Abstract
Key message
The hvbe2a mutations restore the starch-deficient phenotype caused by the hvisa1 and hvflo6 mutations in barley endosperm.
Abstract
The genetic interactions among starch biosynthesis genes can be exploited to alter starch properties, but they remain poorly understood due to the various combinations of mutations to be tested. Here, we isolated two novel barley mutants defective in starch BRANCHING ENZYME 2a (hvbe2a-1 and hvbe2a-2) based on the starch granule (SG) morphology. Both hvbe2a mutants showed elongated SGs in the endosperm and increased resistant starch content. hvbe2a-1 had a base change in HvBE2a gene, substituting the amino acid essential for its enzyme activity, while hvbe2a-2 is completely missing HvBE2a due to a chromosomal deletion. Further genetic crosses with barley isoamylase1 mutants (hvisa1) revealed that both hvbe2a mutations could suppress defects in endosperm caused by hvisa1, such as reduction in starch, increase in phytoglycogen, and changes in the glucan chain length distribution. Remarkably, hvbe2a mutations also transformed the endosperm SG morphology from the compound SG caused by hvisa1 to bimodal simple SGs, resembling that of wild-type barley. The suppressive impact was in competition with floury endosperm 6 mutation (hvflo6), which could enhance the phenotype of hvisa1 in the endosperm. In contrast, the compound SG formation induced by the hvflo6 hvisa1 mutation in pollen was not suppressed by hvbe2a mutations. Our findings provide new insights into genetic interactions in the starch biosynthetic pathway, demonstrating how specific genetic alterations can influence starch properties and SG morphology, with potential applications in cereal breeding for desired starch properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starch, a glucose-based polymer from plants, is extensively utilized in various food and industrial sectors. Its water-insolubility and lack of osmotic activity make it an ideal substance for long-term storage in seeds, grains, and roots. Within these storage organs, starch forms semi-crystalline starch granules (SGs) produced in specialized plastids called amyloplasts (Gunning and Steer 1996). SGs vary in size and shape across different plant species and are broadly categorized as either compound or simple SGs (Tateoka 1962; Matsushima et al. 2013; Chen et al. 2021). Compound SGs consist of assemblies of smaller starch particles, whereas simple SGs are composed of single starch particles. In the case of rice (Oryza sativa) endosperm, the compound SGs measure about 10–20 μm in diameter and are made up of polyhedral starch particles, each measuring 3–8 μm (Matsushima et al. 2010). Meanwhile, the endosperm of barley (Hordeum vulgare) and wheat (Triticum aestivum) features two types of simple SGs, the smaller B-type (~ 5 μm diameter) and the larger A-type (10–30 μm diameter), both existing together within a single cell (Jane et al. 1994; Matsushima and Hisano 2019; Thieme et al. 2023). This type is referred to as bimodal simple SGs. The A-type granules begin to form in the early stages of grain development within amyloplasts, followed by the development of B-type granules in the same amyloplasts, which already contain A-type granules (Langeveld et al. 2000; Matsushima and Hisano 2019; Kamble et al. 2023).
The size and shape of SGs are influenced by specific enzymes involved in synthesizing amylopectin, the primary polymer component of SGs, which is made up of α-1,4-linked and α-1,6-branched glucose chains (Smith and Zeeman 2020; Tetlow and Bertoft 2020). Amylopectin synthesis involves three key reactions: the formation of α-1,4-glycosidic bonds by starch synthases (SSs), which elongate the glucose chains; the creation of α-1,6-glycosidic bonds by branching enzymes (BEs), introducing branches in the structure; and the removal of misplaced glucose branches by hydrolyzing α-1,6 linkages with starch debranching enzymes (DBEs) (Nakamura 2002; Pfister and Zeeman 2016). In the absence of appropriate trimming by DBEs, improperly positioned branches can prevent the formation of the semi-crystalline structure of amylopectin. Deficiency of one of the DBEs, ISOAMYLASE1 (ISA1), leads to the accumulation of phytoglycogen, a water-soluble α-glucan (Pan and Nelson 1984; Nakamura et al. 1997; Burton et al. 2002). Phytoglycogen is characterized by its extensive branching with short glucan chains and has a molecular weight significantly lower than that of amylopectin. In barley ISA1 mutants (hvisa1), the endosperm forms compound SGs, contrasting with the typical bimodal simple SGs found in the wild type (Burton et al. 2002; Matsushima et al. 2023). Cereal mutants exhibiting defects in starch biosynthetic enzymes hold considerable interest in the food industry due to their capability to alter the physicochemical and nutritional properties of starch within the grain (Nakamura 2018). For example, mutations in the ISA1 gene of maize lead to increased soluble sugars, instead of starch in the grains and have been utilized in breeding sweet corn varieties (Gonzales et al. 1976; Pan and Nelson 1984; James et al. 1995). Furthermore, mutants in the BE genes of rice and maize are noted to enhance the production of resistant starch, known for its resistance to digestion by amylase (Xia et al. 2011; Tsuiki et al. 2016; Chen et al. 2022). The intake of resistant starch changes the composition of gut microbiota in mice, marked by an increase in specific beneficial bacteria (Li et al. 2024). This contributes to the improvement in obesity by reducing lipid absorption, decreasing inflammation, and enhancing intestinal barrier strength (Blaak et al. 2020).
Recent studies have identified several non-enzymatic proteins that directly interact with starch biosynthetic enzymes and also affect SG shape and size. These proteins include the Arabidopsis thaliana PROTEIN TARGETING TO STARCH (PTST) 1 to 3, the rice PTST2 ortholog, FLOURY ENDOSPERM 6 (FLO6) and its barley and wheat orthologs, HvFLO6 and B-GRANULE CONTENT1 (BGC1), respectively (Peng et al. 2014; Seung et al. 2015, 2017; Saito et al. 2018; Chia et al. 2020). These proteins possess Carbohydrate-Binding Module 48 (CBM48) domains, known for their affinity towards binding starch and maltooligosaccharides (Peng et al. 2014; Seung et al. 2015, 2017). CBM48s are also found in the sequences of BEs and DBEs, but not in SSs (Pfister and Zeeman 2016). CBM48s probably facilitate the efficient transfer of the substrate to starch biosynthetic enzymes. In the barley hvflo6 mutant (also known as Franubet), the endosperm develops an unusual mix of simple and compound SGs (DeHaas BW and Goering KJ 1983; Chung T-Y 2001; Suh et al. 2004; Matsushima et al. 2023). In wheat, the reduced expression of BGC1 decreases B-type granule content in endosperm (Chia et al. 2017, 2020). In addition, the non-enzymatic protein, LIKE EARLY STARVATION (LESV), was recently reported to be involved in starch biosynthesis in Arabidopsis and rice (Liu et al. 2023; Dong et al. 2024; Yan et al. 2024). In rice, LESV can interact with FLO6 and mediate the localization of starch biosynthetic enzymes to starch (Dong et al. 2024; Yan et al. 2024).
Studies on the genetic interactions among mutations in starch biosynthesis genes have mainly focused on rice and maize (Ferguson et al. 1979; Toyosawa et al. 2016; Lee et al. 2017; Ida et al. 2021; Nagamatsu et al. 2022). In barley, the reduction in starch content and the corresponding increase in phytoglycogen in the hvisa1 grains is further enhanced by the hvflo6 mutation (Matsushima et al. 2023). The enhancement exceeds just an additive effect, indicating potential genetic interactions between the two mutations. The genetic interaction between hvflo6 and hvisa1 is also observed in the pollen grain, which accumulates starch like the endosperm of cereals. In both wild-type and hvisa1 pollen, simple, rod-shaped SGs predominantly are developed. In the hvflo6 pollen, the number of compound SGs is increased, and this increase is significantly pronounced in the hvflo6 hvisa1 double mutant (Matsushima et al. 2023).
In our previous study, we reported on the hvisa1 and hvflo6 mutants in barley, particularly in the elite Japanese malting barley cultivar ‘Haruna Nijo’, using the rapid, simple observation method of SGs (Matsushima et al. 2023). Unlike other procedures, this method does not require chemical fixation and resin embedding of samples, making it suitable for dealing with large number of samples (Matsushima et al. 2010). In this study, we report newly identified barley mutants, hvbe2a-1 and hvbe2a-2, which develop elongated SGs in endosperm, that are not present in the wild type. Both mutants had genetic lesions in the HvBE2a gene coding a major BE in barley endosperm. Our analysis using triple and double mutants of hvisa1, hvflo6 and hvbe2a revealed that hvbe2a mutation has a suppressive effect against hvisa1 mutation in endosperm. hvisa1 mutation altered the SG morphology from a bimodal to a compound type, and the addition of hvbe2a mutation reversed the SG morphology back to the bimodal form. In contrast to the endosperm, the suppressive effect of hvbe2a was not observed in pollen. These findings contribute to our understanding of the genetic network in starch biosynthesis and provide new insights into the determinants of SG morphology.
Materials and methods
Plant material and growth conditions
Barley cultivar’Haruna Nijo’ was provided from NBRP-Barley (http://earth.nig.ac.jp/~dclust/cgi-bin/index.cgi?lang=en). hvisa1-3 and hvflo6-2 were previously reported (Matsushima et al. 2023). hvbe2a-1 and hvbe2a-2 were isolated from the same screening described previously (Matsushima et al. 2023). The mutants were crossed with cv Haruna Nijo, and the progeny exhibiting hvbe2a phenotype were used in this study. Double and triple mutants were generated by artificially crossing individual mutants, and multiple homozygous individuals were selected from the F2 progeny using PCR-based genotyping. Barley plants were grown at 22 °C/18 °C in a growth cabinet (NK Systems, LPH-411S) or around 23 °C continuously at 16 h day/8 h night conditions. The shoot weight and number of tillers were measured 28 days after germination.
Observation of SGs by thin-section microscopy with Technovit 7100 Resin
The preparation of Technovit sections to observe SGs in the endosperm is described previously (Matsushima et al. 2014).
Purification of starch and quantification of starch granule size distributions
Starch purification was essentially conducted following Kamble et al. (2023). A grain was ground using the Multi-beads Shocker MB2000 (YASUI KIKAI, Japan), and the sample was resuspended in 2 mL water. The homogenates were filtered through a 100-μm nylon mesh, then centrifuged at 3,000 × g for 5 min. The pellet was resuspended in 2 mL water. The starch suspension on a 5-mL cushion of 90% (v/v) Percoll in 50 mM Tris–HCl (pH 8) was centrifuged at 2,500 × g for 15 min. The pellet was washed twice in 50 mM Tris–HCl (pH 6.8), 10 mM EDTA, 4% SDS (v/v), 10 mM DTT and then three times in water. Finally, the purified starch was washed with ethanol twice and dried.
To quantify starch granule size distributions, the purified starch was suspended in Isoton II electrolyte solution (Beckman Coulter, Indianapolis), and particle sizes were measured using a Multisizer 4e Coulter Counter (Beckman Coulter) fitted with a 70-μm aperture tube. A minimum of 100,000 particles was measured per sample. These data were used to produce relative percent volume vs. diameter plots. A mixed bimodal distribution (normal and lognormal distributions) was fitted to the relative percent volume vs. diameter plots (Python script available at: https://github.com/DavidSeungLab/Coulter-Counter-Data-Analysis) to calculate mean diameters of A- and B-type granules by fitting normal curves for A-type and lognormal curves for B-type to the granule size distribution traces (Hawkins et al. 2021).
Preparation of antibodies against HvBE2a, HvBE2b and HvBE1
All of the antibodies in this study were raised against synthetic peptides. Anti-HvBE2a antibodies were raised against AAAPGKVLVPDGESDDL (amino acid position from 52 to 68 of HvBE2a) and VDYFTTEHPHDNRPRS (amino acid position from 789 to 804 of HvBE2a). The two antibodies were named anti-HvBE2a-N and anti-HvBE2a-C, respectively. Anti-HvBE2b antibody was raised against AGGPSGEVMI (amino acid position from 58 to 67 of HvBE2b). Anti-HvBE1 antibody was raised against KRGINFVFRSPDKDNK (amino acid position from 810 to 825 of HvBE1). The immunization of rabbits and purification of antibodies were outsourced to Cosmo Bio (Tokyo, Japan) or Eurofins Genomics (Tokyo, Japan).
Immunoblot analysis following SDS-PAGE
Developing grains at 14 days after awn emergence (DAA) were cultivated and stored at − 80 °C before use. The grain was mixed with 150 μL of ice-cold grinding solution (50 mM imidazole–HCl [pH 7.4], 8 mM MgCl2, 12.5% [v/v] glycerol), and then crushed using plastic pestles. The homogenates were centrifugated at 16,000 × g at 4 °C for 5 min. The supernatant (50 μL) was mixed with 50 μL of SDS-sample buffer (2% [w/v] SDS, 100 mM Tris–HCl [pH 6.8], 2% [v/v] 2-mercaptoethanol, 40% [v/v] glycerol) and incubated at 98 °C for 10 min. 8 μL was subjected to SDS-PAGE, and proteins were transferred electrophoretically to a polyvinylidene difluoride membrane (Millipore). The membrane was then incubated in Phosphate-buffered saline (pH 7.4) plus 0.05% (v/v) Tween–20 with the antibodies for 1 h. Dilutions of the antibodies were 1:3,000—5,000 (v/v). The secondary antibody was an Anti-Rabbit IgG, HRP-Linked Whole Ab Donkey (Cytiva, NA934V), which was diluted (1:5,000). The immunoreactive bands were detected with Immobilon Crescendo Western HRP substrate (Millipore, WBLUR0500).
Quantification of starch and phytoglycogen
Total starch and phytoglycogen quantification in a grain are described previously (Matsushima et al. 2023). The Resistant Starch Assay Kit (Megazyme, K-RSTAR) was used according to the manufacturer’s instructions to measure the amount of resistant starch as a percentage of total starch.
Glucan chain length distribution of total α-glucan in grain
The procedures to detect the glucan-chain-length distribution of total α-glucan are the same as in our previous study (Matsushima et al. 2023).
Genotyping of mutations
The base change of hvbe2a-1 mutation was detected by the derived cleaved-amplified polymorphic sequence primers: 5’-TTAGGTGGCGAAGGCTATCTTAATTCCATG-3’ and 5’-GTTCAAATTACAATAAATCGCAACC-3’. The PCR conditions were as follows: 94 °C for 2 min and 35 cycles of 94 °C for 30 s, 53 °C for 45 s, and 68 °C for 1 min. The PCR product was digested with NcoI, and PCR products were subsequently separated by 15% polyacrylamide gel electrophoresis (PAGE) and detected with ethidium bromide staining. In the case of wild type, a PCR product (109 bp) was digested into 83 and 26 bp. In the case of hvbe2a-1, the PCR product was not digested. hvisa1-3 and hvflo6-2 mutation sites were detected according to the previous paper (Matsushima et al. 2023).
Genome sequencing of hvbe2a-2
High-molecular-weight DNA was isolated from leaf material of hvbe2a-2 seedlings using NucleoBond HMW DNA (Takara). A total of 0.5 μg of the isolated DNA underwent sequencing library preparation using the MGIEasy FS PCR-Free DNA library Prep Kit (MGI Tech, Shenzhen, China). Whole-genome sequencing was performed on the DNBSEQ-G400RS platform (MGI Tech) by a commercial vendor (Genome-Lead Co., Kagawa, Japan), yielding 375.9 million reads (2 × 150 nucleotides) of the hvbe2a-2 genome. The genomic sequence reads of barley cv Haruna Nijo (1.5 billion reads, 2 × 100 nucleotides) were obtained from a previous report (Sato et al. 2016). These sequence reads underwent quality control and were trimmed using Trimmomatic version 0.39 (Bolger et al. 2014), then mapped to the reference sequence assembly of Haruna Nijo (Sakkour et al. 2022) using bwa-mem version 0.7.17-r1188 (Li and Durbin 2009) with the default parameters. Only uniquely mapped reads were retained, then the read depth for genomic regions were assessed and visualized using IGV version 2.16.2 (Robinson et al. 2011).
Detection of branching enzyme activity in the developing endosperm following Native-PAGE
After thawing the harvested grains at 14 DAA, the removal of the embryo portion was followed by applying gentle pressure to the opposite side, facilitating the extraction of the endosperm from the grain. The endosperm was homogenized in the ice-cold grinding solution (80 μL), and then crushed using plastic pestles. The homogenates were subjected to centrifugation at 16,000 × g at 4 °C for 5 min. Protein concentration of the supernatant was determined using a Bradford Protein Assay Kit (Takara, Japan, T9310A). The supernatant was mixed with sample buffer (400 mM Tris–HCl [pH 7.0], 33% [v/v] glycerol) to adjust the protein concentration to 1 μg/μL. Proteins (7.5 μg) were subjected to Native-PAGE. BE activity staining was assessed using a gel containing 0.0001% oyster glycogen (Yamanouchi and Nakamura 1992). The immunoblot analysis following the Native-PAGE is identical to the analysis following SDS-PAGE.
Observation of SGs in pollen grains
To stain SGs in mature pollen, anthers at 3–5 DAA were disrupted with forceps in 120-times diluted Lugol solution on a glass cover slide. The released pollen was squashed by putting gentle pressure on a coverslip to release SGs from pollen vegetative cells. The released SGs were observed with a microscope (Olympus, BX53). For the quantification of compound SGs in pollen, the SGs within the 30 μm × 30 μm field of view were classified into simple and compound forms through visual inspection.
Isolation of pollen grains and protein extraction from pollen
Anthers, at 3 DAA, were collected in a 1.5 mL plastic tube and stored at − 80 °C until use. The anthers were mixed with the ice-cold grinding solution (1 mL) and chopped using a surgical scissor (Hammacher, HSB022-12). The homogenates were then filtered through a 100-μm nylon mesh to remove the debris other than pollen. The flow through was centrifuged at 1,000 × g at 4 °C for 1 min. The resulting pellet of pollen grains was resuspended in the ice-cold grinding solution (500 μL) and centrifuged again. The pollen pellet was resuspended once more in the ice-cold grinding solution (50 μL) and disrupted using plastic pestles in the tube. The homogenates were centrifuged at 10,000 × g at 4 °C for 2 min. The supernatant was recovered in another tube and protein concentration was measured using the Bradford Protein Assay kit. The supernatant was mixed with grinding solution and SDS-sample buffer to adjust the protein concentration to 0.5 μg/μL and heated immediately at 98 °C for 10 min. The resulting protein extract containing 5 μg of total protein was subjected to SDS-PAGE and immunoblot analysis.
Results
Barley mutants with elongated starch granules in endosperm
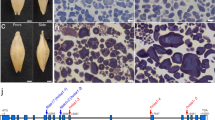
In our screen for barley mutants with altered SG morphology, we examined endosperm SGs in a barley sodium-azide-mutagenized population derived from the Japanese elite malting barley cultivar Haruna Nijo (Matsushima et al. 2023). We isolated mutants, namely hvbe2a-1 and hvbe2a-2, which exhibited elongated SGs in the endosperm (Fig. 1). Subsequent sequencing analysis identified the causative mutations are in the HvBE2a gene of both mutants (described in a subsequent section); for clarity, we will use the hvbe2a-1 and hvbe2a-2 nomenclature to refer to mutants of interest. Haruna Nijo refers to the wild-type reference in this paper.
Isolation of barley mutants with elongated starch granules. a–c Mature grains of Haruna Nijo a and hvbe2a-1 b and hvbe2a-2 c. Front and side views are shown. Bars = 1 mm. d Single grain weight of Haruna Nijo, hvbe2a-1 and hvbe2a-2 at the mature stage (n = 30, 30, 70, respectively). Statistical comparisons were performed using Welch’s t-test (ns, not significant at p = 0.05). e–j Iodine-stained thin sections of endosperm cells of Haruna Nijo (e and f), hvbe2a-1 (g and h), and hvbe2a-2 (i and j). Bars = 20 μm. k–m Granule size distributions of Haruna Nijo, hvbe2a-1 and hvbe2a-2, respectively. The relative percent volume of each diameter was determined using a Coulter Counter (n = 4 each). The graphs are displayed by overlaying the data from four biological replicates with different colors. n–o The average diameter of A- and B-type granules, respectively, extracted from the relative percent volume vs. diameter plots (k-m) by fitting a bimodal mixed normal and lognormal distribution. p Resistant starch amount in total starch of hvbe2a grains (n = 4 each). Statistical comparisons were performed using Wilcoxon rank sum test (*, p < 0.05). q Glucan chain-length distribution of α-glucans in hvbe2a mutant grains. hvbe2a-1, hvbe2a-2 and Haruna Nijo are indicated by green, blue and grey lines, respectively. Data are given as means ± SD. All data were obtained from at least three independent grains. r Difference plot corresponding to the glucan-chain-length distribution profile presented in (q). The value for each chain length of Haruna Nijo was subtracted from that of the hvbe2a mutants
The mature grains of these mutants displayed a similar appearance to those of the wild type (Fig. 1a–c). The grain weight of the hvbe2a-1 and hvbe2a-2 were almost the same as those of Haruna Nijo (Fig. 1d). The dimensions of grains, including length, width, and thickness, from the hvbe2a-1 mutant were almost the same as those of Haruna Nijo. Certain measurements from the hvbe2a-2 mutant showed small changes compared to Haruna Nijo, but they were not consistently significant for both hvbe2a-1 and hvbe2a-2 (Supplementary Fig. 1a–c). This means that they are not caused by the mutation per se.
Iodine-stained Technovit thin sections of the endosperm revealed that Haruna Nijo developed typical bimodal SGs (Fig. 1e, f). In contrast, in hvbe2a-1 (Fig. 1g, h) and hvbe2a-2 (Fig. 1i, j) mutants, some SGs displayed elongated shapes. Coulter Counter analysis demonstrated that SGs from both Haruna Nijo and the hvbe2a mutants exhibited a similar bimodal distribution (Fig. 1k–m). The average diameters of A- and B-type SGs were not significantly different between Haruna Nijo and the hvbe2a mutants (Fig. 1n, o). The minimal changes on the distribution curves detected by the Coulter Counter analysis indicates that the elongated SGs in the hvbe2a mutants were either similar in volume to normal A-type granules, or not the majority. Next, we measured the amount of resistant starch in the hvbe2a mutant grains. Both hvbe2a-1 and hvbe2a-2 grains contained higher amount of resistant starch compared to Haruna Nijo; however, significant variation was observed among the biological replicates (Fig. 1p). The glucan chain length distribution of α-glucan from hvbe2a grains showed subtle differences compared to Haruna Nijo (Fig. 1q). The differential plot showed that both hvbe2a mutants had decreased glucose chains at degree of polymerization (DP) 11, while showing an increase in the frequency of chains at DP 17, relative to Haruna Nijo (Fig. 1r).
Plant appearance at 28 days after germination showed no significant difference in tiller numbers and shoot weights among Haruna Nijo, hvbe2a-1 and hvbe2a-2 mutants (Supplementary Fig. 2a–e). The panicle appearance of hvbe2a mutants was similar to that of Haruna Nijo at 20 DAA and mature stage (Supplementary Fig. 2f–k).
Genetic lesion in BRANCHING ENZYME 2a gene in barley
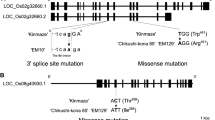
The F1 plant from the cross between hvbe2a-1 and hvbe2a-2 exhibited elongated SGs just like hvbe2a-1 and hvbe2a-2 (Fig. 2a–d). This means that hvbe2a-1 and hvbe2a-2 are allelic to each other. The previous study by Regina (2010) revealed that barley transgenic lines with RNAi-suppressed HvBE2a genes exhibited the occasional alternations of SGs in morphology in endosperm. Furthermore, the RNAi lines showed a decrease in the distribution of glucan chain length around DP10 and an increase around DP15 compared to the parental line (Regina et al. 2010). This pattern closely resembled the profile observed in hvbe2a mutants in Fig. 1r. The phenotypic similarities encouraged us to determine the sequence of the HvBE2a gene in hvbe2a mutants. We amplified the genomic region (chr2H:464,360,851–464,373,337 in Haruna Nijo pseudomolecules v1.) covering the HvBE2a cDNA sequence (AF064560) from hvbe2a-1 and determined the sequence. In hvbe2a-1, the guanine residue located 10,639 bp downstream of the first ATG was replaced by adenine (Fig. 2e). The predicted HvBE2a protein has a plastidial transit peptide at its N-terminus, a CBM48, and a central (β/α)8 catalytic module characteristic of the α-amylase family (Catalytic domain). Additionally, it features β-domains typically found in the C-terminus of α-amylase family members (C-domain) (Fig. 2f). The hvbe2a-1 mutation replaces the glycine residue at position 651with an arginine residue. The glycine residue is conserved across various starch- and glycogen-branching enzymes (Fig. 2g). We designed derived cleaved-amplified polymorphic sequence (dCAPS) primers to detect the base change in hvbe2a-1+/–. The dCAPS primers successfully genotyped the wild-type Haruna Nijo, heterozygous HvBE2a/hvbe2a-1, and homozygous mutation of hvbe2a-1 (Supplementary Fig. 3a). To confirm whether the base change in the hvbe2a-1 mutant co-segregates with the phenotype of the elongated SGs existence in endosperm, we crossed hvbe2a-1 with Haruna Nijo and produced F2 populations. Out of the 70 F2 grains, 19 developed elongated SGs in the endosperm, suggesting that the phenotype segregated in a single recessive manner (χ2 = 0.17, p = 0.68). Of these 19 grains, fifteen were randomly selected and grown into seedlings for genotyping. The genotyping using the dCAPS primer showed that all the 15 plants were homozygous for the hvbe2a-1 base change (Supplementary Fig. 3b). This result supports the idea that the base change in the HvBEIIa gene of the hvbe2a-1 mutant is responsible for the elongated SG phenotype.
Genetic lesions in hvbe2a-1 and hvbe2a-2. a–b Iodine-stained thin sections of endosperm cells of F1 grains from a cross between hvbe2a-1 and hvbe2a-2. Bars = 20 μm. c–d Iodine-stained thin sections of hvbe2a-1 and hvbe2a-1 mutants, respectively. Bars = 20 μm. e The structure of the HvBE2a gene on chr2H:464,360,851..464373337 in Haruna_Nijo_pseudomolecules_v1. The coding and untranslated regions are depicted as blue and white boxes, respectively. Introns are indicated by black lines. The exon–intron structure is based on the reported full-length cDNA (AF064560). The adenine in the translation start codon (ATG) is designated as + 1. hvbe2-1 has a base pair change from G to A at + 10,639, leading to an amino acid substitution of Gly651 by arginine (R). f The protein structure of HvBE2a. The first methionine is designated as + 1. The predicted plastidial transit peptide, carbohydrate-binding module of family 48 (CBM48), the central (β/α)8 catalytic module of α-amylase family (Catalytic domain) and β-domains typically found in the C terminus of α-amylases family members (C-domain) are depicted according to Pfister and Zeeman (2016) and Noguchi et al. (2011). The putative catalytic triad Asp469-Glu524-Asp592 is shown asterisks. g Alignment of sequence around hvbe2-1 mutation with other starch- and glycogen-branching enzymes. HvBE2a, HvBE2b, HvBE1 (HORVU.MOREX.r3.2HG0165780.1, HORVU.MOREX.r3.2HG0170370.1 and HORVU.MOREX.r3.7HG0751660.1), ZmBEIIa, ZmBEIIb and ZmBEI (Zea mays, AAB67316, NP_001105316, and NP_001105370), OsBEIIa, OsBEIIb and OsBEI (Oryza sativa, AB023498, Os02t0528200-01 and Os06t0726400-01), StBEII and StBEI (Solanum tuberosum, CAB40748 and CAA49463), ScGLC3 (Saccharomyces cerevisiae, AAA34632), HsGBEI (Homo sapiens, NM_000158). Perfectly conserved residues are shown in black. Red arrowheads indicate the residues substituted by hvbe2a-1 mutation. The Clustal W program was used for the alignment. h The deletion on chromosome 2H in hvbe2a-2 including the HvBE2a locus. Gray and blue bars represent the relative mapping depth of the NGS short reads for the Haruna Nijo and hvbe2a-2 genomes, respectively, depicted on a logarithmic scale. The relative positions of three annotated genetic loci, including HvBE2a, are marked within the deletion region spanning from 463,944 to 464,477 kb on chromosome 2H
Next-generation sequencing of the hvbe2a-2 mutant genome, followed by the mapping of the obtained short reads against the Haruna Nijo genome, identified the genomic region with missing reads in hvbe2a-2 (Fig. 2h). This region, extending from 464,944 to 464,477 kb on chromosome 2H, includes the HvBE2a gene and two other annotated genes. Thus, hvbe2a-2 is missing the HvBE2a gene due to a deletion.
HvBE2a protein accumulation and branching enzyme activity in hvbe2a mutants
Next, we examined the accumulation of the HvBE2a protein and its enzyme activity in hvbe2a mutants. The amino acid sequences of the synthetic peptides used to create the antibodies are shown in Supplemental Fig. 4. The two anti-HvBE2a antibodies recognize the N-terminus and C-terminus of the mature HvBE2a sequences without the transit peptide, respectively. We refer to the two antibodies as anti-HvBE2a-N and anti-HvBE2a-C. Both antibodies recognized the band around 90 kDa in Haruna Nijo and hvbe2a-1, but not in hvbe2a-2 mutant in the immunoblot analysis of the developing grains (Fig. 3a). The band size is consistent with the expected molecular weight of HvBE2a, which is 87.6 kDa, excluding the transit peptide. The absence of the detected band in the hvbe2a-2 is due to the deletion of the HvBE2a gene (Fig. 2h). In both Haruna Nijo and hvbe2a-1, anti-HvBE2a-C antibody detected the smaller-sized bands together with the 90-kDa band, which could be degradation products of HvBE2a since they were absent in hvbe2a-2. Out of the degradation products, the band around 60 kDa, detected with anti-HvBE2a-C antibody, showed significant intensity comparable to that of the full-length band of HvBE2a, implying that it is the most abundant degradation product. This band was not detected with anti-HvBE2a-N antibody, suggesting that the N-terminus is missing in this degradation product. This suggests that HvBE2a is more prone to degradation from the N-terminus.
Starch branching enzyme activity and protein accumulation in hvbe2a mutants. a Immunoblot analysis with anti-HvBE2a-N and anti-HvBE2a-C antibodies after SDS-PAGE of developing endosperm extracts from Haruna Nijo, hvbe2a-1 and hvbe2a-2. The molecular masses are given on the left in kDa. Membranes are stained with Ponceau-S to verify equal protein loading and transfer. b Native-PAGE activity staining of starch branching enzymes in mutants and immunoblot analysis after Native-PAGE. Proteins from at 14 days after awn emergence (7.5 μg) were loaded on each lane. c–e Immunoblot analysis using anti-HvBE2a-C, anti-HvBE2b and anti-HvBE1, respectively. Closed and open arrowheads indicate the position of HvBE2a and HvBE2b, respectively
Next, we investigated BE activity in the maturing endosperm of hvbe2a mutants. In barley, besides HvBE2a, there are other BE isozymes, namely HvBE2b and HvBE1. These show 76% and 46% amino acid sequence identity with HvBE2a, respectively. To discern their individual activities, we utilized an in-gel Native-PAGE activity assay, which separates these isozymes on the gel. In Haruna Nijo maturing endosperm at 14 DAA, two bands with BE activity were detected with slightly different mobilities on the gel (Fig. 3b). The upper band was major and the lower band was minor (Fig. 3b). In the case of hvbe2a-1 and hvbe2a-2 mutants, the upper band was not detected, while the only lower band were detected to have BE activity. To ascertain the precise locations of the bands corresponding to each isozyme in Native-PAGE, we constructed specific antibodies recognizing HvBE2b and HvBE1, respectively in addition to HvBE2a (Supplemental Fig. 4). Immunoblot analysis following Native-PAGE revealed that the upper and lower bands with BE activities in Haruna Nijo corresponded to HvBE2a and HvBE2b, respectively (Fig. 3c, d). This suggests that HvBE2a is the major BE enzyme in the developing endosperm of Haruna Nijo. The protein accumulation level of HvBE2a in hvbe2a-1 was almost the same as in Haruna Nijo. The absence of BE activity from HvBE2a in hvbe2a-1 indicates that the amino acid substitution in hvbe2a-1 does not impact protein accumulation but is crucial for enzymatic activity of HvBE2a. Although HvBE1 was detected as more than two bands with different mobilities on the immunoblotted membrane, no BE activity was observed at these positions under our experimental conditions (Fig. 3b, e).
Suppressive effect of hvbe2a mutations against starchless phenotype of hvflo6-2 hvisa1-3 grain. a Cross-sections of a mature grain of Haruna Nijo and mutants. Bars = 1 mm. b Single grain weight of triple mutant mature grains (n = 6–8). c Starch amount per grain of triple mutant mature grains (n = 6–8). Data are given as means ± SD. Statistical comparisons were performed using Tukey’s HSD. The same letters above the bars represent statistically indistinguishable groups, and different letters represent statistically different groups (p < 0.05)
hvbe2a suppresses the starchless phenotype of hvflo6 hvisa1
The hvflo6 mutation enhances the hvisa1 phenotype, leading to a significant reduction in starch content in grains and severe grain shrinkage in hvflo6 hvisa1 double mutants (Matsushima et al. 2023). In rice, the isa1 phenotype is mitigated by the mutations of be2a and be2b (Lee et al. 2017; Nagamatsu et al. 2022). We therefore generated the hvflo6 hvisa1 hvbe2a triple mutant to examine the suppressive effect of hvbe2a mutations against the hvflo6 hvisa1 phenotypes. The hvbe2a-1 and hvbe2a-2 mutants did not show any significant differences in grain cross-sections compared to Haruna Nijo (Fig. 4a). The hvflo6-2 hvisa1-3 grains were shrunken. Interestingly, when hvbe2a-1 and hvbe2a-2 mutations were introduced into the hvflo6-2 hvisa1-3 background, the grain shrinkage phenotype was partially rescued in both triple mutants (Fig. 4a). The results show that hvbe2a mutations have a suppressive effect on the hvflo6-2 hvisa1-3 phenotype. We also measured the grain weight and starch content in the grains of the triple mutants (Fig. 4b, c). For the triple mutants, the individual grain weight was higher than that of the hvflo6-2 hvisa1-3 double mutant but lower than that of Haruna Nijo (Fig. 4b). Similarly, the starch content of the grain in triple mutants was higher than that of the hvflo6-2 hvisa1-3 grain but lower than that of Haruna Nijo (Fig. 4c). This indicates that loss of HvBE2a function did not completely suppress the double mutant phenotype of hvflo6-2 hvisa1-3.
To confirm which mutations, hvflo6-2 or hvisa1-3, are targeted by the suppressive effect of hvbe2a, we generated a series of double mutants, including hvisa1-3 hvbe2a-1, hvisa1-3 hvbe2a-2, hvflo6-2 hvbe2a-1 and hvflo6-2 hvbe2a-2. In line with the previous observation (Matsushima et al. 2023), the starch content of the single mutants of hvflo6-2 and hvisa1-3 grains was reduced compared to Haruna Nijo (Fig. 5a). The starch content was higher in the mutants of hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 compared to the hvisa1-3 single mutant. Their starch content was restored to the wild-type level (Fig. 5a). In contrast, there was no significant difference in the starch content of hvflo6-2 hvbe2a-1 and hvflo6-2 hvbe2a-2 grains compared to the hvflo6-2 single mutant (Fig. 5a). This result means that the suppressive effect of the hvbe2a mutations predominantly targets hvisa1-3, rather than hvflo6-2.
Suppressive effect of hvbe2a mutations against hvisa1-3 starch properties. a Starch amount per grain of single and double mutants. Data are given as means ± SD using at least three biological replicates. Statistical comparisons were performed using Welch’s t-test (**, p < 0.01; and ns, not significant at p = 0.05). The starch reduction in hvisa1-3 was recovered by adding hvbe2a mutations. In contrast, the starch reduction in hvflo6-2 was not affected by hvbe2a mutations. b Amount of phytoglycogen per mature grain in double and triple mutants. Data are given as means ± SD using at least three biological replicates. Statistical comparisons were performed using Welch’s t-test (**, p < 0.01). The increases of phytoglycogen in hvisa1-3 and hvflo6-2 hvisa1-3 were suppressed by adding hvbe2a mutations. c Glucan chain-length distribution of α-glucans of mature grains. Haruna Nijo, hvisa1-3, hvisa1-3 hvbe2a-1, hvisa1-3 hvbe2a-2 are indicated by the black, red, green and blue lines, respectively. The values for hvisa1-3 are identical to the data in Matsushima et al. (2023). Data are given as means ± SD. All data were obtained from at least three independent grains
We have previously shown that the hvisa1-3 grains accumulate significantly more phytoglycogen compared to the wild type (Matsushima et al. 2023). Furthermore, this accumulation was found to be even more pronounced with the addition of the hvflo6-2 mutation. To determine whether the hvbe2a mutations suppress the phytoglycogen accumulation of hvisa1-3, we measured the phytoglycogen accumulation in hvisa1-3 hvbe2a double mutants. The amount of phytoglycogen in hvisa-3 hvbe2a-1 and hvisa-3 hvbe2a-2 mutants decreased by 88% and 77%, respectively, compared to hvisa-3 (Fig. 5b). These data demonstrate a noteworthy decrease in phytoglycogen accumulation in both hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 mutants, compared to the single hvisa1-3 mutant (Fig. 5b). This result confirms that the suppressive effect of the hvbe2a mutations primarily targets hvisa1. We also examined the suppressive effect of hvbe2a mutations on phytoglycogen accumulation in the hvflo6-2 hvisa1-3 background. The amount of phytoglycogen in hvflo6-2 hvisa-3 hvbe2a-1 and hvflo6-2 hvisa-3 hvbe2a-2 triple mutants decreased by 45% and 30%, respectively, compared to hvflo6-2 hvisa-3 double mutants (Fig. 5b). In the triple mutants, the phytoglycogen were decreased compared to the hvflo6-2 hvisa1-3, but not to the level of hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 (Fig. 5b). This indicates that hvflo6-2 and hvbe2a are in competition to either enhance or suppress the phenotype of hvisa1-3.
The distribution of glucan chain length in α-glucan from hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 grains closely resembles that of Haruna Nijo (Fig. 5c). While, hvisa1-3 single mutant exhibits a higher abundance of shorter glucose chains (DP < 10) and a lower abundance of longer glucose chains (DP > 10) compared to Haruna Nijo. This also supports the suppressive effect of the hvbe2a mutations against hvisa1-3.
Transformation of starch granule morphology by hvisa1 and hvbe2a
Next, we investigated the impact of hvbe2a mutations on the SG morphology of the hvisa1-3 mutant. In hvisa1-3, compound SGs were well developed in the endosperm, replacing the original bimodal simple SGs typical of barley (Fig. 6a, b). This is consistent with previous observations (Matsushima et al. 2023). In the endosperm of hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2, typical A- and B-type SGs were developed (Fig. 6c–f). When SGs of hvisa1-3 were purified and analyzed using a Coulter Counter, the granule size distribution with typical bimodal peaks of A- and B-type SGs was not observed (Fig. 6g). Instead, a single peak was predominant. The observed peak is most likely attributed to the starch particles that formed the compound SGs of hvisa1-3 and subsequently disintegrated during purification. In the case of hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2, the Coulter Counter analysis consistently showed bimodal peaks (Fig. 6h, i). This suggests that in barley, SGs change from authentic bimodal type to compound type due to the hvisa1 mutation, and the hvbe2a mutation can reverse these compound SGs back to the bimodal type. We have analyzed the average diameters of A- and B-type granules in hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 mutants. Our findings reveal that for A-type granules, the diameter in both hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 mutants was approximately 80% of that in Haruna Nijo (Fig. 6j). Similarly, for B-type granules, the diameter in both mutants was around 85% relative to that in Haruna Nijo (Fig. 6k). The observation of smaller bimodal SGs in the hvisa1-3 hvbe2a mutants, compared to those in Haruna Nijo, indicates that the depletion of HvBE2a does not fully suppress the SG morphological changes induced by hvisa1-3. We also investigated the impact of the hvbe2a mutations on SG morphology in the hvflo6-2 mutant. In hvflo6-2, compound SGs were well developed in the endosperm (Fig. 7a, b), consistent with previous observations (Matsushima et al. 2023). In the endosperm of hvflo6-2 hvbe2a-1 and hvflo6-2 hvbe2a-2, hvbe2a-specific elongated SGs were clearly observed alongside the hvflo6-2-specific compound SGs (Fig. 7c–f). This suggests that the effects of the hvflo6-2 and hvbe2a mutations are cumulative with respect to SG morphology. Regarding the α-glucan chain length distribution, hvflo6-2 exhibited a distribution nearly identical to that of Haruna Nijo (Fig. 7g, h), consistent with previous research (Matsushima et al. 2023). When comparing the hvbe2a-1 mutant with the hvflo6-2 hvbe2a-1 double mutant, there was a slight reduction in glucose chains ranging from DP7 to DP15 in the double mutant (Fig. 7g). Similarly, the hvflo6-2 hvbe2a-2 double mutant showed a slight reduction in glucose chains from DP10 to DP15 compared to the hvbe2a-2 mutant (Fig. 7h). However, no other significant differences in glucose chain length were observed between the double and single mutants (Fig. 7g, h). These findings indicate that the genetic interaction between the hvflo6 and hvbe2a mutations differs from the suppressive interaction observed between the hvisa1 and hvbe2a mutations.
Suppressive effect of hvbe2a mutations on hvisa1-3 starch granule morphology in endosperm. a–f Iodine-stained thin sections of endosperm cells of hvisa1-3 (a and b), hvisa1-3 hvbe2a-1 (c and d), and hvisa1-3 hvbe2a-2 (e and f). Bars = 20 μm. g–i Granule size distributions of hvisa1-3, hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2, respectively. The relative percent volume of each diameter was determined using a Coulter Counter. j–k The average diameter of A- and B-type granules, respectively, extracted from the relative percent volume vs. diameter plots of hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 (h and i) by fitting a bimodal mixed normal and lognormal distributions (n = 4). The values for Haruna Nijo are identical to the data presented in Fig. 1 (n, o). Data are given as means ± SD. Statistical comparisons were performed using Tukey’s HSD. The same letters above the bars represent statistically indistinguishable groups, and different letters represent statistically different groups (p < 0.05)
Starch granule morphology and glucan chain-length distribution of hvflo6 hvbe2a double mutants. a–f Iodine-stained thin sections of endosperm cells of hvflo6-2 (a and b), hvflo6-2 hvbe2a-1 (c and d), and hvflo6-2 hvbe2a-2 (e and f). Bars = 20 μm. g Glucan chain-length distribution of α-glucans of mature grains. Haruna Nijo, hvflo6-2, hvbe2a-1, hvflo6-2 hvbe2a-1 are indicated by the black, red, green and blue lines, respectively. h Glucan chain-length distribution of α-glucans of mature grains. Haruna Nijo, hvflo6-2, hvbe2a-2, hvflo6-2 hvbe2a-2 are indicated by the black, red, green and blue lines, respectively. The values for hvflo6-2 are identical to the data in Matsushima et al. (2023). Data are given as means ± SD. All data were obtained from at least three independent grains
Absence of suppressive effect of hvbe2a against hvflo6 hvisa1 in pollen
Pollen, as well as endosperm, accumulates storage starch. The rod-shaped SGs were well developed in pollen from Haruna Nijo, hvisa1-3, hvbe2a-1 and hvbe2a-2 (Fig. 8a). In the hvflo6-2 mutant, a notable increase in compound SGs was observed in addition to the rod-shaped SGs. The proportion of the compound SGs in hvflo6-2 pollen was up to 66.5%, whereas those in Haruna Nijo, hvisa1-3, hvbe2a-1, and hvbe2a-2 were less than 9% (Fig. 8b). The proportion of the compound SGs in the hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-1 pollens were not statistically significant compared to hvisa1-3 pollen (Supplemental Fig. 5a–b). In contrast, the double mutants, hvflo6-2 hvbe2a-1 and hvflo6-2 hvbe2a-2, exhibited well-developed compound SGs at the same level as hvflo6-2 (Fig. 8a, b). This indicates that hvbe2a does not suppress hvflo6-2 in terms of compound SG formation in pollen. In contrast, the hvisa1-3 mutation significantly enhanced the formation of compound SGs in hvflo6-2 pollen (Fig. 8a). In the hvflo6-2 hvisa1-3 pollen, more than 90% of SGs were of the compound type (Fig. 8b). This implies that HvISA1 is involved in compensating for HvFLO6 function in pollen, especially in the hvflo6-2 background. We also observed pollen SGs in triple mutants, hvflo6-2 hvisa1-3 hvbe2-1 and hvflo6-2 hvisa1-3 hvbe2-2. The proportion of compound SGs in these triple mutants did not significantly differ from that in the hvflo6-2 hvisa1-3 mutants (Fig. 8b). This observation supports the hypothesis that the hvbe2a mutation cannot suppress the formation of compound SGs induced by hvflo6-2 or hvisa1-3 in pollen.
Starch granules in mutant pollen. a Iodine-stained SGs released from squashed pollen grains. Bars = 5 μm. b The percentage of starch granules in compound form, as determined from microscopic images. Data are given as means ± SD. Statistical comparisons were performed using Welch’s t-test (ns, not significant at p = 0.05). c–e Accumulation of HvBE2a, HvBE2b, and HvBE1 in pollen. Protein extracts from developing endosperm at 14 days after awn emergence (DAA) and mature pollen at 3 DAA from Haruna Nijo and mutants were subjected to SDS-PAGE followed by the immunoblot analysis using anti-HvBE2a-C, anti-HvBE2b, and anti-HvBE1 antibodies. Each lane contains 5 μg of protein. The molecular masses are indicated on the left in kDa. Membranes are stained with CBB to verify equal protein loading and transfer. Arrow and asterisk in d indicate the positions of specific and non-specific bands, respectively
To investigate the protein accumulation of HvBE2a, HvBE2b, and HvBE1 in pollen, pollen protein extracts were subjected to the immunoblot analysis (Fig. 8c–e). HvBE2a accumulation was not detected in the mutants’ pollen harboring hvbe2a-2 allele (hvbe2a-2, hvflo6-2 hvbe2a-2 and hvflo6-2 hvisa1-3 hvbe2a-2) because of the deletion of the HvBE2a gene (Fig. 8c). In pollen, HvBE2a accumulation is lower overall, even in Haruna Nijo, when compared to the endosperm (Fig. 8c). The HvBE2a band intensity of the Haruna Nijo pollen is slightly stronger compared to that of hvbe2a-1 pollen (Fig. 8c). This may indicate that the amino acid substitution in hvbe2a-1 affects protein accumulation particularly in pollen. In the hvisa-1-3 and hvflo6-2 mutants, the accumulation of HvBE2a was slightly higher than in Haruna Nijo.
Anti-HvBE2b antibodies recognized the band around 90 kDa in the developing endosperm (Fig. 8d, arrow). The band size is consistent with the expected molecular weight of HvBE2b (87.9 kDa). The bands with the same size were detected in pollen of hvflo6-2 hvbe2a-1, hvflo6-2 hvbe2a-2, hvflo6-2 hvisa1-3, hvflo6-2 hvisa1-3 hvbe2a-1, and hvflo6-2 hvisa1-3 hvbe2a-2. It is noted that these mutants are characterized by the increased proportion of compound SGs in their pollen (Fig. 8b). Anti-HvBE2b antibodies also detected bands around 100 kDa only in the pollen samples (Fig. 8d, asterisk). These bands are significantly larger than the expected molecular weight of HvBE2b, and may be non-specific bands unique to the pollen samples. Anti-HvBE1 antibodies recognized the band around 90 kDa in developing endosperm of Haruna Nijo, which is consistent with the expected molecular weight of HvBE1 (86.5 kDa) (Fig. 8e). In hvisa-1-3 and hvbe2a-2 pollen, HvBE1 was significantly accumulated compared to Haruna Nijo.
Fertility of pollen with compound starch granules
In order to evaluate the fertility of hvflo6-2 pollen with compound SGs, we studied the SGs in mature pollens from heterozygous plant (HvFLO6/hvflo6-2). We measured the segregation ratio of hvflo6-2 mutant pollen (having compound SGs) to wild-type pollen (containing normal rod-shaped simple SGs) (Table 1). The segregation ratio was almost 1:1. This suggests that the hvflo6-2 mutation behaves in a gametophytic manner in pollen and that hvflo6-2 pollen matures successfully without aborting during the developmental stage. We then obtained self-fertilized F2 grains from HvFLO6/hvflo6-2 heterozygous plants and examined the segregation ratio of hvflo6-2 grains to wild-type grains by observing SG morphology in each grain (Table 2). The segregation ratio was 1:3 without any segregation distortion. This indicates that the fertility of hvflo6-2 pollen is not significantly different from that of wild-type pollen. These results suggest that the increase in compound SGs in hvflo6-2 pollen does not significantly affect the pollen fertility.
In our previous paper (Matsushima et al. 2023), we presented the co-segregation analysis of shrunken grains caused by the hvflo6-2 hvisa1-3 genotype. This analysis used the selfed population of HvFLO6/hvflo6-2 hvisa1-3 plants, where hvflo6-2 is heterozygous, and hvisa1-3 is homozygous. In the selfed population, the segregation ratio of shrunken grains to normal grains was approximately 1:3 (Matsushima et al. 2023). Genotyping of the grains revealed that all shrunken grains were consistent with the hvflo6-2 hvisa1-3 double homozygous genotype. On the other hand, normal grains were either HvFLO6/hvflo6-2 hvisa1-3 or HvFLO6 hvisa1-3. This indicates that the hvflo6-2 mutation segregates as a single recessive allele in the hvisa1-3 background. This undistorted co-segregation experiment suggests that the fertility of hvflo6-2 hvisa1-3 pollen (having compound SGs) is not significantly different from that of hvisa1-3 pollen (containing normal rod-shaped simple SGs).
Discussion
Isolation of mutants defective in HvBE2a gene
BE is an enzyme that creates branches during amylopectin synthesis (Nakamura 2002; Pfister and Zeeman 2016). In barley and wheat endosperms, BE2a is the major BE isozyme in endosperm (Regina et al. 2006, 2010). In this study, two barley mutants with genetic lesions in HvBE2a gene were isolated through screening based on the SG morphology (Fig. 1). In hvbe2a-1 and hvbe2a-2 mutants, elongated SGs were observed in the endosperm cells (Fig. 1g–j). Previously, transgenic barley with suppressed HvBE2a expression using RNAi were created, but mutants have not been isolated (Regina et al. 2010). The hvbe2a-1 mutant had a nucleotide change causing an amino acid substitution, and the hvbe2a-2 mutant had the complete deletion of the HvBE2a gene (Fig. 2). In the hvbe2a-1 mutant, the HvBE2a-1 protein accumulated in similar amounts to the wild-type HvBE2a protein in Haruna Nijo. However, no BE activity was detected from the HvBE2a-1 protein (Fig. 3b, c). Therefore, the substituted amino acid residue in the HvBE2a-1 protein does not affect protein accumulation but is crucial for the enzymatic activity. This observation aligns with the location of the amino acid substitution near the catalytic triad, and the fact that this residue is conserved among BEs of various plant species as well as glycogen BEs from animals and bacteria (Fig. 2g).
BEIIb is the primary BE in rice endosperm (Mizuno et al. 1993). Rice beIIb mutant EM10 fails to accumulate BEIIb protein, while another beIIb mutant, ssg3, carries a base substitution leading to an amino acid change in BEIIb (Mizuno et al. 1993; Matsushima et al. 2010). ssg3 mutation allows the accumulation of BEIIb protein but eliminates its enzymatic activity. EM10 and ssg3 exhibit similar phenotypes, characterized by the reduced polygonal starch particles and altered glucan chain length distributions (Matsushima et al. 2010; Nagamatsu et al. 2022). However, they differ in starch biosynthetic enzyme complex formation (Crofts et al. 2018). This difference is thought to arise from the inactive enzyme still having the ability to contribute to complex formation (Crofts et al. 2018). In this study, in the glucan chain length distribution, hvbe2a-1 exhibited a more pronounced reduction around DP11 compared to hvbe2a-2 (Fig. 1q, r). The difference may be due to the distinct protein complexes of starch biosynthetic enzymes. In the hvbe2a-1 mutant, the HvBE2a protein is present without biochemical activity, which may prevent other isozymes from replacing it. This could lead to a more severe phenotype compared to the complete absence of HvBE2a in hvbe2a-2. However, we did not observe any morphological differences in SGs between hvbe2a-1 and hvbe2a-2 (Fig. 1g–j). This is because both mutants have a substantial number of normal SGs along with elongated ones, making it difficult to distinguish differences in SG morphology between hvbe2a mutants.
Suppressive impact of hvbe2a against hvisa1 in endosperm
In hvisa1-3 grains, there was a reduction in total starch and an increase in phytoglycogen. However, these phenotypes were less pronounced in the hvbe2a-1 and hvbe2a-2 background. (Fig. 5a, b). This suggests that the hvbe2a-1 and hvbe2a-2 mutations are epistatic to the hvisa1-3 mutation. BEs are essential for catalyzing glucose chain branching during amylopectin biosynthesis. However, BEs can sometimes lead to branching at inappropriate positions. In contrast, ISA1 effectively removes glucose chains attached to inappropriate positions on amylopectin to avoid excessive branching caused by BEs (Nakamura 2002; Smith and Zeeman 2020). The absence of HvBE2a, a major BE in barley endosperm, is expected to reduce the formation of incorrect glucose chains. This may decrease the need for trimming by HvISA1. As a result, the loss of HvBE2a has the potential to alleviate the phenotype of HvISA1.
Previous reports in barley RNAi experiments showed that silencing HvBE2b had less impact on amylopectin chain length distribution than HvBE2a. However, the double silencing of both genes exhibited an enhanced phenotype compared to the HvBE2a single silencing (Regina et al. 2010). This suggests a potential functional overlap or compensatory mechanism between HvBE2a and HvBE2b. In the hvbe2a mutant, we detected HvBE2b activity through Native-PAGE activity staining. Thus, HvBE2b is likely to function in the hvbe2a endosperm. It appears that HvBE2b has a minor role in creating inappropriate glucose chains in the endosperm, as there is a significant suppression of phytoglycogen production in hvisa1-3 hvbe2a-1 and hvisa1-3 hvbe2a-2 compared to the hvisa1-3 single mutant (Fig. 5b). It is also possible that additional DBEs, such as pullulanase, play a crucial role in trimming the inappropriate glucose chains generated by HvBE2b, alongside ISA1. Notably, in rice, pullulanase has been proposed to be involved in debranching inappropriate glucose chains in isa1 mutant (Fujita et al. 2009).
The mutation in BE genes has been shown to alleviate the isa1 phenotype in rice (Lee et al. 2017; Nagamatsu et al. 2022). Specifically, the beIIb rice mutation reduces the reduction in grain weight of isa1 mutants and changes the glucan chain length distribution of α-glucan from the isa1-type to the beIIb-type (Nagamatsu et al. 2022). This result indicates that beIIb is epistatic to isa1 in rice. Additionally, the beIIa mutation also can mitigate the phenotype caused by isa1, even though it shows minimal phenotype in rice (Satoh et al. 2003; Lee et al. 2017). These results indicate that the antagonistic relationship between isa1 and be mutations is common in rice and barley.
The hvisa1 mutant forms compound SGs, while hvisa1-3 hvbe2a double mutants develop bimodal simple SGs (Fig. 6a–i). This result suggests that in barley, mutations in the HvISA1 and HvBE2a genes enable the conversion between compound and bimodal types of SGs. Soluble phytoglycogen molecules or their degradation products, such as maltooligosaccharides, are proposed to act as substrates for nucleating new granule initiation (Burton et al. 2002). The higher level of phytoglycogen in hvisa1 mutants may increase the nucleation events and lead to more starch particles within an amyloplast, resulting in the formation of compound SGs (Burton et al. 2002). This idea is supported by the development of simple SGs instead of compound SGs in hvisa1-3 hvbe2a double mutants where the level of phytoglycogen was reduced (Figs. 5b, 6a–i). The fact that HvISA1 is not necessary for developing bimodal SGs under conditions with reduced HvBE2a activity suggests that HvISA1 does not play an exclusive function in developing bimodal SGs.
These mutations are unlikely to be involved in the natural variation in SG morphology across different plant species. For example, wild-type rice develops compound SGs, but the rice beIIb mutant increases the number of small spherical starch particles without forming the typical bimodal SGs observed in barley and wheat. The differences in glucan chain length distribution of amylopectin among species are not as pronounced as those caused by mutations in major starch biosynthetic enzymes (Jane et al. 1999). This suggests that the variation in SG morphology is influenced by factors other than mutations in starch biosynthetic enzymes.
The minor impact of HvBE2a on starch granule formation in pollen
In cereal pollen, vegetative cells accumulate large amounts of starch (Lee et al. 2022). This starch acts as a nutritional source during pollen germination. Some starch-related genes expressed in pollen often function similarly in the endosperm. For instance, waxy mutants of rice, maize and sorghum, possessing mutations in the amylose-synthesizing enzyme, show an absence of amylose in both the endosperm and pollen (Terada et al. 2002; Pedersen et al. 2004; Talukder et al. 2022). The shape of SGs in pollen is commonly consistent among plant species, typically appearing as smaller, rod-shaped, and simple-type (Matsushima and Hisano 2019). The consistency of SG shapes in pollen contrasts with the wide range of SG morphologies found in the endosperm. Nevertheless, mutations affecting SG morphologies in the endosperm often lead to alterations in SG shape in pollen (Matsushima et al. 2014, 2016, 2023).
In the pollen of Haruna Nijo and the mutants, including hvisa1-3, hvbe2a-1, and hvbe2a-2, the ratio of compound SGs were less than 9%. (Fig. 8b). In contrast, the hvflo6 pollen exhibited a significantly higher proportion of compound SGs, approximately 66.5% (Fig. 8b). Furthermore, the hvflo6 phenotype was notably enhanced by the hvisa1 mutation, resulting in over 90% compound SGs in the pollen (Fig. 8b). This suggests that the HvISA1 gene plays a role in pollen, at least in the hvflo6-2 background. The level of pollen compound SGs in hvflo6-2 hvbe2a-1 and hvflo6-2 hvbe2a-2 were found to be comparable to hvflo6-2, indicating no significant reduction. Similarly, there were no significant difference observed among hvflo6-2 hvisa1-3 hvbe2a triple mutants and hvflo6-2 hvisa1-3 double mutants (Fig. 8b). These findings indicate that mutations in hvbe2a did not alleviate the hvflo6-2 hvisa1-3 phenotype in pollen, which contrasts with the traits identified in the endosperm.
The level of HvBE2a accumulation in pollen was lower than in the endosperm (Fig. 8c). This suggests that HvBE2a has a less significant role in pollen compared to the endosperm. Notably, the accumulation of HvBE2b was increased in hvflo6-2 hvbe2a-1, hvflo6-2 hvbe2a-2, hvflo6-2 hvisa1-3 hvbe2a-1, and hvflo6-2 hvisa1-3 hvbe2a-2 (Fig. 8d). All of these lines developed compound SGs in pollen despite having the hvbe2a mutations (i.e., the suppressive effect of the hvbe2a mutations is not observed). The HvBE2a depletion leads to the increase of HvBE2b, and the induced HvBE2b may compensate for the HvBE2a function (Fig. 8d). In the endosperm, due to its high dependency on HvBE2a, HvBE2b may not be able to fully compensate for the depletion of HvBE2a.
Another possible explanation for the different suppressive effects of HvBE2a depletion between pollen and endosperm is that the pathways leading to the increase of compound SGs differ between pollen and endosperm. In the endosperm, the depletion of HvISA1 leads to an increase in compound SGs (Fig. 6a, b). In contrast, in pollen, the depletion of HvISA1 does not increase compound SGs (Fig. 8a). This suggests that pollen does not require HvISA1 to maintain normal SG morphology. On the other hand, the absence of HvFLO6 leads to an increase in compound SGs in both pollen and endosperm. This indicates that there are independent pathways involving HvISA1 and HvFLO6 to maintain normal SG morphology in barley. Given that the compound SG increase in hvflo6-2 pollen but not in hvisa1-3, it is likely that in wild-type pollen, the HvFLO6 pathway primarily ensures the formation of normal simple SGs in pollen. The suppressive effect of HvBE2a depletion on starch accumulation is not significant in hvflo6-2 (Fig. 5a). This may explain why the suppressive effect of HvBE2a depletion is not observed in pollen, where the HvFLO6 pathway is dominant. However, even considering this possibility, it is difficult to explain why the suppressive effect of HvBE2a depletion is not observed in the hvflo6 hvisa1 double mutant background. In the future, it will be necessary to create additional mutants of HvBE2b and HvBE1 in barley to investigate the contributions of these BEs in pollen.
Conclusion
Mutations in starch-related genes play a crucial role in cereal breeding as they can affect taste, digestibility, and processing qualities. Cereals are versatile crops used for food, feed, malting, and industrial purposes, and their grain starch properties determine their suitability for these different applications. This study demonstrated that genetic interactions among specific mutations can control the amount and characteristics of starch and the SG morphology. Combining mutations to uncover genetic interactions that enhance or suppress traits are crucial for controlling starch properties. Multiple mutants could be used as breeding materials in future breeding efforts.
Data availability
The sequence of HvBE2a, HvBE2b and HvBE1 are available in the Ensemble Plants database (http://plants.ensembl.org/index.html) as HORVU.MOREX.r3.2HG0165780.1, HORVU.MOREX.r3.2HG0170370.1 and HORVU.MOREX.r3.7HG0751660.1, respectively. The raw NGS sequence data are available in the DNA Data Bank of Japan Sequence Read Archive (DDBJ DRA) database under specific accession numbers PRJDB17596 for hvbe2a-2 genome and PRJDB4103 for Haruna Nijo genome.
Abbreviations
- DAA:
-
Days after awn emergence
- DP:
-
Degree of polymerization
- HvBE2:
-
Hordeum vulgare BRANCHING ENZYME2
- HvISA1:
-
Hordeum vulgare ISOAMYLASE1
- HvFLO6:
-
Hordeum vulgare FLOURY ENDOSPERM 6
- SG:
-
Starch granule
References
Blaak EE, Canfora EE, Theis S et al (2020) Short chain fatty acids in human gut and metabolic health. Benef Microbes 11:411–455. https://doi.org/10.3920/BM2020.0057
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Burton RA, Jenner H, Carrangis L et al (2002) Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J 31:97–112. https://doi.org/10.1046/J.1365-313X.2002.01339.x
Chen J, Hawkins E, Seung D (2021) Towards targeted starch modification in plants. Curr Opin Plant Biol 60:102013. https://doi.org/10.1016/j.pbi.2021.102013
Chen Y, Luo L, Xu F et al (2022) Carbohydrate repartitioning in the rice starch branching enzyme IIb mutant stimulates higher resistant starch content and lower seed weight revealed by multiomics analysis. J Agric Food Chem 70:9802–9816. https://doi.org/10.1021/acs.jafc.2c03737
Chia T, Adamski NM, Saccomanno B et al (2017) Transfer of a starch phenotype from wild wheat to bread wheat by deletion of a locus controlling B-type starch granule content. J Exp Bot 68:5497–5509. https://doi.org/10.1093/jxb/erx349
Chia T, Chirico M, King R et al (2020) A carbohydrate-binding protein, B-GRANULE CONTENT 1, influences starch granule size distribution in a dose-dependent manner in polyploid wheat. J Exp Bot 71:105–115. https://doi.org/10.1093/jxb/erz405
Chung T-Y (2001) Inheritence, linkage and possible use of fractured starch mutant in barley (Hordeum vulgare L.). J Plant Biotechnol 3:151–157
Crofts N, Iizuka Y, Abe N et al (2018) Rice mutants lacking starch synthase I or branching enzyme IIb activity altered starch biosynthetic protein complexes. Front Plant Sci 9:1817. https://doi.org/10.3389/fpls.2018.01817
DeHaas BW, Goering KJ (1983) Barley starch. VII. New barley starches with fragmented granules. Cereal Chem 60:327–329
Dong N, Jiao G, Cao R et al (2024) OsLESV and OsESV1 promote transitory and storage starch biosynthesis to determine rice grain quality and yield. Plant Commun 5:100893. https://doi.org/10.1016/j.xplc.2024.100893
Ferguson JE, Dickinson DB, Rhodes AM (1979) Analysis of endosperm sugars in a sweet corn inbred (Illinois 677a) which contains the sugary enhancer (se) gene and comparison of se with other corn genotypes. Plant Physiol 63:416–420. https://doi.org/10.1104/pp.63.3.416
Fujita N, Toyosawa Y, Utsumi Y et al (2009) Characterization of pullulanase (PUL)-deficient mutants of rice (Oryza sativa L.) and the function of PUL on starch biosynthesis in the developing rice endosperm. J Exp Bot 60:1009–1023. https://doi.org/10.1093/jxb/ern349
Gonzales JW, Rhodes AM, Dickinson DB (1976) Carbohydrate and enzymic characterization of a high sucrose sugary inbred line of sweet corn. Plant Physiol 58:28–32. https://doi.org/10.1104/pp.58.1.28
Gunning BES, Steer MW (1996) Plastids (9): Amyloplasts. In: Wise Robert R, Kenneth Hoober J (eds) Plant cell biology: structure and function. Jones and Bartlett publishers, Massachusetts
Hawkins E, Chen J, Watson-Lazowski A et al (2021) STARCH SYNTHASE 4 is required for normal starch granule initiation in amyloplasts of wheat endosperm. New Phytol 230:2371–2386. https://doi.org/10.1111/nph.17342
Ida T, Crofts N, Miura S et al (2021) Structure and properties of starch in rice double mutants lacking starch synthase (SS) IIa and starch branching enzyme (BE) IIb. J Appl Glycosci 68:31–39. https://doi.org/10.5458/jag.jag.jag-2021_0002
James MG, Robertson DS, Myers AM (1995) Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 7:417–429. https://doi.org/10.1105/tpc.7.4.417
Jane J, Chen YY, Lee LF et al (1999) Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem 76:629–637. https://doi.org/10.1094/cchem.1999.76.5.629
Jane J-L, Kasemsuwan T, Leas S et al (1994) Anthology of starch granule morphology by scanning electron microscopy. Starch 46:121–129. https://doi.org/10.1002/star.19940460402
Kamble NU, Makhamadjonov F, Fahy B et al (2023) Initiation of B-type starch granules in wheat endosperm requires the plastidial α-glucan phosphorylase PHS1. Plant Cell 35:4091–4110. https://doi.org/10.1093/plcell/koad217
Langeveld SMJ, van Wijk R, Stuurman N et al (2000) B-type granule containing protrusions and interconnections between amyloplasts in developing wheat endosperm revealed by transmission electron microscopy and GFP expression. J Exp Bot 51:1357–1361. https://doi.org/10.1093/jexbot/51.349.1357
Lee Y, Choi MS, Lee G et al (2017) Sugary endosperm is modulated by STARCH BRANCHING ENZYME IIa in rice (Oryza sativa L.). Rice 10:33. https://doi.org/10.1186/s12284-017-0172-3
Lee SK, Lee J, Jo M, Jeon JS (2022) Exploration of sugar and starch metabolic pathway crucial for pollen fertility in rice. Int J Mol Sci 23:14091. https://doi.org/10.3390/ijms232214091
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Li H, Zhang L, Li J et al (2024) Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat Metab 6:578–597. https://doi.org/10.1038/s42255-024-00988-y
Liu C, Pfister B, Osman R et al (2023) Like early starvation 1 and early starvation 1 promote and stabilize amylopectin phase transition in starch biosynthesis. Sci Adv. https://doi.org/10.1126/sciadv.adg7448
Matsushima R, Hisano H (2019) Imaging amyloplasts in the developing endosperm of barley and rice. Sci Rep 9:3745. https://doi.org/10.1038/s41598-019-40424-w
Matsushima R, Maekawa M, Fujita N, Sakamoto W (2010) A rapid, direct observation method to isolate mutants with defects in starch grain morphology in rice. Plant Cell Physiol 51:728–741. https://doi.org/10.1093/pcp/pcq040
Matsushima R, Yamashita J, Kariyama S et al (2013) A phylogenetic re-evaluation of morphological variations of starch grains among Poaceae species. J Appl Glycosci 60:37–44. https://doi.org/10.5458/jag.jag.jag-2012_006
Matsushima R, Maekawa M, Kusano M et al (2014) Amyloplast-localized SUBSTANDARD STARCH GRAIN4 protein influences the size of starch grains in rice endosperm. Plant Physiol 164:623–636. https://doi.org/10.1104/PP.113.229591
Matsushima R, Maekawa M, Kusano M et al (2016) Amyloplast membrane protein SUBSTANDARD STARCH GRAIN6 controls starch grain size in rice endosperm. Plant Physiol 170:1445–1459. https://doi.org/10.1104/pp.15.01811
Matsushima R, Hisano H, Galis I et al (2023) FLOURY ENDOSPERM 6 mutations enhance the sugary phenotype caused by the loss of ISOAMYLASE1 in barley. Theor Appl Genet 136:94. https://doi.org/10.1007/s00122-023-04339-5
Mizuno K, Kawasaki T, Shimada H et al (1993) Alteration of the structural properties of starch components by the lack of an isoform of starch branching enzyme in rice seeds. J Biol Chem 268:19084–19091. https://doi.org/10.1016/s0021-9258(17)46738-X
Nagamatsu S, Wada T, Matsushima R et al (2022) Mutation in BEIIb mitigates the negative effect of the mutation in ISA1 on grain filling and amyloplast formation in rice. Plant Mol Biol 108:497–512. https://doi.org/10.1007/s11103-022-01242-3
Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: Rice endosperm as a model tissue. Plant Cell Physiol 43:718–725. https://doi.org/10.1093/pcp/pcf091
Nakamura Y (2018) Rice starch biotechnology: Rice endosperm as a model of cereal endosperms. Starch 70:1600375. https://doi.org/10.1002/star.201600375
Nakamura Y, Kubo A, Shimamune T et al (1997) Correlation between activities of starch debranching enzyme and α-polyglucan structure in endosperms of sugary-1 mutants of rice. Plant J 12:143–153. https://doi.org/10.1046/j.1365-313x.1997.12010143.x
Noguchi J, Chaen K, Vu NT et al (2011) Crystal structure of the branching enzyme I (BEI) from Oryza sativa L with implications for catalysis and substrate binding. Glycobiology 21:1108–1116. https://doi.org/10.1093/glycob/cwr049
Pan D, Nelson OE (1984) A debranching enzyme deficiency in endosperms of the Sugary-1 mutants of maize. Plant Physiol 74:324–328. https://doi.org/10.1104/pp.74.2.324
Pedersen JF, Bean SR, Funnell DL, Graybosch RA (2004) Rapid iodine staining techniques for identifying the waxy phenotype in sorghum grain and waxy genotype in sorghum pollen. Crop Sci 44:764–767. https://doi.org/10.2135/cropsci2004.7640
Peng C, Wang Y, Liu F et al (2014) FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J 77:917–930. https://doi.org/10.1111/tpj.12444
Pfister B, Zeeman SC (2016) Formation of starch in plant cells. Cell Mol Life Sci 73:2781–2807. https://doi.org/10.1007/s00018-016-2250-x
Regina A, Bird A, Topping D et al (2006) High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc Natl Acad Sci USA 103:3546–3551. https://doi.org/10.1073/pnas.0510737103
Regina A, Kosar-Hashemi B, Ling S et al (2010) Control of starch branching in barley defined through differential RNAi suppression of starch branching enzyme IIa and IIb. J Exp Bot 61:1469–1482. https://doi.org/10.1093/jxb/erq011
Robinson JT, Thorvaldsdóttir H, Winckler W et al (2011) Integrative genomics viewer. Nat Biotechnol 29:24–26. https://doi.org/10.1038/nbt.1754
Saito M, Tanaka T, Sato K et al (2018) A single nucleotide polymorphism in the “Fra” gene results in fractured starch granules in barley. Theor Appl Genet 131:353–364. https://doi.org/10.1007/s00122-017-3006-1
Sakkour A, Mascher M, Himmelbach A et al (2022) Chromosome-scale assembly of barley cv. ‘Haruna Nijo’ as a resource for barley genetics. DNA Res 29:1–8. https://doi.org/10.1093/dnares/dsac001
Sato K, Tanaka T, Shigenobu S et al (2016) Improvement of barley genome annotations by deciphering the Haruna Nijo genome. DNA Res 23:21–28. https://doi.org/10.1093/dnares/dsv033
Satoh H, Nishi A, Fujita N et al (2003) Isolation and characterization of starch mutants in rice. J Appl Glycosci 50:225–230. https://doi.org/10.5458/jag.50.225
Seung D, Boudet J, Monroe J et al (2017) Homologs of PROTEIN TARGETING TO STARCH control starch granule initiation in Arabidopsis leaves. Plant Cell 29:1657–1677. https://doi.org/10.1105/tpc.17.00222
Seung D, Soyk S, Coiro M et al (2015) PROTEIN TARGETING TO STARCH is required for localising GRANULE-BOUND STARCH SYNTHASE to starch granules and for normal amylose synthesis in Arabidopsis. PLoS Biol 13:e1002080. https://doi.org/10.1371/journal.pbio.1002080
Smith AM, Zeeman SC (2020) Starch: a flexible, adaptable carbon store coupled to plant growth. Annu Rev Plant Biol 71:217–245. https://doi.org/10.1146/annurev-arplant-050718-100241
Suh DS, Verhoeven T, Denyer K, Jane JL (2004) Characterization of Nubet and Franubet barley starches. Carbohydr Polym 56:85–93. https://doi.org/10.1016/j.carbpol.2003.12.005
Talukder ZA, Muthusamy V, Zunjare RU et al (2022) Pollen staining is a rapid and cost-effective alternative to marker-assisted selection for recessive waxy1 gene governing high amylopectin in maize. Physiol Mol Biol Plants 28:1753–1764. https://doi.org/10.1007/s12298-022-01256-7
Tateoka T (1962) Starch grains of endosperm in grass systematics. Bot Mag Tokyo. https://doi.org/10.15281/jplantres1887.75.377
Terada R, Urawa H, Inagaki Y et al (2002) Efficient gene targeting by homologous recombination in rice. Nat Biotechnol 20:1030–1034. https://doi.org/10.1038/nbt737
Tetlow IJ, Bertoft E (2020) A Review of Starch Biosynthesis in Relation to the Building Block-Backbone Model. Int J Mol Sci 21:7011. https://doi.org/10.3390/ijms21197011
Thieme M, Hochmuth A, Ilse TE et al (2023) Detecting variation in starch granule size and morphology by high-throughput microscopy and flow cytometry. Carbohydr Polym 299:120169. https://doi.org/10.1016/j.carbpol.2022.120169
Toyosawa Y, Kawagoe Y, Matsushima R et al (2016) Deficiency of starch synthase IIIa and IVb alters starch granule morphology from polyhedral to spherical in rice endosperm. Plant Physiol 170:1255–1270. https://doi.org/10.1104/pp.15.01232
Tsuiki K, Fujisawa H, Itoh A et al (2016) Alterations of starch structure lead to increased resistant starch of steamed rice: Identification of high resistant starch rice lines. J Cereal Sci 68:88–92. https://doi.org/10.1016/j.jcs.2016.01.002
Xia H, Yandeau-Nelson M, Thompson DB, Guiltinan MJ (2011) Deficiency of maize starch-branching enzyme I results in altered starch fine structure, decreased digestibility and reduced coleoptile growth during germination. BMC Plant Biol 11:95. https://doi.org/10.1186/1471-2229-11-95
Yamanouchi H, Nakamura Y (1992) Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol 33:985–991. https://doi.org/10.1093/oxfordjournals.pcp.a078351
Yan H, Zhang W, Wang Y et al (2024) Rice LIKE EARLY STARVATION1 cooperates with FLOURY ENDOSPERM6 to modulate starch biosynthesis and endosperm development. Plant Cell 36:1892–1912. https://doi.org/10.1093/plcell/koae006
Acknowledgements
The authors would like to thank the National BioResource Project for barley, which is run by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) for distributing the barley grains, and also gratefully thank Brendan Fahy at John Innes Centre for providing technical assistance.
Funding
Open Access funding provided by Okayama University. This work was funded by a MEXT Grant-in-Aid for Scientific Research (No. 23K05167 to RM) and grants from G-7 Scholarship Foundation, ASAHI GROUP Foundation, the Foundation for Dietary Scientific Research, and Intensive Support for Young Promising Researchers program by New Energy and Industrial Technology Development Organization (to HH), and the Ohara Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ryo Matsushima; Methodology: Ryo Matsushima, Hiroshi Hisano, June-Sik Kim, Rose McNelly, Naoko F. Oitome, David Seung, Naoko Fujita, Kazuhiro Sato; Formal analysis and investigation: Ryo Matsushima; Writing—original draft preparation: Ryo Matsushima; Writing—review and editing: Ryo Matsushima; Funding acquisition: Ryo Matsushima, Hiroshi Hisano; Resources: Hiroshi Hisano, Kazuhiro Sato.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Kevin Smith.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsushima, R., Hisano, H., Kim, JS. et al. Mutations in starch BRANCHING ENZYME 2a suppress the traits caused by the loss of ISOAMYLASE1 in barley. Theor Appl Genet 137, 212 (2024). https://doi.org/10.1007/s00122-024-04725-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-024-04725-7