding sites in each type of orf138 mRNA.
Sequencing of Rfs genes and the product proteins
We conducted PCR to amplify the DNA of the region containing Rfs candidate after finding the candidate locus of Rfs. The DNA sequences of ‘Okute-Sakurajima’ shown in Plant GARDEN were used for primer designing, and the PCR product of ‘SK’ was directly sequenced by the methods described in Yamagishi et al. (2021a). The DNA sequences of the primers were FW03: GGATTTTCGGGTATTTCGGTA for the forward primer and RV03: GGAAATAGCAACACCTGCATACTC for the reverse primer.
The DNA sequence of ‘SK’ was used for BLAST search against the 17 genome assemblies deposited to GenBank (https://www.ncbi.nlm.nih.gov/data-hub/genome/?taxon=3725) including ‘Okute-Sakurajima.’ Although 22 radish accessions are deposited in NCBI, three (Modifier name; MSURR, Aokubi S–h, and Aokubi) were omitted from the analysis because their genome assemblies were incomplete yet. Further, the three accessions (‘GCA_902824885.1,’ ‘GCA_963506615.2,’ and ‘GCA_963506625.1’) were from a same radish cultivar, ‘QZ16,’ deposited by the same institute. Thus, the latter two accessions were discarded from the analysis. Based on the results of BLAST, the DNA sequence of the coding region of Rfs locus for ‘SK’ was annotated (DDBJ Accession number LC802790). Also, the sequences of the corresponding site to Rfs in the deposited radishes were investigated using the NCBI data. Further, the DNA sequence of the coding region corresponding to Rfs was determined for ‘MS-G’ that is a male sterile radish having rfs (DDBJ Accession number LC802791). ‘MS-G’ has been used as a control line of Ogura CMS in our experiments.
Thereafter, the amino acid (a.a.) sequence of the product of Rfs was deduced from the DNA sequences, and the structure of the protein was inferred from the amino acid sequences by the scan in PROSITE (SIB, Swiss, Institute of Biometrics: https://prosite.expasy.org). The PPR codes of the product of candidate PPR genes in ‘Okute-sakurajima’ and each radish in NCBI were estimated from the protein structure and used for the prediction of binding sites in orf138 mRNA.
RT-PCR
Total RNA (1.5 µg) prepared using the RNeasy Plant Mini Kit (QIAGEN) was reverse transcribed with an oligo(dT) primer and ReverTra Ace (TOYOBO, Osaka) in a 20 µL reaction, following the manufacturer's protocol. PCR was performed on the cDNA template using KOD One Blue (TOYOBO) according to the manufacturer's protocol. Two primer pairs were used for amplification. The first primer pair (F04: GTTGAAACTTGGGTATGAGCC and R06: CCTTTCGAAGGTAGGCTACAG) was located in the coding region of the At1g12300 homolog. In the second primer pair, one (F07: GCGCCAAATGATTGTACGTAC) was located in the coding region of At1g12300, whereas the other (R08: CCGCTTCAGACAATGAACC) was in the At1g12620-like homolog. Following an initial denaturation (98 °C, 1 min), 35 cycles of denaturation (98 °C, 10 s), annealing (58 °C, 10 s), and extension (68 °C, 10 s) were performed in a 20 µL solution. 5 µL of the PCR product was electrophoresed on a 1% agarose gel.
Prediction of the target site of the products of the candidate genes and Rfs in orf138 mRNA
To predict the potential binding sites in orf138 and the affinity of the products of candidate genes, Rfs from ‘SK’ and other radishes, we used the FIMO program in the MEME suite (https://meme-suite.org>tool>fimo) as mentioned in Takenaka et al. (2013). We used different haplotypes of orf138 for the prediction of the target site of Rfs product.
Results
Effects of Rfo and Rfs on the ancestral type of orf138, Type B
We demonstrated, previously, that Rfo had no effect on the Type H orf138 haplotype, while it restored the fertility of Type A (Yamagishi et al. 2021a). Further, the fertility of Type H plants was recovered by Rfs, but Rfs did not restore the fertility of Type A plants. The reason for the different effectiveness of Rfo was due to one of the two single nucleotide substitutions between Type A and Type H haplotypes (Yamagishi et al. 2021a). On the other hand, it has been unclear which or both of the substitutions are critical for the effectiveness of Rfs. Therefore, we focused on Type B haplotype, which is an ancestral type of Type A and Type H (Yamagishi and Terachi 2001). A nucleotide substitution from adenine (A) to cytosine (C) at the 61st of Type B generated Type H, whereas a substitution from guanine (G) to adenine (A) at the 99th of Type B resulted in Type A. Investigation of effectiveness of Rfo and Rfs on the Type B orf138 should answer which substitutions are critical for the effectiveness of Rfs to orf138. Thus, we crossed a male sterile line with Type B that had neither Rfo, Rfs, nor Rft as a female parent and a line that had Rfs but not Rft as a pollen parent.
One of the sterile plants (F2 (Znp-8 × UG) No. 1) had Type B orf138 and possessed the full-length orf138 mRNA, showing that the mRNA was not processed in the coding region (Table 1, Fig. 1). PCR–RFLP and PCR demonstrated that Rfo genotype of the sterile plant was rforfo and it lacked Rft (Table 1). We presumed that Rfs is absent in this plant, and we used this male sterile plant as a female parent with an ancestral orf138, Type B and rforfo/rftrft/rfsrfs. On the other hand, as the pollen parent, we used (‘MR × (BK × SK)’ No. 2) plant, which had both Rfo and Rfs in the heterozygote state and lacked Rft, i.e., Rforfo/rftrft/Rfsrfs and Type H orf138 as in Yamagishi et al. (2021a).
Segregation of phenotype, Rfo, and orf138 mRNA cleavage
Table 2 shows the segregation of the CMS phenotype, the genotype of the Rfo locus, and the presence of the orf138 mRNA cleavage observed by the crossing experiment. The progeny population was segregated into 27 fertile and five sterile plants. The segregation evidenced our hypothesis that the female parent did not possess Rfs. If it was sterile in spite of having Rfs, the population would have been segregated into 1 fertile: 1 sterile ratio, but the actual segregation was significantly deviated from this ratio (χ2(1:1) = 15.13 (p < 0.005)). Two randomly selected plants were of the Type B orf138 haplotype, as expected, irrespective of the phenotype (data not shown). The five sterile plants had the rforfo genotype and the full-length orf138 mRNA (Table 2, Fig. 1). On the other hand, six plants with the Rforfo genotype which showed cleavage of orf138 mRNA were male fertile. In addition to the six plants, 12 plants with the Rforfo genotype and full-length orf138 mRNA, and nine plants with the rforfo genotype and the cleaved orf138 mRNA, were all fertile (Table 2, Fig. 1). The segregation ratio of the pollen fertility phenotype fitted well to a 3 fertile: 1 sterile ratio (χ2 (3: 1) = 1.50 ( p> 0.1)). This ratio demonstrated that both Rfo and the gene for mRNA cleavage were involved in the restoration of fertility in those progenies with a Type B orf138 haplotype.
The heterozygosity of the two loci, i.e., the Rfo locus and the one for the cleavage, in the pollen parent was ascertained by the segregation ratios of Rfo and orf138 mRNA cleavage in the progeny population. The PCR–RFLP analysis of Rfo revealed that segregation occurred into 18 plants with the Rforfo genotype and 14 plants with the rforfo genotype (Table 2). This ratio fitted to 1: 1 (χ2 = 0.50 (p > 0.1)). Similarly, the progeny was segregated into 15 plants that exhibited cleavage and 17 that did not (Table 2). As before, the ratio fitted to 1: 1 (χ2 = 0.125 (p > 0.5)).
While the full-length mRNA of orf138 was approximately 1.4 kb, all of the cleaved mRNA measured approximately 1.1 kb (Fig. 1). We determined the processing site by circular RT-PCR for one of the fertile plants and determined that the 5′ end was at the 157th (2 clones) or the 159th (1 clone) nucleotide from the start of the coding sequence. The location of this site corresponded with that reported by Giancola et al. (2007) and Yamagishi et al. (2021a). Therefore, the mRNA cleavage observed in Type B was caused by Rfs, the new Rf gene that was effective to Type H orf138 and that we named previously (Yamagishi et al. 2021a). Rfs was derived from the variety ‘SK,’ the progenitor of the pollen parent of the population studied here.
Furthermore, the combination of the two Rf loci had a segregation ratio of 6:12:9:5 (Table 2). This segregation ratio indicated the independence of the two loci, by not deviating from 1:1:1:1 ratio (χ2 = 3.75 ( p > 0.25)). The results clarified that the two Rf genes (Rfo and Rfs), which were inherited independently from each other, restored the CMS induced by the Type B orf138 (Fig. 2). Moreover, the results suggest that the 99th G to A substitution that occurred in Type A is the reason for the loss of effect of Rfs on Type A orf138, and that the binding site for the Rfs product is in the region containing the nucleotide substitution.
Position and the candidate of Rfs
For the mapping of Rfs, we used the population (‘MR x (BK x SK)’) that was studied in our previous paper (Yamagishi et al. 2021a). The obtained progeny population was segregated into 62 fertile (Rfsrfs) and 66 sterile (rfsrfs) plants fitting to the ratio of 1: 1. The phenotypic segregation showed the linkage with the DNA markers on the fifth chromosome (RSAskr1.0R5g) (Table 3). The SNP markers at the position of 33,240,166 and 34,223,552 on the chromosome showed one and two recombinants with Rfs, respectively. The distance between the two markers was about 983 kbp. The four DNA markers between the two, three SNPs and one Indel markers, indicated the complete linkage with the phenotype (Table 3). Namely, all the fertile plants had heterozygous genotypes and the sterile ones were homozygous for the markers. According to the Plant GARDEN’s annotation, ‘Okute-Sakurajima’ has 219 genes in this region, and six among them were found to be genes of PPR-containing protein to which most of Rf genes identified to date belong. Although it was reported that the mitochondrial transcription termination factor (mTERF) was linked to fertility restoration in barley (Bernhard et al. 2019), there were no genes for mTERF in the region.
The six PPR genes, their positions in RSAskr1.0R5g, and the subclasses of the products in PPR protein are shown in Table 4. Based on the deduced amino acid sequences, the PPR codes were obtained, and we predicted the binding affinity of the PPR protein to orf138 mRNA (Table 4). Table 4, further, indicates the orthologue in Arabidopsis thaliana for each gene. Three of the PPR genes (59,289, 59,291, 59,374) encoded the P-class protein to which most of Rf proteins belong (Dahan and Mireau 2013), but the other three (59,387, 59,416, 59,473) produced the PLS-E subclass protein, which is exclusively involved in RNA editing, and were discarded from the candidate of Rfs. Among the three genes of P-subclass PPR protein, the PPR codes of 59,289 product showed the highest affinity for orf138 mRNA, followed by those of 59,374 product (Table 4). However, the latter PPR codes had very low specificity of nucleotide preference, eight of 13 PPR codes having neutral preference (data not shown). The PPR codes of 59,291 product did not exhibit the affinity with orf138 mRNA (P > 1.0E-02). The gene RSAskr1.0R5g59289 was an orthologue of At1g12300 that encodes restorer of fertility like (RFL) PPR protein (RFL2).
RFL2 encoded by At1g12300 contains 16 PPRs (Fujii et al. 2016). Fujii et al. (2016) studied the function of RFL2 in A. thaliana and found that the protein bound to the transcript of mitochondrial orf291 in the coding region. After binding, RFL2 recruited RNase P that cleaved the mRNA of orf291; however, the function of orf291 was unknown. Even though, in Brassica napus, Farooq et al. (2022) have shown through gene editing that loss-of-function mutation of At1g12300 orthologue (BnaRFL11) leads to male sterility. On the other hand, the orthologue of RSAskr1.0R5g59374 in A. thaliana (At3g48250) produced a PPR protein, Buthionine Sulfoximine-Insensitive Roots 6 (BIR6) that participated in nad7 intron1 splicing (Koprivova et al. 2010) and regulated plant resistance to Phytophthora parasitica (Yang et al. 2022), in place of fertility restoration. Based on these observations together, it was judged that RSAskr1.0R5g59289 was the most promising candidate of Rfs.
Sequence of Rfs
The DNA sequence of the region containing the locus of RSAskr1.0R5g59289 whose length was 4,228 bp in ‘Okute-Sakurajima’ was determined in ‘SK’ by the direct sequencing. Supplementary Fig. S1 shows the DNA sequence of ‘SK’ corresponding to the region from the start to stop codons including the intron in the annotated gene of ‘WK10039’ as described below.
By the BLAST search of the determined DNA sequence of ‘SK’ against the radish genomes deposited to NCBI, it was found that six assemblies had higher identity with ‘SK’ than ‘Okute-Sakurajima’ (Table 5). ‘Okute-Sakurajima’ had 99.53% identity and 8 gaps because of the nucleotide substitutions and Indels. Among the six assemblies demonstrating higher identity, the radish with the modifier name of ‘WK10039’ had only six nucleotide substitutions and no gaps in 4,230 bp and showed 99.86% identity (Table 5). In the region of the 4,230 bp of ‘WK10039,’ two genes were annotated according to the GenBank data (GCA_000801105.3). The products of the two genes are, respectively, pentatricopeptide repeat-containing protein of 628 a.a., and pentatricopeptide repeat-containing protein of 309 a.a. The former one was described as ‘pentatricopeptide repeat-containing protein At1g12300, mitochondrial-like’ in the GenBank of ‘WK10039’ similarly to RSAskr1.0Rg59289 of ‘Okute-Sakurajima.’ As aforementioned, At1g12300 encodes restorer of fertility like protein (RFL2). On the other hand, the latter protein of 309 a.a. was described as pentatricopeptide repeat-containing protein At1g12620-like. It was recently clarified that At1g12620 encodes a protein that is involved in processes forming the 5’ ends of nad3-rps12 transcripts (RFP8) (Schleicher and Binder 2021). The annotations of the two genes were evidenced by RNAseq as shown by Cho et al. (2022), while the annotation of the single gene, RSAskr1.0R5g59289, was not supported by any transcriptomic analysis. Therefore, the locus of Rfs was judged to be the DNA sequence of 1,887 bp encoding 628 a.a., and not the 4,228 bp of RSAskr1.0R5g59289 consisting of 6 exons and 5 introns as shown by the gene model in the Plant GARDEN.
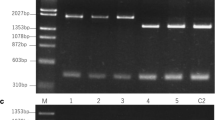
To investigate the expression of the candidates of Rfs experimentally, we carried out RT-PCR. The RT-PCR with the primer pair of F07 and R08, the stop codon of the 1,887 bp sequence being present between them, did not amplify DNA fragments in any plants tested (Fig. 3). Not only the sterile parents (‘MR’ and ‘BK’) and the sterile plants in the progeny population of ‘MR x (BK x SK),’ but also the fertile parent (‘SK’) and the fertile progeny plants did not show the transcription of RSA1.05g59289 annotated in the Plant GARDEN (Fig. 3). On the other hand, all the plants showed the DNA amplification by the primer pair of F04 and R06 (Fig. 3). The result indicated that the DNA sequence in the 1,887 bp was transcribed both in the fertile and sterile plants. The RT-PCR experiments ascertained that the locus of Rfs is 1,887 bp encoding 628 a.a. The results also demonstrate that both Rfs and rfs are transcribed, and thus, suggest that the difference of the fertility restoration ability between the two alleles is not caused by the difference of their expression.
RT-PCR analysis of fertile (lanes 5 and 6) and sterile (lanes 7 and 8) plants in the progeny populations of ‘MR × (BK × SK).’ The parental plants used in the crossing experiment (lanes 1, 2 and 3), along with MS-G (lane 4) as a control for rfs, are also analyzed. The predicated genome structure of the Rf locus, along with the position and orientation of the primers, is schematically illustrated below the gel
Both the genes of ‘SK’ and ‘WK10039’ possessed one coding sequence containing 1,887 nucleotides and encoded a 628 a.a. protein (Supplementary Fig. S2). The coding region of ‘MS-G’ determined by the direct sequencing also had 1,887 bp, but the stop codon changed from TAA of ‘SK’ to TAG because of the insertion of G at the 1,887th position (Supplementary Fig. S2). Table 6 shows the variations of NCBI assemblies from ‘SK’ for the DNA sequences of 1,887 bp found by the BLAST search. ‘Okute-Sakurajima’ and other six accessions had four nucleotide substitutions and two of them accompanied amino acid substitutions (Table 6). The largest variations were observed in ‘SKDG’ and ‘QZ16.’ The two accessions shared an identical sequence, containing 80 nucleotide substitutions and 40 amino acid substitutions compared with ‘SK’ (Table 6). The six accessions that had one Indel commonly possessed the insertion of G at the position of the 1,887th nucleotide as in the case of ‘MS-G.’ Thus, all the assemblies in NCBI were considered to have an allele of Rfs locus encoding the 628 a.a. protein.
Deduced domain structure of the product of Rfs
The amino acid sequence was deduced for ‘SK’ and ‘MS-G’ from the determined DNA sequences (Fig. 4). Protein motif scanning by PROSITE indicated that the 628 a.a. length of ‘SK’ contained 15 PPR motifs (Fig. 4). We name here the product by Rfs of ‘SK’ PPR628-Rfs and the corresponding product of ‘MS-G’ PPR628-MS-G. An orthologue of Rfs in A. thaliana, RFL2, has 16 PPR motifs, and the first PPR motif starts from the 53rd a.a. (Fujii et al. 2016; Liu et al. 2017), while PPR628-Rfs had 9 a.a. substitutions in the region corresponding to the first PPR motif of RFL2, reducing similarity to the PPR signature. Thus, the sequence of this region was not counted as a PPR motif similarly to the case of RFP that restores the pol CMS of B. napus (Liu et al. 2017). In fact, the PROSITE scan showed that the PPR motifs of PPR628-Rfs start from the 78th a.a., and that PPR motifs of RFL2 start from the 87th a.a. The 9th PPR motif of ‘SK’ had 36 a.a., but all the other PPR motifs were composed of 35 a.a. (Fig. 4).
Amino acid sequences of PPR628-Rfs and PPR628-MS-G. Differences in the amino acid sequence of PPR628-MS-G compared to PPR628-SK are indicated by letters below the PPR628-SK sequence. The mitochondrial targeting sequence predicted by the program TargetP-2.0 (https://services.healthtech.dtu.dk/services/TargetP-2.0/) is shown with a double underline
The combination of the 5th and the last (35th or 36th) a.a. in each PPR motif is called PPR code that determines the binding affinity between the PPR and the nucleotide in the target mRNA (Cheng et al. 2016). We compared the PPR codes of PPR628-Rfs and PPR628-MS-G, and other NCBI accessions. The PPR codes of the radishes deposited in NCBI were classified into three types (PPR codes-I, II, III), as shown in Table 7. The PPR codes-I which is identical to those of PPR628-Rfs of ‘SK’ is common in 12 accessions including ‘WK10039’ and ‘Okute-Sakurajima.’ The PPR codes-II, which contains five different codes from the PPR628-Rfs (the 10th, 11th, 13th, 14th, and 15th), was observed in one accession (‘NA-2021’) (Table 7). Meanwhile, the PPR codes–III found in the four assemblies (‘SR01(rat tail radish),’ ‘JC5,’ ‘SKDG,’ and ‘QZ16’) had four different PPR codes from those of PPR628-Rfs (the 10th, 11th, 13th, and 15th) (Table 7). PPR628-MS-G also belonged to the third type (Fig. 4).
The predicted target site of the product of PPR628-Rfs in Type H orf138 mRNA contains a single nucleotide substitution in Type A
To evaluate the functional relationship between the three types of Rfs products and each type of orf138, we predicted the target site of PPR codes-I, II, and III in orf138 mRNA with their binding affinities. The highest affinity (P = 3.61E-04) of the PPR628-Rfs (PPR codes-I) was observed at the site of 15 nucleotides ranging from the 89th to 103rd in the coding sequence of Type H orf138 (Table 8). This relatively higher affinity well explains the fertility restoration of Type H by Rfs. In the range of the 15 nucleotides, Type H and Type B share the identical nucleotide sequence (Fig. 2). This is the reason why Rfs was effective to the ancestral Type B as aforementioned. At the binding site, the PPR codes-I contained only two mismatches with Type H (the 5th and the 15th PPR) (Table 9). But the number of mismatches increased to three with Type A because of the nucleotide substitution at the 99th nucleotide of orf138, the counterpart of the 11th PPR code (Table 9). This induces the decrease of binding affinity to p = 2.81E-03 (Table 8) and would be the reason why the male sterility was not rescued by Rfs in Type A as observed in our previous experiment (Yamagishi et al. 2021a). Both PPR codes-II and PPR codes-III showed a lower binding affinity with Type H and Type A orf138 (Table 8).
At the site of 15 nucleotides to which PPR628-Rfs binds, Type C and Type G orf138 possesses a nucleotide substitution, respectively, relative to Type B and Type H. Type C has A instead of T at the 95th nucleotide, whereas Type G was generated by a substitution from A to C at the 90th nucleotide (Yamagishi and Terachi 2001). Thus, we extended the binding site prediction to Type C and Type G orf138. PPR628-Rfs with PPR codes-I showed fewer affinities with Type C and Type G as well as with Type A, suggesting Rfs does not restore the fertility of radish having Type C and Type G orf138 (Table 8). Interestingly, PPR codes-II showed a high affinity with Type G orf138 (p = 4.94E-04) at the 15 nucleotides from the 87th to 101st, which is two nucleotides shifted from the predicted binding site of PPR628-Rfs in Type H, with only two mismatches (Tables 8, 10). By contrast, PPR codes-II showed lower affinity (p = 3.89E-3) with Type H at the same site due to an additional mismatch that arose by a nucleotide substitution at the 90th from C (Type G) to A (Type H) (Tables 8, 10), suggesting that the gene encoding PPR codes-II was expected to specifically restore the fertility of radish having Type G orf138, though this must be experimentally proven.
PPR628-MS-G having PPR codes-III showed very low affinities with all of Types H, A, C, and G orf138 (Table 8). In comparison to PPR codes-I, PPR codes-III had a higher number of mismatches (four) to both Type H and Type A orf138 by the changes of PPR codes of the 11th and 13th PPRs (Table 9). Therefore, the gene of ‘MS-G’ encoding PPR628-MS-G is a recessive allele, rfs, lacking the restoring ability.
Overall, the results suggested that Rfs encodes the PPR protein with PPR codes-I and restores the fertility in Type H (and the ancestral Type B), but not in Types A, C, and G. The Rfs gene encoding PPR codes-II has the possibility to restore Type G. On the other hand, the gene that produces the PPR codes-III cannot restore the fertility in any types, and this is the recessive allele, rfs.
Discussion
To our knowledge, this is the first study to clarify the effectiveness of multiple Rf genes with distinct functions to the same mitochondrial CMS gene containing nucleotide substitutions. The two Rf genes produce PPR proteins alike that bind to the transcript of mitochondrial orf138 of the Ogura CMS, but one induces mRNA cleavage, and another blocks the translation. The expression of the ancestral orf138 (Type B) is prevented by both mRNA cleavage and translation block. However, it was shown that a single nucleotide substitution in the coding region depresses the binding of PPR proteins and diminishes the effect of each Rf gene (Fig. 2). The results demonstrate that the restoration of fertility of the CMS trait is not only determined by variations in nuclear Rf genes, but also by nucleotide differences in the CMS gene. This study also suggests the presence of a new allele of Rfs that specifically suppresses one type of orf138. These findings on the CMS-Rf system add further insights into the relationship between nuclear and mitochondrial genomes in the evolution of plants.
To date, the molecular mechanisms by which different Rf genes restore the same CMS have been clarified in rice and wheat. Wang et al. (2006) identified two related PPR motif genes that restore male sterility in rice with BoroII cytoplasm. The two genes, members of a multigene cluster, are located at the classical locus Rf–1 and blocked CMS protein production. The product of Rf1a had the function in endonucleolytic cleavage of the mRNA of the CMS-causing orf79, while Rf1b induced degradation of the mRNA. Although the modes differed between the two genes, both were involved in RNA pathways. Recently, Melonek et al. (2021) identified two Rf genes in the T–CMS of wheat. The two genes on the homoeologous chromosomes (1A and 1B), Rf1 and Rf3, encode RFL proteins that belong to the PPR protein family. Both the RFL proteins bound to the transcript of mitochondrial orf279 causing T–CMS, but the binding sites were different and produced different cleavage sites. In these cases, the two Rf genes silence the CMS gene in the processing pathway of the mRNA. By contrast, Luo et al. (2013) found that CMS-WA of rice was suppressed by different mechanisms. They observed that the amounts of transcripts of the CMS gene (WA352) were decreased to ~ 20–25% by Rf4 but were not affected by Rf3. Thus, Rf4 functions at the transcription level to reduce the CMS-RNA abundance, whereas Rf3 functions post-transcriptionally to bring about fertility restoration. Similarly, the two Rf genes act on the Ogura CMS through different mechanisms of fertility restoration, and the more detailed mechanisms were clarified here (Fig. 2). Although the two Rf genes (Rfo and Rfs) commonly produce the PPR proteins that bind to the orf138 transcript, they are on the different chromosomes and the PPRs bind to separate sites of orf138 mRNA, acting differently. Rfs induces the cleavage of orf138 mRNA downstream of the binding site in a similar manner to the case observed in rice and wheat mentioned above. However, Rfo encodes a PPR protein that blocks the translation of orf138 (Wang et al. 2021; Yamagishi et al. 2021a).
In the long history of studies on CMS and Rf genes, especially after the identification of mitochondrial CMS genes, almost all researches have focused on the cloning and molecular characterization of corresponding Rf genes without considering the variations in the CMS gene. One exception was a study on orf108 found in a wide variety of Brassicaceae taxa that induces CMS in Brassica and Raphanus crops. Naresh et al. (2016) identified interspecific mutations in the mitochondrial orf108 between Moricandia arvensis and Brassica oxyrrhina and observed that the Rf gene of M. arvensis that cleaved the orf108–atp1 transcript was rendered ineffective by the mutations. A similar effect of such mutations in orf108 on mRNA cleavage was also observed in Brassica maurorum (Yamagishi et al. 2021b). However, the Rf gene against orf108 has not yet been identified, and thus, the molecular relationship between orf108 and the Rf genes is still unclear. The findings of this study and the study of Yamagishi et al. (2021a) indicate, for the first time, the molecular relationships between the variations in the CMS gene and Rf genes, all of which are identified and characterized well. Eight of the haplotypes among Type A–I share Type B as their ancestor, and Type H and Type A were derived from Type B by a single nucleotide substitution (Yamagishi and Terachi 2001) (Fig. 2). The nucleotide substitution from Type B to Type H (A to C) occurred at the 61st nucleotide in the coding region of orf138. As shown in Yamagishi et al. (2021a), this mutation in the 17 nucleotides to which 17 of the PPR of the ORF687 encoded by Rfo bind drastically decreases the binding affinity, and diminishes the effect of Rfo.
We newly identified Rfs the product of which induces the cleavage of the orf138 mRNA at around the 157th nucleotide in the coding region. The map-based cloning showed that Rfs is located on the fifth chromosome, while BLAST search of the NCBI assemblies indicates that Rfo is present on the ninth chromosome. This fact supports the independent inheritance of the two Rf genes experimentally shown in Table 2. Table 2 also indicates that all the plants having the genotype of Rforfo/Rfsrfs are male fertile because of the cleavage of orf138 mRNA. From the result, Rfs is inferred to be epistatic to Rfo in the meaning that Rfs hides the effect of Rfo on the phenotype of pollen fertility. Since the product of Rfs functions upstream of orf138 mRNA pathways, the binding of ORF687 encoded by Rfo to orf138 mRNA would show no effect on the fertility restoration. However, more detailed molecular studies remain to be conducted that clarify the binding affinity of ORF687 to orf138 mRNA cleaved by the product of Rfs.
Rfs encodes the PPR protein (PPR628-Rfs) and the 15 PPR motifs were predicted to bind most strongly to the 89th ~ 103rd nucleotides of orf138 mRNA according to the PPR codes (Table 8). Typically, RFL proteins cleave the targeted transcript within 100 nucleotides of the 3’-end of the RFL-binding site (Colas des Francs-Small et al. 2018). Thus, the PPR628-Rfs works as typical RFL proteins. Moreover, Huynh et al. (2023), recently, found that a majority of RFL proteins possess a distinct domain of about 60–70 amino acids at their C-terminus called Rf CTD (Restorer of fertility C-terminal domain). The presence of RfCTD correlates with the ability to induce cleavage of the mitochondrial RNA target. In the RfCTD, there are conserved amino acid residues that differ from a typical P-class motif, such as arginine (at Position 9), leucine (at Position 12), and phenylalanine (at Position 32) etc. (Huynh et al. 2023). It is of importance that both PPR628-Rfs and PPR628-MS-G share most of these characteristics of RfCTD (Fig. 4). On the other hand, Rfo whose function is not mRNA cleavage, but translation blocking (Wang et al. 2021) does not have the RfCTD as shown in Huynh et al. (2023).
We found in our previous paper that a single nucleotide substitution in the binding region of ORF687 of Rfo diminished the effect of fertility restoration in Type H orf138. Here, similar phenomenon was observed for Rfs and Type A orf138. The prediction indicated that PPR628-Rfs binds strongly to Type H (Table 8). However, because of the nucleotide substitution from G of Type H (also the ancestral Type B) to A of Type A, the binding ability decreased, and Rfs could not restore the fertility of Type A (Table 8). Among the two nucleotide substitutions between Type H and Type A, one at the 61st determines the effectiveness of Rfo and another at the 99th determines the effectiveness of Rfs. The nucleotide substitution at the 99th is the synonymous mutation as shown in Yamagishi and Terachi (2001). This fact reveals that the nucleotide substitution in the mitochondrial CMS gene on its own but not the amino acid substitution determines the response to the Rf gene present in the nuclear genome. As shown in Fig. 2, male sterility of the ancestral type orf138 (Type B) is suppressed by both Rfo and Rfs. A single mutation in orf138 played a crucial role for the radish plants to escape from the suppression by one of the Rf genes and, as a result, orf138 disturbed the production of fertile pollens. Both the two single mutations increased the number of mismatches between PPR codes and the target nucleotides and lowered the binding affinity.
The cloning of Rfs experimentally conducted here is of considerable interest as it will permit clarification of the response of Type C and Type G orf138 to Rfs. As mentioned above, the nucleotide substitution that differentiates them from Type B is in the region where PPR628-Rfs binds (i.e., at the 90th nucleotide (Type G) and the 95th nucleotide (Type C)) (Yamagishi and Terachi 2001). The fact that the variable region of orf138 is concentrated in the site where PPR628-Rfs binds corresponds with the finding of Fujii et al. (2016) that mitochondrial orf291 of A. thaliana is hypervariable in the region to which RFL2 binds. Our prediction indicated that the binding of PPR628-Rfs is lowered in Type C and Type G than Type H (equal to Type B) (Table 8). The results suggest that the three mutations in the region result in the escape of most haplotypes except for Type B and Type H from the suppression by Rfs (See Yamagishi and Terachi 2001). Furthermore, it is much interesting that one accession deposited in NCBI has the PPR protein (PPR code-II) that was predicted to have high binding affinity with Type G orf138. The variations of the PPR protein genes in the nuclear genome produce the new allele that is likely to suppress the escaped type of orf138. However, the Rf genes encoding RFL2-like PPR protein that inhibit the expression of Type A and Type C are still to be identified.
Relating to this, further studies of Rft that was found in a Japanese wild radish (Yasumoto et al. 2009) would be informative. We previously observed that Rft processes the orf138 mRNA similarly to Rfs. However, Rft had different characteristics from Rfs as follows. First, Rft restored the fertility of the radish with Type A orf138 such as ‘MS-G’ to which Rfs is ineffective. Second, the restoration by Rft was not always complete and partially fertile plants were observed in high frequency, whereas such semi-fertile plants were not observed at all in the experiments of this article on Rfs. Third, the processing by Rft was at around the 103rd nucleotide in the coding sequence, while Rfs induces the cleavage at around the 157th. These comparisons suggest that Rft is one of the Rfs-like genes effective to Type A orf138, but the more detailed analyses based on accurate identification of Rft would be necessary to clarify the relationship between Rfs and Rft. Additionally, a wider survey of genetic variations in the Rfs locus for wild and cultivated radishes would be expected to find an allele that restores Type C orf138.
Recently, Melonek and Small (2022) described that it remains to be tested whether the ‘RNA cleavage’ and ‘translation block’ pathways are fundamentally different. In this context, it would be possible, from our results, to think that Rfs is an older gene, and Rfo for the translation blocking evolved later as if in response to the cumulative mutations in the site where Rfs acts to orf138. The mutated orf138 that escaped from the RNA cleavage is suppressed in turn by translation block with Rfo having more strict binding affinity in the downstream stage of orf138 expression process. Clarification of the functional mechanisms of Rfs to the orf138 in various haplotypes would provide a deeper understanding of the complex relationships between the sequence variations in the CMS gene and Rf genes with distinct functions. The mechanisms may open new avenues for studying the evolutionary relationships between these mitochondrial genes and their corresponding nuclear genes.
References
Bernhard T, Koch M, Snowdon RJ, Friedt W, Wittkop B (2019) Undesired fertility restoration in msm1 barley associates with two mTERF genes. Theor Appl Genet 132:1335–1350
Bonhomme S, Budar F, Lancelin D, Small I, Defrance M-C, Pelletier G (1992) Sequence and transcript analysis of the Nco2.5 Ogura-specific fragment correlated with cytoplasmic male sterility in Brassica cybrids. Mol Gen Genet 235:340–348
Brown GG, Formanová N, Jim H, Wargachuk R, Dendy C, Patil P, Laforest M, Zhang J, Cheng WY, Landy BS (2003) The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J 35:262–272
Chen Z, Zhao N, Li S, Grover CE, Nie H, Wendel JF, Hua J (2017) Plant mitochondrial genome evolution and cytoplasmic male sterility. Crit Rev Plant Sci 36:55–69
Cheng S, Gutmann B, Zhong X, Ye Y, Fisher MF et al (2016) Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J 85:532–547
Cho A, Jang H, Baek S, Kim M-J, Yim B, Huh S, Kwon S-H, Yu H-J, Mun J-H (2022) An improved Raphanus sativus cv. WK10039 genome localizes centromeres, uncovers variation of DNA methylation and resolves arrangement of the ancestral Brassica genome blocks in radish. Theor Appl Genet 135:1731–1750
des Francs-Small C Colas, Sanglard L Vincis Pereira, Small I (2018) Targeted cleavage of nad6 mRNA induced by a modified pentatricopeptide repeat protein in plant mitochondria. Commun Biol 1:166
Dahan J, Mireau H (2013) The Rf and Rf-like PPR in higher plants, a fast-evolving subclass of PPR genes. RNA Biol 10:1469–1476
Desloire S, Ghebri H, Laloui W, Marhadour S, Cloet V et al (2003) Identification of the fertility restoration locus, Rfo, in radish as a member of the pentatricopeptide-repeat protein family. EMBO Rep 4:588–594
Farooq Z, Riaz MN, Farooq MS, Li Y, Wang H et al (2022) Induction of male sterility by targeted mutation of a restorer-of-fertility gene with CRISPR/Cas9-mediated genome editing in Brassica napus L. Plants 11:3501
Fujii S, Suzuki T, Giege P, Higashiyama T, Koizuka N, Shikanai T (2016) The restorer-of-fertility-like 2 pentatricopeptide repeat protein and RNase are required foe the processing of mitochondrial orf291 RNA in Arabidopsis. Plant J 86:504–513
Giancola S, Rao Y, Chaillos S, Hiard S, Martin-Canadell A, Pelletier G, Budar F (2007) Cytoplasmic suppression of Ogura cytoplasmic male sterility in European natural populations of Raphanus raphanistrum. Theor Appl Genet 114:1333–1343
Huynh SD, Melonek J, des Francs-smallBondSmall C ColasCSI (2023) A unique C-terminal domain contributes to the molecular function of Restorer-of-fertility proteins in plant mitochondria. New Phytol 240:830–845
Kim Y-J, Zhang D (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci 23:53–65
Koizuka N, Imai R, Fujimoto H, Hayakawa T, Kimura Y, Kohno-Murase J, Sakai T, Imamura J (2003) Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J 34:407–415
Koprivova A, des Francs-SmallCalderMugfordTanzLeeZechmannSmallKopriva CCGSTSBRBIS (2010) Identification of a pentatricopeptide repeat protein implicated in splicing of intron1 of mitochondrial nad7 transcript. J Biol Chem 285:32192–32199
Liu Z, Dong F, Wang X, Wang T, Su R et al (2017) A pentatricopeptide repeat protein restores nap cytoplasmic male sterility in Brassica napus. J. Experi. Bot. 68:4115–4123
Luo D, Xu H, Liu Z, Guo J, Li H et al (2013) A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 45:573–577
Melonek J, Small I (2022) Triticeae genome sequences reveal huge expansions of gene families implicated in fertility restoration. Curr Opini Plant Biol 66:102166
Melonek J, Duarte J, Martin J, Beuf L, Murigneux A et al (2021) The genetic basis of cytoplasmic male sterility and fertility restoration in wheat. Nature Commun 12:1036
Naresh V, Singh SK, Watts A, Kumar P, Kumar V, Rao KS, Bhat SR (2016) Mutations in the mitochondrial orf108 render Moricandia arvensis restorer ineffective in restoring male fertility to Brassica oxyrrhina-based cytoplasmic male sterile line of B juncea. Mol Breeding. https://doi.org/10.1007/s11032-016-0489-4
Ogura H (1968) Studies on the new male sterility in Japanese radish, with special reference to the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ 6:39–78
Perrin R, Meyer EH, Zaepfel M, Kim Y-J, Mache R, Grienenberger J-M, Gualberto M, Gagliardi D (2004) Two exoribonucleases act sequentially to process mature 3’-ends of atp9 mRNAs in Arabidopsis mitochondria. J Biol Chem 229:25440–25446
Schleicher S, Binder S (2021) In Arabidopsis thaliana mitochondrial 5’ end polymorphisms of nad4L-atp4 and nad3-rps12 transcript are linked to RNA PROCESSING FACTORs 1 and 8. Plant Mol Biol 106:335–348
Shirasawa K, Hirakawa H, Fukino N, Kitashiba H, Isobe S (2020) Genome sequence and analysis of a Japanese radish (Raphanus sativus) cultivar named ‘Sakurajima Daikon’ possessing giant root. DNA Res 27(2):1–6
ShiraswaKitashiba KH (2017) Genetic maps and whole genome sequences of Radish. In: Nishio T, Kitashiba H (eds) The Radish Genome. Springer, Cham, pp 31–42
Takenaka M, Zehmann A, Brennicke A, Graichen K (2013) Improved computational target site prediction for pentatricopeptide repeat RNA editing factors. PLoS ONE 8:e65343
Wang Z, Zou Y, Li X, Zhang Q, Chen L et al (2006) Cytoplasmic male sterility of rice with Boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18:676–687
Wang C, Lezhneva L, Arnal N, Quadrado M, Mireau H (2021) The radish Ogura fertility restorer impedes translation elongation along its cognate CMS-causing mRNA. Proc Natl Acad Sci USA 118:e2105274118
Yamagishi H, Bhat SR (2014) Cytoplasmic male sterility in Brassicaceae crops. Breed Sci 64:38–47
Yamagishi H, Terachi T (1996) Molecular and biological studies on male-sterile cytoplasm in the Cruciferae. III. Distribution of Ogura-type cytoplasm among Japanese wild radishes and Asian radish cultivars. Theor Appl Genet 93:325–332
Yamagishi H, Terachi T (2001) Intra- and inter-specific variations in the mitochondrial gene orf138 of Ogura-type male-sterile cytoplasm from Raphanus sativus and Raphanus raphanistrum. Theor Appl Genet 103:725–732
Yamagishi H, Jikuya M, Okushiro K, Hashimoto A, Fukunaga A, Takenaka M, Terachi T (2021) A single nucleotide substitution in the coding region of Ogura male sterile gene, orf138, determines effectiveness of a fertility restorer gene, Rfo, in radish. Mol Genet Genom 296:705–717
Yamagishi H, Hashimoto A, Fukunaga A, Bang SW, Terachi T (2021) Intraspecific variations of the cytoplasmic male sterility genes orf108 and orf117 in Brassica maurorum and Moricandia arvensis, and the specificity of the mRNA processing. Genome 64:1081–1089
Yang Y, Zhao Y, Zhang Y, Niu L, Li W et al (2022) A mitochondrial RNA processing protein mediates plant immunity to a broad spectrum of pathogens by modulating the mitochondrial oxidative burst. Plant Cell 34:2343–2363
Yasumoto K, Matsumoto Y, Terachi T, Yamagishi H (2008) Restricted distribution of orf687 as the pollen fertility restorer gene for Ogura male sterility in Japanese wild radish. Breed Sci 58:177–182
Yasumoto K, Terachi T, Yamagishi H (2009) A novel Rf gene controlling fertility restoration of Ogura male sterility by RNA processing of orf138 found in Japanese wild radish and its STS markers. Genome 52:495–504
Acknowledgements
This research was supported in part by a grant from the Center for Plant Sciences, Kyoto Sangyo University. The authors thank Mai Tsujimura for the technical assistance.
Funding
This research was supported in part by a grant from the Center for Plant Sciences, Kyoto Sangyo University.
Author information
Authors and Affiliations
Contributions
HY and TT designed research. HY, AH and AF performed research. HY, AH and MT analyzed data. HY and MT wrote the paper. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that the experiments complied with current laws of the country where they were performed.
Additional information
Communicated by Richard G. F. Visser.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamagishi, H., Hashimoto, A., Fukunaga, A. et al. Identification and variation of a new restorer of fertility gene that induces cleavage in orf138 mRNA of Ogura male sterility in radish. Theor Appl Genet 137, 231 (2024). https://doi.org/10.1007/s00122-024-04736-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-024-04736-4


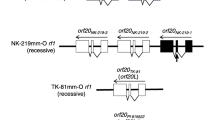


 and
and  indicate that the Rf genes suppresses
indicate that the Rf genes suppresses  or does not suppress
or does not suppress  orf138, respectively. b The arrows show the nucleotide substitutions from the ancestral type B to type H and type A
orf138, respectively. b The arrows show the nucleotide substitutions from the ancestral type B to type H and type A
