Abstract
Key message
Japanese weedy melon exhibits unique sex expression with interactions between previously reported sex determination genes and two novel loci.
Abstract
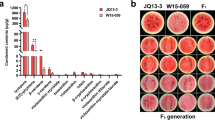
Sex expression contributes to fruit quality and yield in the Cucurbitaceae. In melon, orchestrated regulation by sex determination genes explains the mechanism of sex expression, resulting in a great variety of sexual morphologies. In this study, we examined the Japanese weedy melon UT1, which does not follow the reported model of sex expression. We conducted QTL analysis using F2 plants for flower sex on the main stem and the lateral branch and mapped “occurrence of pistil-bearing flower on the main stem” locus on Chr. 3 (Opbf3.1) and “type of pistil-bearing flower” (female or bisexual) loci on Chr. 2 (tpbf2.1) and Chr. 8 (tpbf8.1). The Opbf3.1 included the known sex determination gene CmACS11. Sequence comparison of CmACS11 between parental lines revealed three nonsynonymous SNPs. A CAPS marker developed from one of the SNPs was closely linked to the occurrence of pistil-bearing flowers on the main stem in two F2 populations with different genetic backgrounds. The UT1 allele on Opbf3.1 was dominant in F1 lines from crosses between UT1 and diverse cultivars and breeding lines. This study suggests that Opbf3.1 and tpbf8.1 may promote the development of pistil and stamen primordia by inhibiting CmWIP1 and CmACS-7 functions, respectively, making the UT1 plants hermaphrodite. The results of this study provide new insights into the molecular mechanisms of sex determination in melons and considerations for the application of femaleness in melon breeding.




Similar content being viewed by others
Data availability
Datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
References
Ainsworth C (2000) Boys and girls come out to play: the molecular biology of dioecious plants. Ann Bot 86:211–221. https://doi.org/10.1006/anbo.2000.1201
Akagi T, Henry IM, Tao R, Comai L (2014) A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science 346:646–650. https://doi.org/10.1126/science.1257225
Akagi T, Henry IM, Ohtani H et al (2018) A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit. Plant Cell 30:780–795. https://doi.org/10.1105/tpc.17.00787
Akagi T, Pilkington SM, Varkonyi-Gasic E et al (2019) Two Y-chromosome-encoded genes determine sex in kiwifruit. Nat Plants 5:801–809. https://doi.org/10.1038/s41477-019-0489-6
Akagi T, Shirasawa K, Nagasaki H et al (2020) The persimmon genome reveals clues to the evolution of a lineage-specific sex determination system in plants. PLoS Genet 16:1–26. https://doi.org/10.1371/journal.pgen.1008566
Arends D, Prins P, Jansen RC, Broman KW (2010) R/qtl: High-throughput multiple QTL mapping. Bioinformatics 26:2990–2992. https://doi.org/10.1093/bioinformatics/btq565
Bai SL, Ben PY, Cui JX et al (2004) Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 220:230–240. https://doi.org/10.1007/s00425-004-1342-2
Barrett SCH (2002) The evolution of plant sexual diversity. Nat Rev Genet 3:274–284. https://doi.org/10.1038/nrg776
Boualem A, Fergany M, Fernandez R et al (2008) A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science 321:836–838. https://doi.org/10.1126/science.1159023
Boualem A, Troadec C, Camps C et al (2015) A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350:688–691. https://doi.org/10.1126/science.aac8370
Bu F, Chen H, Shi Q et al (2016) A major quantitative trait locus conferring subgynoecy in cucumber. Theor Appl Genet 129:97–104. https://doi.org/10.1007/s00122-015-2612-z
Byers RE, Baker LR, Sell HM et al (1972) Ethylene: a natural regulator of sex expression of Cucumis melo L. Proc Natl Acad Sci 69:717–720. https://doi.org/10.1073/pnas.69.3.717
Charlesworth B, Charlesworth D (1978) A model for the evolution of dioecy and gynodioecy. Am Soc Nat 112:975–997
Chen H, Sun J, Li S et al (2016) An ACC oxidase gene essential for cucumber carpel development. Mol Plant 9:1315–1327. https://doi.org/10.1016/j.molp.2016.06.018
Chen J, Sui X, Ma B et al (2022) Arabidopsis CPR5 plays a role in regulating nucleocytoplasmic transport of mRNAs in ethylene signaling pathway. Plant Cell Rep 41:1075–1085
Cohen Y, Eyal H, Cohen A (1993) ‘Gylan’— A gynoecious muskmelon. HortScience 28:855
Grumet R, Taft J (2012) Sex expression in cucurbits. In: Behera TK, Kole C (eds) Wang, Y-H. Genetics, Genomics and breeding of cucurbits, pp 351–375
Hao YJ, Wang DH, Ben PY et al (2003) DNA damage in the early primordial anther is closely correlated with stamen arrest in the female flower of cucumber (Cucumis sativus L.). Planta 217:888–895. https://doi.org/10.1007/s00425-003-1064-x
Harkess A, Zhou J, Xu C et al (2017) The asparagus genome sheds light on the origin and evolution of a young y chromosome. Nat Commun. https://doi.org/10.1038/s41467-017-01064-8
Harkess A, Huang K, van der Hulst R et al (2020) Sex determination by two Y-linked genes in garden asparagus. Plant Cell 32:1790–1796. https://doi.org/10.1105/tpc.19.00859
Hartwig T, Chuck GS, Fujioka S et al (2011) Brassinosteroid control of sex determination in maize. Proc Natl Acad Sci U S A 108:19814–19819. https://doi.org/10.1073/pnas.1108359108
Karch Z (1970) Effects of 2-chloroethanephosphonic acid on flower types and flowering sequences in muskmelon. J Am Soc Hortic Sci 95:515–518
Kater MM, Franken J, Carney KJ et al (2001) Sex determination in the monoecious species cucumber is confined to specific floral whorls. Plant Cell 13:481–493. https://doi.org/10.1105/tpc.13.3.481
Kenigsbuch D, Cohen Y (1990) The inheritance of gynoecy in muskmelon. Genome 33:317–320
Kesh H, Kaushik P (2021) Advances in melon (Cucumis melo L) breeding: An update. Sci Hortic 282:110045. https://doi.org/10.1016/j.scienta.2021.110045
Kishor DS, Noh Y, Song WH et al (2021) SNP marker assay and candidate gene identification for sex expression via genotyping-by-sequencing-based genome-wide associations (GWAS) analyses in Oriental melon (Cucumis melo L.var.makuwa). Sci Hortic 276:109711. https://doi.org/10.1016/j.scienta.2020.109711
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. https://doi.org/10.1038/nmeth.1923
Latrasse D, Rodriguez-Granados NY, Veluchamy A et al (2017) The quest for epigenetic regulation underlying unisexual flower development in Cucumis melo. Epigenetics Chromatin 10:1–17. https://doi.org/10.1186/s13072-017-0132-6
Li H, Handsaker B, Wysoker A et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Li Z, Han Y, Niu H et al (2020) Gynoecy instability in cucumber (Cucumis sativus L.) is due to unequal crossover at the copy number variation-dependent femaleness (F) locus. Hortic Res 7:32. https://doi.org/10.1038/s41438-020-0251-2
Little HA, Papadopoulou E, Hammar SA, Grumet R (2007) The influence of ethylene perception on sex expression in melon (Cucumis melo L.) as assessed by expression of the mutant ethylene receptor, At-etr1-1, under the control of constitutive and floral targeted promoters. Sex Plant Reprod 20:123–136. https://doi.org/10.1007/s00497-007-0049-5
Loy JB, Natti TA, Zack CD, Fritts SK (1979) Chemical regulation of sex expression in a gynomonoecious line of muskmelon. J Am Soc Hortic Sci 104:100–101. https://doi.org/10.21273/jashs.104.1.100
Manzano S, Martínez C, García JM et al (2014) Involvement of ethylene in sex expression and female flower development in watermelon (Citrullus lanatus). Plant Physiol Biochem 85:96–104. https://doi.org/10.1016/j.plaphy.2014.11.004
Martin A, Troadec C, Boualem A et al (2009) A transposon-induced epigenetic change leads to sex determination in melon. Nature 461:1135–1138. https://doi.org/10.1038/nature08498
Masuda K, Akagi T, Esumi T, Tao R (2019) Epigenetic flexibility underlies somaclonal sex conversions in hexaploid persimmon. Plant Cell Physiol 61:393–402. https://doi.org/10.1093/pcp/pcz207
Miao M, Yang X, Han X, Wang K (2011) Sugar signalling is involved in the sex expression response of monoecious cucumber to low temperature. J Exp Bot 62:797–804. https://doi.org/10.1093/jxb/erq315
Müller NA, Kersten B, Leite Montalvão AP et al (2020) A single gene underlies the dynamic evolution of poplar sex determination. Nat Plants 6:630–637. https://doi.org/10.1038/s41477-020-0672-9
Murase K, Shigenobu S, Fujii S et al (2017) MYB transcription factor gene involved in sex determination in Asparagus officinalis. Genes Cells 22:115–123. https://doi.org/10.1111/gtc.12453
Papadopoulou E, Little HA, Hammar SA, Grumet R (2005) Effect of modified endogenous ethylene production on sex expression, bisexual flower development and fruit production in melon (Cucumis melo L.). Sex Plant Reprod 18:131–142. https://doi.org/10.1007/s00497-005-0006-0
Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am J Bot 101:1588–1596. https://doi.org/10.3732/ajb.1400196
Renner SS (2016) Pathways for making unisexual flowers and unisexual plants: moving beyond the “two mutations linked on one chromosome” model. Am J Bot 103:587–589. https://doi.org/10.3732/ajb.1600029
Rodriguez-Granados NY, Ramirez-Prado JS, Brik-Chaouche R et al (2022) CmLHP1 proteins play a key role in plant development and sex determination in melon (Cucumis melo). Plant J 109:1213–1228. https://doi.org/10.1111/tpj.15627
Rudich J, Halevy AH, Kedar N (1969) Increase in femaleness of three cucurbits by treatment with ethrel, an ethylene releasing compound. Planta 86:69–76. https://doi.org/10.1007/BF00385305
Shirasawa K, Hirakawa H, Isobe S (2016) Analytical workflow of double-digest restriction site-associated DNA sequencing based on empirical and in silico optimization in tomato. DNA Res 23:145–153. https://doi.org/10.1093/dnares/dsw004
Takahashi H, Suge H (1980) Sex expression in cucumber plants as affected by mechanical stress. Plant Cell Physiol 21:303–310. https://doi.org/10.1093/oxfordjournals.pcp.a076003
Taylor J, Butler D (2017) R package ASMap: efficient genetic linkage map construction and diagnosis. J Stat Softw 79:1–29. https://doi.org/10.18637/jss.v079.i06
Tsugama D, Matsuyama K, Ide M et al (2017) A putative MYB35 ortholog is a candidate for the sex-determining genes in Asparagus officinalis. Sci Rep 7:1–9. https://doi.org/10.1038/srep41497
Wang F, Wang L, Qiao L et al (2017) Arabidopsis CPR5 regulates ethylene signaling via molecular association with the ETR1 receptor. J Integr Plant Biol 59:810–824. https://doi.org/10.1111/jipb.12570
Wang Z, Zhang S, Yang Y et al (2022) Novel bisexual flower control gene regulates sex differentiation in melon (Cucumis melo L.). J Agric Food Chem. https://doi.org/10.1021/acs.jafc.2c05998
Win KT, Zhang C, Silva RR et al (2019) Identification of quantitative trait loci governing subgynoecy in cucumber. Theor Appl Genet 132:1505–1521. https://doi.org/10.1007/s00122-019-03295-3
Zhang H, Li X, Yu H et al (2019) A high-quality melon genome assembly provides insights into genetic basis of fruit trait improvement. iScience 22:16–27. https://doi.org/10.1016/j.isci.2019.10.049
Zhang S, Tan F, Chung C et al (2022) The control of carpel determinacy pathway leads to sex determination in cucurbits. Science 378:543–549
Acknowledgements
We acknowledge funding for this work from JSPS KAKENHI and JST SPRING. We thank N. Fujishita at Osaka Prefecture University for providing seeds of Japanese weedy melon. We are grateful to N. Nishi and H. Matsumura for their technical assistance.
Funding
This research was funded by JSPS KAKENHI Grant Nos. JP22J10569 to AN and JP22H02333 to YY and JST SPRING Grant No. JPMJSP2124 to AN.
Author information
Authors and Affiliations
Contributions
YY conceived and supervised the study. AN, HM, KT, and FF developed the populations, evaluated the phenotypes, and analyzed the data. SI and KS carried out the ddRAD-seq analysis. AN and HM performed DNA marker and sequence analyses. AN wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sanwen Huang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
122_2023_4381_MOESM1_ESM.xlsx
Supplementary file1 Table S1 Primers for sequencing and CAPS analysis used in this study. Table S2 Phenotypic variation of sex type on the main stem in 2015. Table S3 Genes within the 1.5-LOD support interval of a QTL for type of pistil-bearing flowers on Chr. 8 detected in 2020. Table S4 Genes within the 1.5-LOD support interval of a QTL for the occurrence pistil-bearing flowers on the main stem on Chr. 3 detected in 2020. Table S5 Genotypes of two sex determination genes and the SNP markers adjacent to CmCPR5, and sexual type segregation in F2 population in 2020. Table S6 Frequency of pistil-bearing flowers, male flowers, and mixed phenotypes on the main stem in F2 population derived from a cross between a non-netted andromonoecious fixed line (HOF) and UT1. (XLSX 37 KB)
122_2023_4381_MOESM2_ESM.docx
Supplementary file2 Fig. S1 Molecular characterization of the CmACS11 gene. CmACS11 sequence variation among ‘DHL92’ (reference genome), EF, UT1, and seven cultivars. SNP positions are numbered from the first nucleotide of the start codon. Gray shading indicates SNPs in the coding sequence. Amino acid substitutions in ‘DHL92’ or EF relative to UT1 are shown in bold; the first amino acid corresponds to ‘DHL92’ or EF and the second one to mutant ones. Fig. S2 CmCPR5 sequence variation among ‘DHL92’ (reference genome), EF and UT1 (sequenced in this study), and seven cultivars. Allele was identified at CmCPR5. Allele 1a was identical as 1 except for the no data part. Gray shading indicates SNPs in the coding sequence. Amino acid substitutions in ‘DHL92’ or EF relative to UT1 is shown in bold; the first amino acid corresponds to EF and the second one to mutant ones. NA is no data. (DOCX 153 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nashiki, A., Matsuo, H., Takano, K. et al. Identification of novel sex determination loci in Japanese weedy melon. Theor Appl Genet 136, 136 (2023). https://doi.org/10.1007/s00122-023-04381-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04381-3