Abstract
Key message
This study identified a novel SNP and developed a highly efficient KASP marker for drought tolerance in wheat by genotyping NILs targeting a major QTL for drought tolerance using an SNP array and validation with commercial varieties.
Abstract
Common wheat (Triticum aestivum L.) is an important winter crop worldwide and a typical allopolyploid with a large and complex genome. With global warming, the environmental volatility and incidence of drought in wheat-producing areas will increase. Molecular markers for drought tolerance are urgently needed to enhance drought tolerance breeding. Here, we genotyped four near-isogenic line (NIL) pairs targeting a major QTL qDSI.4B.1 on wheat chromosome arm 4BS for drought tolerance using the 90K SNP Illumina iSelect array and discovered a single nucleotide polymorphism (SNP) (Excalibur_c100336_106) with consistent genotype–phenotype associations among all four NIL pairs and their parents. Then, we converted the SNP into a Kompetitive Allele-Specific PCR (KASP) marker, with an accuracy of 100% for the four NIL pairs and their parents and as high as 81.8% for the 44 tested wheat lines with known phenotypes collected from Australia and China. Two genes near this SNP were suggested as candidate genes for drought tolerance in wheat after checking the Chinese Spring reference genome annotation version 1.1. One gene, TraesCS4B02G085300, encodes an F-box protein reportedly related to the ABA network, a main pathway for drought tolerance, and another gene, TraesCS4B02G085400, encodes a calcineurin-like metallophos-phoesterase transmembrane protein, which participates in Ca2+-dependent phosphorylation regulatory system. Based on this work and previous research on pre-harvest sprouting, we established a quick and efficient general SQV-based approach for KASP marker development, integrating genotyping by SNP arrays (S) using NILs targeting major QTL for a specific trait (Q) and validating them with commercial varieties (V). The identified SNP and developed KASP marker could be applied to marker-assisted selection in drought breeding, and further study of the candidate genes may improve our understanding of drought tolerance in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common wheat (Triticum aestivum L.) is one of the most important cereal crops and a staple food grain for approximately 35% of the world’s population (Grote et al. 2021). Wheat is widely planted in the temperate zones north to a latitude of 67°and south to a latitude of 45° (King et al. 2018; Olmstead and Rhode 2011). However, abiotic stress significantly affects wheat quality and yield. As global temperatures have risen in recent years, drought has become a major abiotic stress for wheat, especially in rain-fed or limited irrigation environments (Akpınar et al. 2013; Hikmet et al. 2013). Unfortunately, the unavoidable causes of drought include global warming, underground water depletion, and erratic rainfall patterns, leading to water scarcity in agroecosystems worldwide, particularly in semi-arid, sub-tropical, and tropical drylands. Therefore, an effective response to drought effects is urgently needed in modern wheat breeding. The most promising and economically viable solution is using new cultivars with high drought tolerance.
Drought tolerance is a complex trait with several physiological and biochemical processes at the cellular and organism level and different stages of plant development (Ferdous et al. 2015; Langridge and Reynolds 2015; Lippmann et al. 2019). Plants adapt to drought stress in various ways, for example, by enhancing water uptake through deep root systems, reducing water loss by increasing stomatal sensitivity, and accumulating cellular osmolytes (Li et al. 2022). The complexity of drought tolerance has hindered the development of drought-tolerant varieties using only a single breeding approach. Hence, modern molecular breeding approaches can be developed to complement other methods to produce new cultivars with high tolerance to drought. In view of this, finding more stable and effective markers and genes conferring drought tolerance in wheat is crucial.
Compared to traditional marker-assisted selection, which is well recognized for its gap between expected and actual impacts, high-density single nucleotide polymorphism (SNP) genotyping arrays provide a much more efficient tool for discovering molecular markers. In the SNP genotyping, the 90K gene-associated SNP chip array (90k Wheat Illumina Infinium array) has been used to characterize genetic variation in allohexaploid wheat populations (Avni et al. 2014; Wang et al. 2014) for its excellent value in terms of genome-coverage. In addition, the recently created Kompetitive Allele-Specific PCR (KASP) markers have been used in marker-assisted plant breeding in pre-harvest sprouting, stripe rust, and powdery mildew resistance due to their cost-effectiveness and high throughput (Han et al. 2020; Liu et al. 2015; Makhoul et al. 2020). However, very few KASP markers have been developed for drought tolerance, and none have been validated in commercial cultivars. Therefore, it is vital to develop efficient and reliable KASP markers for drought tolerance in wheat using the 90K gene-associated SNP chip.
Besides the effective screening methodology, the NIL materials used in this study are also important which could reduce the genetic background noise in marker analysis. In earlier studies, recombinant inbred lines have been mainly used for molecular marker development for drought tolerance, which can require significant lengths of time for researchers to identify the target loci from all the 21 wheat chromosomes. Near-isogenic lines (NILs) could be used as a more valuable resource for studying specific genome positions as they transform complicated quantitative traits into a Mendelian factor (qualitative trait). Our laboratory recently developed a set of NILs targeting drought tolerance on chromosome arm 4BS and reported that the presence of the drought tolerance alleles increased drought tolerance by up to 14% across the NILs (Mia et al. 2019). Therefore, it will be valuable to use these NILs to develop molecular markers and identify genes closely related to this source of drought tolerance in wheat.
Taking advantage of combining NILs targeting drought tolerance, the 90K SNP chip assay, and the Chinese Spring wheat genome sequence data (Appels et al. 2018), this research developed a highly efficient and reliable KASP marker to assist drought tolerance breeding in wheat and identifies two putative genes for drought tolerance in wheat, which may provide a valuable reference for future drought regulation research.
Materials and methods
Plant materials and trait phenotyping
Four NIL pairs significantly differing in drought tolerance—developed by our laboratory from a cross of C306× Dharwar Dry (C306 is the donor parent of the tolerant allele of the QTL) using the heterogeneous inbred family (HIF) method coupled with immature embryo culture-based fast generation-cycling system with 8 generation of selfing. (Mia et al. 2019; Yan et al. 2017; Zheng et al. 2013)—were used as plant material. The genetic background of the isolines in each NIL pair, namely qDSI.4B.1-2, qDSI.4B.1-3, qDSI.4B.1-6, and qDSI.4B.1-8 (Mia et al. 2019), is almost identical, except at the target 4BS QTL, QDSI.4B.1, a major QTL explaining up to 14% of the phenotypic variation for drought tolerance (Kadam et al. 2012). The phenotypes of the NILs were previously tested in a post-anthesis water stress experiment in a temperature-controlled and naturally lit glasshouse (Mia et al. 2017).
Thirty-four newly released or historically domesticated commercial cultivars with known drought response, collected from Australia and China, were used for KASP marker validation. Their phenotypic data for drought response were obtained from previous studies by Grains Research and Development Corporation (GRDC), Australia (McDonald 2016; Shackley et al. 2019; Shackley et al. 2020; Shackley et al. 2021; State of Victoria 2018; GRDC 2019; Brown and Harris 2019) and Hebei Agricultural University (HAU), China (Tables S1, S2 and S3) based on their phenotypic data for drought response related traits (drought index,growing geography, and cultivar character descriptions). Because each state and country has its own ways of showing drought response level, we unified them into one standard, i.e., T for tolerant (drought index > 0.04 or grown in arid/semi-arid zone), S for sensitive (drought index ≤ 0.01 or grown in mid-high rainfall zone). For Australian cultivars, the drought response phenotype was collected from 2013 to 2022 in five states based on Australia’s crop sowing guide handbooks (Western Australia, Queensland, South Australia, New South Wales, and Victoria). The cultivars reported across at least two states and/or at least two years were chosen for this study. For Chinese cultivars, their drought tolerance phenotypes were collected from their National or Provincial seed ID descriptions based on the standard of technical specification of identification and evaluation for drought resistance in wheat (GB/T 21127-2007). Of the 34 cultivars, 16 are sensitive to drought, and 18 are tolerant (Table 1).
DNA extraction and 90K SNP genotyping
Two-week-old young leaves were collected for genomic DNA extraction. The protocol for DNA extraction followed the CTAB method (Murray and Thompson 1980). Genotyping of the four NIL pairs and their parents (Dharwar Dry and C306) was carried out using the 90K SNP Illumina iSelect array. SNP clustering and genotype call steps were determined using GenomeStudio 2.0 software (Illumina) (Wang et al. 2018a). SNPs with a call frequency < 0.8 (i.e., missing data points > 20% or SNPs with no polymorphism) or minor allele frequency (MAF) < 0.05, and SNPs with > 0.25 heterozygous calls, were removed to obtain the raw SNP data.
Screening SNP markers for drought tolerance
The raw SNP data were filtered to generate the genotypic data according to the reference SNP chromosome position (Mayer et al. 2014; Wang et al. 2014). The filtered SNPs were classified into two classes (tolerant or susceptible) based on the corresponding phenotype data. The SNPs on chromosome arm 4BS with > 50% genotype–phenotype associations among the four NIL pairs were selected for further study.
KASP marker development and validation
The selected SNPs with polymorphism in or near the target QTL (QDSI.4B.1) for drought tolerance on chromosome arm 4BS were converted into KASP markers for genotyping in the validation population. KASP primers were designed using PolyMarker (http://www.polymarker.info/designed_primers) by selecting the standard primers in the Wheat iSelect 90 k section. The junction sequence of the fluorescent moiety FAM (6-carboxy-fluorescein) and HEX (Hexachlorofluorescein) was added to each of the two KASP 5’ ends of forward primers for genotyping. Protocols for the KASP marker preparation and PCR conditions are given in the KASP manual (https://www.biosearchtech.com/support/education/kasp-genotyping-reagents/running-kasp-genotyping-reactions) using SNPline™ instrumentation. The PCR was performed in a total volume of 3 μl using a 384-well plate format. The KASP genotyping assays were screened and read by Kraken™ software (v16.3.16.16288). The KASP primer mix, KASP 2× Mastermix, and 384-well plates were purchased from LGC Science Shanghai Ltd. (LGC). The KASP-related experiments were performed on the SNPline™ platform by LGC. The KASP marker was validated by testing the four NIL pairs and their two parents, and then, 34 commercial cultivars were collected from Australia and China with phenotype data for drought tolerance, obtained from published papers and the Grains Research and Development Corporation (GRDC).
Identification and annotation of candidate genes
The SNPs on chromosome arm 4BS that could generate high-efficiency KASP markers (over 75% accuracy rate) were chosen to identify candidate genes for drought resistance. The genes containing or flanking these SNPs were subject to a BLASTN (Basic search) on the IWGSC Chinese Spring genome database (https://urgi.versailles.inra.fr/jbrowseiwgsc/gmod_jbrowse) using IWGSC Annotation v1.1. The candidate gene functions were searched in the online GrainGenes database (https://wheat.pw.usda.gov/GG3/), PlantGDB (http://www.plantgdb.org/), NCBI (https://www.ncbi.nlm.nih.gov/), EnsemblPlants (http://plants.ensembl.org/), Uniprot (https://www.uniprot.org/), IWGSC Annotation v1.0 reference to obtain more annotation information, including domain, gene family, structure, and biological functions.
In silico expression of candidate genes
The selected candidate genes were further screened in WheatOmics (http://wheatomics.sdau.edu.cn/) to reveal gene expression in the Hexaploid Wheat Expression Database (IWGSC Annotation v1.1) under drought stress in their gene-expression databases (Ma et al. 2021).
Statistical analysis
Student’s t-test was used to perform significance analysis in Excel 2013.
Results
Screening of candidate SNPs for drought tolerance
81,587 SNPs were identified using the 90K iSelect genotyping array and four NIL pairs targeting the drought tolerance locus on chromosome arm 4BS created from a cross of C306 and Dharwar Dry. Of which, 31,821 were called across the 21 wheat chromosomes after removing the SNPs that did not show polymorphism or did not satisfy the selection criteria, with 7157 SNPs assigned to the targeted chromosome 4B. Six SNPs had genotype–phenotype associations between tolerant and susceptible isolines of at least two of the four NIL pairs for drought tolerance. Of the six SNPs, Excalibur_c100336_106 (in bold in Table 2) had 100% genotype–phenotype matching among all four NIL pairs (Table 2).
Based on our similar study on pre-harvest sprouting, we found that the SNPs with perfect genotype–phenotype associations in at least two NIL pairs could be useful for marker selection (Liu et al. 2022). Therefore, the above-mentioned six SNPs were all chosen for further analysis. Their sequences were subjected to BLASTN searches on the IWGSC reference genome sequence (V1.1) and GrainGenes databases to identify whether they flank or fall within the target QTL (QDSI.4B.1) on chromosome arm 4BS (Fig. 1). Five of the SNPs (Excalibur_c39876_403, IAAV5564, Kukri_c64195_1044, Kukri_c64195_346, and Kukri_c64195_432) are on the 4B long arm, with the other (Excalibur_c100336_106) located on 4BS near the target QTL. This SNP has 100% genotype–phenotype association in all four NIL pairs and is close to the peak of the target QTL (QDSI.4B.1). Therefore, this SNP was selected for further analysis.
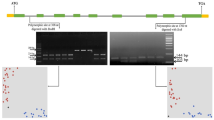
Locations of the six selected SNPs with their physical positions on chromosome 4B. SNP marked as bold shows genotype–phenotype associations in all four NIL pairs; red line is the peak region of the target QTL (qDSI.4B.1); QTL markers are bolded; the details of each SNP’s physical distance (based on Chinese Spring genome version 1.1) were listed in the embedded table
Conversion of the selected SNP into KASP marker and its efficiency validation
The selected SNP, Excalibur_c100336_106, was converted into a KASP marker, designated DE106 (Fig. 2). The KASP marker was used to check all four NIL pairs and their parents, which separated perfectly in the KASP assay (Fig. 2) and expressed the same results as the phenotype screening (Table 1). Therefore, the KASP marker DE106 had 100% accuracy for the drought response phenotype in the four NIL pairs and their two parents and was reliable and worthy of further validation.
Genotyping plot of KASP assays for the KASP marker DE106 on 44 wheat cultivars/lines with different drought resistance performance (left part) and information on the KASP primers (right part) designed based on the sequence of the qualified SNP Excalibur_c100336_106. Note: X-axis: Allele 1, reported by FAM-type fluorescence; Y-axis, Allele 2, reported by HEX-type fluorescence; Blue dots are homozygous allele group 1 (resistance genotype allele BB); Red dots are homozygous allele group 2 (susceptible genotype allele AA); Black dots are blank controls
Thirty commercial wheat cultivars (Table 1) with different drought responses were used to validate the efficiency of the KASP marker DE106. The results are in Table 1 and Fig. 2.
The 44 genotypes, including the four NIL pairs and their two parents, were separated into two clusters (Fig. 2): allele group 1 (blue dots are susceptible alleles) and allele group 2 (red dots are resistant alleles). The KASP marker had 81.8% conformity between genotype and phenotype (Tables 2 and 3).
Candidate genes for drought tolerance on chromosome arm 4BS
The SNP, Excalibur_c100336_106, used to generate a high-efficiency KASP marker for drought tolerance evaluation, was subsequently used to search candidate genes for drought tolerance in the chromosome arm 4BS of the Chinese Spring reference wheat genome (version 1.1, accessed 5 November, 2022). Thirteen high-confidence and 14 low-confidence genes were detected within 1 Mbp distance from the SNP. Three high-confidence genes and eight low-confidence genes were upstream of the candidate SNP, and ten high-confidence genes and six low-confidence genes were downstream (Fig. 3). Detailed information on the 27 genes is in Table 4. Downstream, TraesCS4B02G085400 is the closest high-confidence gene to the SNP, with a distance of 41,468 bp, while TraesCS4B02G106200LC is the closest low-confidence gene to the SNP, with a distance of only 570 bp.
The relative CDS sequences of the above-mentioned 27 genes were obtained from the reference Chinese Spring wheat genome database RefV1.1 (https://tri-ticeaetoolbox.org/wheat//). By blasting in NCBI (https://www.ncbi.nlm.nih.gov), 11 high-confidence genes had function annotations in other plant species and two had putative functions, while nine low-confidence genes had function annotations and five had putative functions (Table 4).
Among the above-mentioned genes, TraesCS4B02G085300 is a gene coding F-box protein, reportedly related to ABA metabolism (Lim et al. 2019; Peng et al. 2012), one of the main pathways for drought tolerance. It is the nearest upstream high-confidence gene with a distance of 0.19 Mbp to the qualified SNP (Excalibur_c100336_106). TraesCS4B02G085400, a high-confidence gene nearest to the SNP (41,468 bp) downstream, encodes a calcineurin-like metallophosphoesterase transmembrane protein and participates in the Ca2+-dependent phosphorylation regulatory system, enabling plants to tolerate various environmental stresses including drought (Sun et al. 2015; Toyota et al. 2018). The low-confidence gene TraesCS4B02G106200LC, annotated as a similar functional annotation with gene TraesCS4B02G085400, is the nearest to the SNP (Excalibur_c100336_106) with a physical distance of only 570 bp downstream. The second nearest gene (low confidence), TraesCS4B02G106300LC, encodes phytochelatin synthase 2.
In silico expression of candidate genes
Three candidate genes (TraesCS4B02G106200LC, TraesCS4B02G085300, and TraesCS4B02G085400) were further screened in WheatOmics (accessed 5 February, 2023) in gene-expression databases to analyze their expression under drought stress. The gene expression result (Fig. 4) shows that TraesCS4B02G106200LC expressed large variation in root and leaf tissue in drought-resistant cultivar Atay 85 (Budak 2001) and drought-susceptible cultivar Zubkov, TraesCS4B02G085400 expressed large variation in grain tissue in the drought-resistant and susceptible cultivars, while TraesCS4B02G085300 only expressed large variation in root tissue in the susceptible cultivar.
Discussion
In many parts of the world, abiotic stresses such as drought have become a major factor limiting wheat production due to climate change (De Vita and Taranto 2019; Qiao et al. 2022; Zhang et al. 2022). Thus, farmers need new wheat varieties with good adaptation to the future climate. Furthermore, with the increasing shortage of water resources, there is an urgent need to breed new wheat cultivars with improved drought tolerance. Marker-assisted selection is an effective way to accelerate the breeding process and improve breeding efficiency.
This study takes advantage of newly developed NILs and the Illumina 90 K SNP chip in wheat to identify an SNP (Excalibur_c100336_106) with 100% genotype–phenotype associations among all four NIL pairs (targeting a major chromosome 4BS QTL qDSI.4B.1 for drought tolerance) and their parents (C306 and Dharwar Dry). The SNP was converted into a KASP marker, with a confirmed 81.8% accuracy rate for evaluating drought tolerance in wheat germplasm. Consequently, the KASP marker, DE106, is a suitable molecular marker for the four tested NIL pairs and their parents and could be a highly useful molecular marker for identifying drought tolerance in practical germplasm evaluation and drought tolerance breeding of wheat.
Drought tolerance is a complicated quantitative trait, with major QTL for drought tolerance explaining limited phenotypic variation-a challenge for developing high-efficiency markers for drought tolerance (Khalid et al. 2019). Recently developed Kompetitive Allele-Specific PCR (KASP) markers are desirable for marker-assisted breeding due to their cost-effectiveness and high throughput (Han et al. 2020; Liu et al. 2015, 2022; Makhoul et al. 2020). However, very few efficient KASP markers have been reported for drought tolerance (Hao et al. 2017; Ur Rehman et al. 2021). In this study, we developed a novel KASP marker (DE106) for wheat drought tolerance using the SNP Excalibur_c100336_106 identified from four NIL pairs targeting a major QTL qDSI.4B.1 that explained up to 14% of the phenotypic variation of drought tolerance. The KASP marker had 100% accuracy for the drought tolerance phenotype in the four tested NIL pairs and their two parents and up to 77.8% when considering 34 commercial cultivars and as high as 81.8% for the total 44 tested wheat lines collected from Australia and China with known drought tolerance/susceptibility. There are three possible reasons why the accuracy is 77.8% rather than 100%. First, drought in wheat is controlled by multiple genes, some cultivars which show drought-tolerant/susceptible phenotype may not be controlled by the QTL we investigated. Second, drought tolerance involves different metabolism pathways, not all the drought-tolerant wheats are regulated by the same pathway. Finally, some other major QTL on other chromosomes may exist which could affect the accuracy of this single QTL validation. Of course, the genetic/physical distance between the marker and the genes controlling drought tolerance/susceptibility may be the major reason. The linkage between the marker and the genes may disassociate through recombination in some genotypes resulting in lower accuracy. However, this KASP marker, associated with one of the major drought tolerance loci, could still be useful for high-throughput evaluation and marker-assisted selection of the drought tolerance trait.
According to the present work and our previous research on pre-harvest sprouting in wheat (Liu et al. 2022), integrating an SNP array, NILs targeting a QTL for a specific trait, and validation using commercial varieties with differences in the specific trait—named SQV (S = SNP, Q = QTL and V = Validation)—is a quick and efficient approach for developing KASP markers and identifying candidate genes for quantitative traits (Fig. 5). The SQV-based approach has multiple advantages. First, it could dramatically reduce researcher workload by using more than three NIL pairs with known and validated phenotypes (most tolerant/most susceptible) when screening the genetic marker data at the initial data filtering stage based on genome-wide association studies, genome re-sequencing, and/or other related methods such as DNA arrays. This study selected six candidate SNPs from the first screening by blasting the four NIL pairs targeting a QTL for drought tolerance. Other studies have also reported that using NILs as material could easily identify around 30 candidate SNPs near the target QTL region in wheat for pre-harvest sprouting resistance, heat tolerance, plant height, and root traits (Halder et al. 2021; Liu et al. 2022; Lu et al. 2020; Zhang et al. 2021), reducing the huge workload of SNP screening in wheat. Second, using target QTL makes it much easier for scientists to narrow the number of candidate SNPs. In this study, five SNPs were filtered from six after physically positioning the target QTL qDSI.4B.1 in the reference genome. The excluded five SNPs were converted to KASP markers for further screening to verify the importance of the targeted QTL localization; the results were consistent with the prediction that these markers are inconsistent, less informative, and hence, could not be used. Third, validation with commercial cultivars could further check the broad spectrum and effectiveness of the KASP markers, an important step in our earlier study validating KASP markers developed for pre-harvest sprouting resistance (Liu et al. 2022), which narrowed ten KASP markers to four for final use. The layer-by-layer screening in the SQV approach can quickly obtain KASP markers with end-use potential (Fig. 4). Besides, this method can be used downstream in conjunction with gene mining, molecular breeding, phenotype identification, and resource variety evaluation.
The SQV-based approach should help researchers select cultivars with biotic and abiotic stress tolerance more efficiently by quickly screening and validating molecular markers and putative genes. Overall, this approach could be a simple model for developing KASP markers for quantitative wheat traits.
Drought tolerance is controlled by a number of genes and external factors. However, most external factors (e.g., light, temperature, rainfall) are hard to control in the field. Selection based on molecular markers related to drought resistance is more user-friendly and reliable for breeders. We identified a candidate gene near the SNP Excalibur_c100336_106 that generated the highly efficient KASP marker (DE106) for drought tolerance. The gene TraesCS4B02G085300, within 0.19 Mb of the SNP, encodes the F-box domain related to the ABA system involved in drought tolerance regulation. The F-box domain-containing protein family is one of the two largest gene families in the plant kingdom. Proteins encoded by F-box genes respond to plant abiotic stresses (Abd-Hamid et al. 2020). Among the F-box gene family, FOA1 is an ABA signaling-related gene (Peng et al. 2012). Some F-box genes, together with their interacting partners, can negatively regulate the ABA-dependent defense signaling response (Lim et al. 2019). So, TraesCS4B02G085300 might be highly correlated with drought tolerance. In silico gene expression on WheatOmics revealed that TraesCS4B02G085300 had doubled the gene expression in root tissue under drought stress compared with control, indicating that it participates in the drought response system. In addition, TraesCS4B02G085400 (closest to the SNP with only 0.04 Mb in distance) and TraesCS4B02G106200LC, both encoding calcineurin-like metallophosphoesterase transmembrane protein, are ser/thr protein kinases with vital roles in plant growth, development, and multiple stress responses (Zhang et al. 2017). When calcineurin-like metallophosphoesterase transmembrane proteins interact with protein kinase TaCIPK27, Arabidopsis plants expressed drought tolerance and exogenous ABA sensitivity (Wang et al. 2018b). Besides, Calcineurin B-like proteins (CBLs) together with CBL-interacting protein kinases (CIPKs) are an important regulatory network (CBL-CIPK) in response to abiotic stresses (Toyota et al. 2018). The CBL-CIPK signaling system regulates many ion transport proteins. At the same time, CBL-CIPK plays an important role in the response to C/N nutrients and uptake of Mg and Fe. This Ca2+-dependent phosphorylation regulatory system functions to ensure plant growth and tolerate various environmental stresses, including drought (Léran et al. 2015; Ragel et al. 2019; Saito and Uozumi 2020; Sanyal et al. 2020; Zhu 2016). Another study found that calcineurin-like metallophosphoesterase transmembrane protein controls seed-coat impermeability in wild soybean (Sun et al. 2015). Hence, it might also be involved in regulating drought tolerance in wheat. The low-confidence gene TraesCS4B02G106200LC is the nearest one to the SNP (Excalibur_c100336_106) with a physical distance of only 570 bp downstream, with similar functional annotation as the gene TraesCS4B02G085400. These two genes may be adjacent genes that co-regulate the drought response. In silico gene expression on WheatOmics showed that TraesCS4B02G085400 and TraesCS4B02G106200LC expressed two-fold or more differences in gene expression in root and grain tissue under drought stress, indicating that they also participate in the drought response system. Consequently, our study improves our understanding of the genes and genetic pathways controlling drought tolerance in wheat.
Conclusion
We identified a novel SNP, Excalibur_c100336_106, with 100% consistency of genotype–phenotype associations among four NIL pairs targeting a major 4BS QTL (QDSI.4B.1) responsible for drought tolerance and their two parents in wheat. A highly efficient KASP marker generated from this SNP had 81.8% conformity of the drought-tolerant genotype and phenotype when tested in 30 commercial cultivars with known drought responses, the four NIL pairs, and their parents. The SNP-converted KASP marker can be suitably employed in future genetic studies on drought tolerance and for high-throughput germplasm evaluation and marker-assisted breeding in wheat. Two candidate genes were found near this SNP—TraesCS4B02G085400 (0.04 Mb) encoding a calcineurin-like metallophosphoesterase transmembrane protein and TraesCS4B02G085300 (0.19 Mb) encoding an F-box protein—which might be responsible for drought tolerance in wheat.
Data availability
The data supporting this study’s findings are available in the supplementary material.
References
Abd-Hamid N-A, Ahmad-Fauzi MI, Zainal Z, Ismail I (2020) Diverse and dynamic roles of F-box proteins in plant biology. Planta 251(3):68–68. https://doi.org/10.1007/s00425-020-03356-8
Akpınar BA, Lucas SJ, Budak H (2013) Genomics approaches for crop improvement against abiotic stress. The Scientific World 2013:361921–361929. https://doi.org/10.1155/2013/361921
Appels R, Eversole K, Stein N, Feuillet C, Rogers J, Pozniak CJ, Choulet F, Distelfeld A, Poland J, Barad O, Baruch K, Keeble-Gagnère G, Mascher M, Josselin A-A, Himmelbach A, Balfourier F, Gutierrez-Gonzalez J, Koh C, Rigault P, Haberer G (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science (Am Assoc Adv Sci) 361(6403):661. https://doi.org/10.1126/science.aar7191
Avni R, Nave M, Eilam T, Sela H, Alekperov C, Peleg Z, Dvorak J, Korol A, Distelfeld A (2014) Ultra-dense genetic map of durum wheat × wild emmer wheat developed using the 90K iSelect SNP genotyping assay. Mol Breed 34(4):1549–1562. https://doi.org/10.1007/s11032-014-0176-2
Brown S, Harris K (2019) 2020 Victorian crop sowing guide. Department of Jobs, Precincts and Regions, Australia
Budak N (2001) Breeding durum and bread wheat lines resistant to high temperatures during grain filling period. Cereal Res Commun 29(3/4):351–358. https://doi.org/10.1007/bf03543681
De Vita P, Taranto F (2019) Durum wheat (Triticum turgidum ssp. durum) breeding to meet the challenge of climate change. Advances in plant breeding strategies: cereals. Springer, Berlin, pp 471–524. https://doi.org/10.1007/978-3-030-23108-8_13
Ferdous J, Hussain S, Shi BJPBJ (2015) R Role of microRNAs in plant drought tolerance. Plant Biotechnol J 13(3):293–305. https://doi.org/10.1111/pbi.12318
Glenn McDonald D (2016) Drought tolerance for wheat varieties. Available from: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2016/02/drought-tolerance-fo-wheat-varieties
Grains Research and Development Corporation (GRDC) (2019) 2019 Queensland winter crop variety sowing guide. Grains Research and Development Corporation, Canberra
Grote U, Fasse A, Nguyen TT, Erenstein O (2021) Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2020.617009
Halder T, Liu H, Chen Y, Yan G, Siddique KHM (2021) Identification of candidate genes for root traits using genotype-phenotype association analysis of near-isogenic lines in hexaploid wheat (Triticum aestivum L.). Int J Mol Sci 22(7):3579. https://doi.org/10.3390/ijms22073579
Han G, Liu S, Jin Y, Jia M, Ma P, Liu H, Wang J, An D (2020) Scale development and utilization of universal PCR-based and high-throughput KASP markers specific for chromosome arms of rye (Secale cereale L.). BMC Gen 21(1):206–206. https://doi.org/10.1186/s12864-020-6624-y
Hao X, Yang T, Liu R, Hu J, Yao Y, Burlyaeva M, Wang Y, Ren G, Zhang H, Wang D, Chang J, Zong X (2017) An RNA sequencing transcriptome analysis of grasspea (Lathyrus sativus L.) and development of SSR and KASP markers. Front Plant Sci 8:1873–1873. https://doi.org/10.3389/fpls.2017.01873
Hikmet B, Melda K, Kuaybe YK (2013) Drought tolerance in modern and wild wheat. Scient World 2013:548246–548316. https://doi.org/10.1155/2013/548246
Kadam S, Singh K, Shukla S, Goel S, Vikram P, Pawar V, Gaikwad K, Khanna-Chopra R, Singh N (2012) Genomic associations for drought tolerance on the short arm of wheat chromosome 4B. Funct Integr Gen 12(3):447–464. https://doi.org/10.1007/s10142-012-0276-1
Khalid M, Afzal F, Gul A, Amir R, Subhani A, Ahmed Z, Mahmood Z, Xia X, Rasheed A, He Z (2019) Molecular characterization of 87 functional genes in wheat diversity panel and their association with phenotypes under well-watered and water-limited conditions. Front Plant Sci 10:717. https://doi.org/10.3389/fpls.2019.00717
King M, Altdorff D, Li P, Galagedara L, Holden J, Unc A (2018) Northward shift of the agricultural climate zone under 21st-century global climate change. Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-26321-8
Langridge P, Reynolds MP (2015) Genomic tools to assist breeding for drought tolerance. Curr Opin Biotechnol 32:130–135. https://doi.org/10.1016/j.copbio.2014.11.027
Léran S, Edel KH, Pervent M, Hashimoto K, Corratgé-Faillie C, Offenborn JN, Tillard P, Gojon A, Kudla J, Lacombe B (2015) Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci Signal 8(375):ra43. https://doi.org/10.1126/scisignal.aaa4829
Li J, Yao X, Yao Y, An L, Feng Z, Wu K (2022) Genome-wide association mapping of hulless barley phenotypes in drought environment. Front Plant Sci 13:924892–924892. https://doi.org/10.3389/fpls.2022.924892
Lim J, Lim CW, Lee SC (2019) Functional analysis of pepper F-box Protein CaDIF1 and its interacting partner CaDIS1: Modulation of ABA signaling and drought stress response. Front Plant Sci 10:1365–1365. https://doi.org/10.3389/fpls.2019.01365
Lippmann R, Babben S, Menger A, Delker C, Quint MJCbC, (2019) Development of wild and cultivated plants under global warming conditions. Curr Biol 29(24):R1326–R1338. https://doi.org/10.1016/j.cub.2019.10.016
Liu S, Sehgal SK, Lin M, Li J, Trick HN, Gill BS, Bai G (2015) Independent mis-splicing mutations in TaPHS1 causing loss of preharvest sprouting (PHS) resistance during wheat domestication. New Phytol 208(3):928–935. https://doi.org/10.1111/nph.13489
Liu G, Mullan D, Zhang A, Liu H, Liu D, Yan G (2022) Identification of KASP markers and putative genes for pre-harvest sprouting resistance in common wheat (Triticum aestivum L.). Crop J. https://doi.org/10.1016/j.cj.2022.09.002
Lu L, Liu H, Wu Y, Yan G (2020) Development and characterization of near-isogenic lines revealing candidate genes for a major 7AL QTL responsible for heat tolerance in wheat. Front Plant Sci 11:1316–1316. https://doi.org/10.3389/fpls.2020.01316
Ma S, Wang M, Wu J, Guo W, Chen Y, Li G, Wang Y, Shi W, Xia G, Fu D, Kang Z, Ni F (2021) WheatOmics: a platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol Plant 14(12):1965–1968. https://doi.org/10.1016/j.molp.2021.10.006
Makhoul M, Rambla C, Voss-Fels KP, Hickey LT, Snowdon RJ, Obermeier C (2020) Overcoming polyploidy pitfalls: a user guide for effective SNP conversion into KASP markers in wheat. Theor Appl Genet 133(8):2413–2430. https://doi.org/10.1007/s00122-020-03608-x
Mayer KFX, Rogers J, Doležel J, Pozniak C, Eversole K, Feuillet C, Gill B, Friebe B, Lukaszewski AJ, Sourdille P, Endo TR, Kubaláková M, Číhalíková J, Dubská Z, Vrána J, Šperková R, Šimková H, Febrer M, Clissold L, McLay K, Singh K, Chhuneja P, Singh NK, Khurana J, Akhunov E, Choulet F, Alberti A, Barbe V, Wincker P, Kanamori H, Kobayashi F, Itoh T, Matsumoto T, Sakai H, Tanaka T, Wu J, Ogihara Y, Handa H, Maclachlan PR, Sharpe A, Klassen D, Edwards D, Batley J, Olsen O-A, Sandve SR, Lien S, Steuernagel B, Wulff B, Caccamo M, Ayling S, Ramirez-Gonzalez RH, Clavijo BJ, Wright J, Pfeifer M, Spannagl M, Martis MM, Mascher M, Chapman J, Poland JA, Scholz U, Barry K, Waugh R, Rokhsar DS, Muehlbauer GJ, Stein N, Gundlach H, Zytnicki M, Jamilloux V, Quesneville H, Wicker T, Faccioli P, Colaiacovo M, Stanca AM, Budak H, Cattivelli L, Glover N, Pingault L, Paux E, Sharma S, Appels R, Bellgard M, Chapman B, Nussbaumer T, Bader KC, Rimbert H, Wang S, Knox R, Kilian A, Alaux M, Alfama F, Couderc L, Guilhot N, Viseux C, Loaec M, Keller B, Praud S (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345(6194):1251788–1251788. https://doi.org/10.1126/science.1251788
Mia MS, Liu H, Wang X, Lu Z, Yan G (2017) Response of wheat to post-anthesis water stress, and the nature of gene action as revealed by combining ability analysis. Crop Pasture Sci 68:534–543. https://doi.org/10.1071/Cp17112
Mia MS, Liu H, Wang X, Yan G (2019) Multiple near-isogenic lines targeting a QTL hotspot of drought tolerance showed contrasting performance under post-anthesis water stress. Front Plant Sci 10:271–271. https://doi.org/10.3389/fpls.2019.00271
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325. https://doi.org/10.1093/nar/8.19.4321
Olmstead AL, Rhode PW (2011) Adapting North American wheat production to climatic challenges, 1839–2009. Proceed Nat Acad Sci - PNAS 108(2):480–485. https://doi.org/10.1073/pnas.1008279108
Peng J, Yu D, Wang L, Xie M, Yuan C, Wang Y, Tang D, Zhao X, Liu X (2012) Arabidopsis F-box gene FOA1 involved in ABA signaling. Sci China Life Sci 55(6):497–506. https://doi.org/10.1007/s11427-012-4332-9
Qiao L, Wang X, Smith P, Fan J, Lu Y, Emmett B, Li R, Dorling S, Chen H, Liu S, Benton TG, Wang Y, Ma Y, Jiang R, Zhang F, Piao S, Mϋller C, Yang H, Hao Y, Li W, Fan M (2022) Soil quality both increases crop production and improves resilience to climate change. Nat Clim Chang 12(6):574–580. https://doi.org/10.1038/s41558-022-01376-8
Ragel P, Raddatz N, Leidi EO, Quintero FJ, Pardo JM (2019) Regulation of K+ nutrition in plants. Front Plant Sci 10:281–281. https://doi.org/10.3389/fpls.2019.00281
Saito S, Uozumi N (2020) Calcium-regulated phosphorylation systems controlling uptake and balance of plant nutrients. Front Plant Sci 11:44–44. https://doi.org/10.3389/fpls.2020.00044
Sanyal SK, Mahiwal S, Nambiar DM, Pandey GK (2020) CBL-CIPK module-mediated phosphoregulation: facts and hypothesis. Biochem J 477(5):853–871. https://doi.org/10.1042/BCJ20190339
Shackley B, Paynter B, Troup G, Bucat J, Seymour M, Blake A (2019) 2020 Western Australian crop sowing guide. Department of Primary Industries and Regional Development, State of Western Australia
Shackley B, Paynter B, Troup G, Bucat J, Seymour M, Blake A (2020) 2021 Western Australian crop sowing guide. Department of Primary Industries and Regional Development, State of Western Australia
Shackley B, Paynter B, Bucat J, Seymour M, S Power (2021) 2022 Western Australian crop sowing guide. Department of Primary Industries and Regional Development, State of Western Australia
State of Victoria (2018) 2019 Victorian winter crop summary. Department of Economic Development, Jobs, Transport and Resources, Australia
Sun L, Miao Z, Cai C, Zhang D, Zhao M, Wu Y, Zhang X, Swarm SA, Zhou L, Zhang ZJ, Nelson RL, Ma J (2015) GmHs1-1, encoding a calcineurin-like protein, controls hard-seededness in soybean. Nat Genet 47(8):939–943. https://doi.org/10.1038/ng.3339
Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S (2018) Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361(6407):1112–1115. https://doi.org/10.1126/science.aat7744
Ur Rehman S, Ali Sher M, Saddique MAB, Ali Z, Khan MA, Mao X, Irshad A, Sajjad M, Ikram RM, Naeem M, Jing R (2021) Development and exploitation of KASP assays for genes underpinning drought tolerance among wheat cultivars from Pakistan. Front Genet 12:684702–684702. https://doi.org/10.3389/fgene.2021.684702
Wang X, Liu H, Mia MS, Siddique KHM, Yan G (2018a) Development of near-isogenic lines targeting a major QTL on 3AL for pre-harvest sprouting resistance in bread wheat. Crop Pasture Sci 69(9):864–872. https://doi.org/10.1071/CP17423
Wang Y, Li T, John SJ, Chen M, Chang J, Yang G, He G (2018b) A CBL-interacting protein kinase TaCIPK27 confers drought tolerance and exogenous ABA sensitivity in transgenic Arabidopsis. Plant Physiol Biochem 123:103–113. https://doi.org/10.1016/j.plaphy.2017.11.019
Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske J, International Wheat Genome Sequencing C, Lillemo M, Mather D, Appels R, Dolferus R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo MC, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden M, Akhunov E (2014) Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol J 12(6):787–796. https://doi.org/10.1111/pbi.12183
Yan G, Liu H, Wang H, Lu Z, Wang Y, Mullan D, Hamblin J, Liu C (2017) Accelerated generation of selfed pure line plants for gene identification and crop breeding. Front Plant Sci 8:1786–1786. https://doi.org/10.3389/fpls.2017.01786
Zhang H, Wei C, Yang X, Chen H, Yang Y, Mo Y, Li H, Zhang Y, Ma J, Yang J, Zhang X (2017) Genome-wide identification and expression analysis of calcium-dependent protein kinase and its related kinase gene families in melon (Cucumis melo L.). PLoS ONE 12:e0176352–e0176352. https://doi.org/10.1371/journal.pone.0176352
Zhang Y, Liu H, Yan G (2021) Characterization of near-isogenic lines confirmed QTL and revealed candidate genes for plant height and yield-related traits in common wheat. Mol Breed. https://doi.org/10.1007/s11032-020-01196-8
Zhang T, He Y, DePauw R, Jin Z, Garvin D, Yue X, Anderson W, Li T, Dong X, Zhang T, Yang X (2022) Climate change may outpace current wheat breeding yield improvements in North America. Nat Commun 13(1):5591–5591. https://doi.org/10.1038/s41467-022-33265-1
Zheng Z, Wang HB, Chen GD, Yan GJ, Liu CJ (2013) A procedure allowing up to eight generations of wheat and nine generations of barley per annum. Euphytica 191(2):311–316. https://doi.org/10.1007/s10681-013-0909-z
Zhu J-K (2016) Abiotic stress signaling and responses in plants. Cell 167(2):313–324. https://doi.org/10.1016/j.cell.2016.08.029
Acknowledgements
This work was funded by the Global Innovation Linkage program (GIL53853) from the Australian Department of Industry, Science, Energy and Resources, an Australian Government RTP Scholarship (International), and a University Postgraduate Award (UPA). We thank Prof. Jacqueline Batley and Dr. Aneeta Pradhan for their assistance with the 90K SNP array genotyping. The whole manuscript has been revised and edited by a professional English language editor, Dr Chris Davies of Tweak Editing.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
GY, DM, and GL designed the research. GL, HL, DM, AZ, and DL prepared the plant samples. MdSM developed the near-isogenic lines. GL undertook DNA extraction, 90k SNP array genotyping, KASP marker identification, and gene function annotation. GL wrote the manuscript, and GY, AZ, DL, DM, HL, and MdSM reviewed and edited the manuscript. All authors approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest or personal relationships that could appear to influence the work reported in this paper.
Additional information
Communicated by Albrecht E. Melchinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, G., Liu, D., Zhang, A. et al. Identification of KASP markers and candidate genes for drought tolerance in wheat using 90K SNP array genotyping of near-isogenic lines targeting a 4BS quantitative trait locus. Theor Appl Genet 136, 190 (2023). https://doi.org/10.1007/s00122-023-04438-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04438-3