Abstract
Improving phosphorus (P) crop nutrition has emerged as a key factor toward achieving a more resilient and sustainable agriculture. P is an essential nutrient for plant development and reproduction, and phosphate (Pi)-based fertilizers represent one of the pillars that sustain food production systems. To meet the global food demand, the challenge for modern agriculture is to increase food production and improve food quality in a sustainable way by significantly optimizing Pi fertilizer use efficiency. The development of genetically improved crops with higher Pi uptake and Pi-use efficiency and higher adaptability to environments with low-Pi availability will play a crucial role toward this end. In this review, we summarize the current understanding of Pi nutrition and the regulation of Pi-starvation responses in plants, and provide new perspectives on how to harness the ample repertoire of genetic mechanisms behind these adaptive responses for crop improvement. We discuss on the potential of implementing more integrative, versatile, and effective strategies by incorporating systems biology approaches and tools such as genome editing and synthetic biology. These strategies will be invaluable for producing high-yielding crops that require reduced Pi fertilizer inputs and to develop a more sustainable global agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential element for all living forms and one of the most restrictive nutrients for plant growth and reproduction (Ågren et al. 2012; Hou et al. 2020). Low-phosphate (Pi) availability in agricultural soils severely compromises crop productivity; thus, global P accessibility and supply have a direct implication on the food production chain worldwide. World’s population is projected to reach about 10 billion by 2050 (UN Department of Economics and Social Affairs 2015). Important challenges should be overcome to feed the world, including an increase in crop productivity from 25 to 70% without increasing agricultural land and lowering environmental pollution at the same time (Hunter et al. 2017). Favoring low-Pi tolerance in crops through improvement of Pi uptake and use efficiency will play an essential role toward developing high-yielding and better adapted plants to changing environmental conditions (Heuer et al. 2017). This represents a great challenge because of the non-renewable fate of the P reserves and the high dependency of current agricultural production systems on heavy application rates of inorganic Pi-fertilizers (Cordell and White 2015). Furthermore, the P-market economy also has a profound impact on the food production chain (Cordell et al. 2015). In this context, plant biology, biotechnology, and genetic engineering are compelled to generate innovative solutions, some of them built on traditional breeding efforts, specifically focused on improving crop varieties to reduce Pi-fertilizer inputs and enable a more sustainable agriculture.
In this review, we first discuss the role of P as an essential piece to achieve the above-mentioned goals. Then, we provide a brief description of the biological role of Pi in plants and summarize the current understanding of Pi nutrition and the regulation of Pi-starvation responses in plants. We also propose how this knowledge could be harnessed for targeted breeding of Pi uptake and use efficiency in crops. Our final purpose is to reveal the ample repertoire of genetic mechanisms behind low-Pi tolerance that can be utilized to produce low-Pi tolerant plants that have higher yield with less Pi-fertilizer input. In this review, we will define Pi-utilization efficiency as total crop dry matter production per unit of Pi applied, and uptake efficiency as the plant´s ability to obtain Pi from the soil (Wang et al. 2010).
Phosphorus: an essential piece toward agricultural sustainability
Modern crop varieties and different inorganic Pi-fertilizers (e.g., mono- and di-ammonium-Pi, triple super-Pi, single super-Pi, and nitrogen-phosphorus-potassium (NPK)) are now widely marketed and utilized at large scale. These two components have become the pillars that sustain our society’s food production systems (Pingali 2012; John and Babu 2021). Pi-fertilizer use has increased continuously over the last decades, and its demand is projected to increase to more than 50 million tons in 2022 (FAO 2020). Forecasted demand of Pi-fertilizers is expected to increase mainly in Asia and moderately in Latin and Central America. However, no substantial increase is projected in Africa, where the Food and Agricultural Organization of the United Nations (FAO) estimated 33% of the total undernourished people in 2020 (FAO, IFAD, UNICEF, WFP, WHO 2021).
Pi-rock deposits, the only source of Pi-fertilizers, are non-renewable. Therefore, a potential global P crisis has been discussed extensively during the last several years (see Cordell and White 2014). Forecasts project the potential P peak, a global Pi depletion, to occur within the next 5 to 40 decades (Mohr and Evans 2013; Van Vuuren et al. 2010; Cordell and White 2014). World resources of Pi-rock are calculated globally in over 300 billion tons of which only about 71 billion tons are considered economically extractable reserves (Jasinski 2021). Pi-rock reserves are not equally distributed, and about 90% of them are found in only six countries: Morocco and Western Sahara, China, Algeria, Syria and Brazil (Jasinski 2021). Moreover, compared to the N- and K-fertilizers market, the Pi-fertilizer market has had great fluctuation over the years suggesting that Pi supply and cost is influenced by numerous factors. It can be argued that socio-economic/political and environmental factors, rather than the geo-localization of Pi rock availability, will be the real cause of a P crisis. Nevertheless, because there are no substitutes for Pi in agriculture and the threat of Pi-limitation is imminent, humanity will unavoidably face a P crisis at some point. Science-driven, novel, and effective strategies together with inclusive and equitable environmental-social-economic policies are needed to avoid or delay the effects of a P crisis. In this context, improving the crop genetics and physiology to develop varieties that perform better under low-Pi environments becomes essential and is the focal point of this review (Fig. 1).
Paths toward phosphorus (P) and food security. Science-driven strategies should be implemented to be able to produce enough food to feed the growing population for the coming years. These strategies include for instance: substantial improvement of agricultural practices (i.e., fertile land and water use), development and improvement of technologies for P recycling from agricultural and industrial wastewater, deployment of symbionts, and improvement of crops traits. Improvement of plant P uptake and utilization plays a crucial role toward P and food security. Some elements in this figure were created with BioRender
The challenge for modern agriculture is to produce more food and improve food quality in a sustainable way for all humans and to do so by significantly reducing dependency on Pi-based fertilizers (Fig. 1). Based on historical yield increase rates (0.9–1.6%), global production of four key crops, maize (Zea mays), rice (Oryza sativa), wheat (Triticum aestivum), and soybean (Glycine max) would be far below what is required in 2050 (Ray and Foley 2013). Thus, it is urgent to transform current food production systems. This implies implementing effective and re-oriented governance strategies which should also include ways to boost the development and introduction into the market of improved crop varieties. Improved plants, better adapted to thrive under low-Pi environments and/or more efficient in the use of Pi-fertilizers, can be generated through modern techniques such as genomic-assisted-breeding, transgenesis, and gene editing and by incorporating research and development at the systems level and synthetic biology approaches.
Limiting factors of phosphate nutrition
P is a constituent of essential biomolecules such as nucleic acids, membrane phospholipids and ATP, and is involved in crucial biological processes. The primary source of P is the Earth’s crust, and its abundance is from 0.10 to 0.12% (on a weight basis), with most of P existing as inorganic salts like calcium (Ca)-, potassium (K)- and aluminum (Al)-Pi, and to a lesser extend as Pi-organic compounds (Mackey and Paytan 2009). Plants take up Pi from the soil solution via membrane transporters and then reach a concentration of up to 100 mg L−1 in the xylem sap and around 4000 mg kg−1 in the seed (Tiessen 2008; Versaw and Garcia 2017), indicating that adequate Pi nutrition is required for maintaining crop productivity. At a first level, physiological and metabolic mechanisms allow the plant to cope with both excessive and deficient Pi levels so it can thrive under either condition. When Pi levels are too high, the plant prevents Pi toxicity by increasing Pi efflux and storage in the vacuoles and reducing the activity of high affinity Pi transporters (Schachtman et al. 1998). When Pi is low, the plant activates a series of mechanisms including the activation of a high affinity Pi transporter system, the remobilization and recycling of Pi from non-essential molecules and an enhanced interaction with the rhizosphere to take advantage of dissolved Pi. All these mechanisms constitute the general response of the plant to low-Pi availability and will be explained in more detail in the following sections.
There are numerous external factors influencing Pi availability and plant uptake rates, including: Pi chemical properties, soil properties (pH, cations), environmental conditions, and agricultural practices. For example, soil Pi availability is limited because Pi is very susceptible to adsorption and precipitation making Pi unavailable for plant uptake (Yadav et al. 2012). Furthermore, cations such as Ca2+, Al3+, and Mg2+ strongly interact with Pi, limiting even more its availability. Another factor contributing to low-Pi availability is soil erosion (Alewell et al. 2020). A heatmap that summarizes the main factors limiting Pi availability and their estimated influence on aboveground plant productivity is presented in Fig. 2a (based on the data analysis provided in Hou et al. 2020 and Yu et al. 2021). Because all these factors influence Pi-fertilization efficiency, a heatmap summarizing their effect on Pi-fertilization efficiency and its consequences on aboveground plant production is presented in Fig. 2b. It is worth mentioning that the most important cereal crops including maize, rice, wheat, and barley (Hordeum vulgare) have low-Pi fertilizer use efficiency which is in average less than 10% (Fig. 2c; Yu et al. 2021).
Current phosphorus (P) fertilization practices are largely inefficient and determined by several factors besides the P fertilizer amount applied to soil. Heatmap illustrating a the size of the effect that the listed factors can have in limiting the variance of above-ground crop production; b the effect that P fertilization can have in increasing aboveground plant production in function of the properties of the cropland (aridity, climate zone, soil weathering) and the P fertilization regime (amount and fertilizer type); and c P use efficiency in common cereals. P fertilizer use efficiency for grain (left) is calculated as follows: (P content in grain with P fertilization—P content in grain without P fertilization)/P fertilizer amount applied × 100%. P fertilizer use efficiency for above-ground mass production (right) was calculated as follows (P content in aboveground biomass with P fertilizer—P content in aboveground biomass without P fertilizer)/P fertilizer amount × 100%. a, b Heatmaps are based on the data published by Hou et al. (2020). The heatmap presented in (c) is based on the data published by Yu et al. (2021). Heat maps were prepared using ComplexHeatmap R package (Gu et al. 2016b)
After the plant has taken up Pi from the soil, the remaining Pi gets fixed into soil particles and becomes part of the so-called pool of residual P (difference between P inputs and P outputs) or legacy P (Pavinato et al. 2020). Importantly, large fractions of legacy P can be made naturally available, i.e., by changes in soil properties either caused by the climate conditions, the plant and/or its associated microbiome, and further removed and taken up in subsequent years (Khan et al. 2007; Sharpley et al. 2013; Pavinato et al. 2020). Therefore, legacy P might play an essential role in increasing or at least maintaining crop productivity and decreasing Pi-fertilizers use in the near future (Sattari et al. 2012; Alewell et al. 2020). Interestingly, a recent study suggests that increasing Pi-fertilization by 7% in the Pi-depleted soils in sub-Sahara Africa would boosts crop production and contribute to meet the global food demand by 2050, and thereby, decrease cropland expansion (Mogollón et al. 2021). The latter strategy should be undoubtedly accompanied by improved crops provided with adequate traits to succeed under such adverse environmental growth conditions.
Plant adaptive mechanisms associated with low-phosphate conditions
Several crops and model plants have served to study plant responses to Pi-deprivation at different levels (i.e., metabolic, physiological and molecular). This has broadened our understanding of metabolic and organ plasticity in response to Pi-starvation, Pi-sensing mechanisms, and how key molecular regulators trigger adaptive molecular responses. Plant molecular responses to Pi-availability can be categorized into two types of responses: local and systemic responses (Péret et al. 2011). Local responses depend on the external concentrations of Pi that the root is in contact with and modulate modifications of root architecture and the exudation of anions of organic acids mainly malate and citrate (Raghothama and Karthikeyan 2005; Plaxton and Tran 2011; Péret et al. 2011). Systemic responses comprehend the activation of physiologic, metabolic, and molecular adjustments at the whole plant level when internal Pi homeostasis is compromised. However, evidence from transcriptomic analysis of mutants defective in the local response to low-Pi indicates that local and systemic responses are not completely independent and there is some degree of crosstalk between these two signaling pathways (Mora-Macías et al. 2017; Raya-González et al. 2021). However, this dissection provides a framework that we will use to explain and summarize findings on both the plant adaptive mechanisms, and the molecular components that sense and control responses to internal and external Pi levels, and how these regulatory modules can be used to improve low-Pi tolerance.
Systemic responses
Phosphate uptake and translocation
Pi is generally required at a higher concentration inside the plant cell (5–10 mM) than it is in the soil solution in which it seldom exceeds 10 μM (Raghothama and Karthikeyan 2005; Marschner 2011). Thus, it can be actively taken up against a concentration gradient by Pi transporter proteins present in the plasma membrane of epidermal root cells. Pi is then distributed symplastically across the root to reach the xylem and the aerial parts of the plant (López-Arredondo et al. 2014; Malhotra et al. 2018). Two types of Pi transporter proteins enable Pi acquisition: low-affinity Pi transporters with affinity constant (Km) values in the 50–300 μM range, and high-affinity Pi transporters with a Km in the 3–10 μM range (Raghothama 1999; Hasan et al. 2016). High-affinity Pi transporters in plants are encoded by PHOSPHATE TRANSPORTER (PHT) genes which are phylogenetically classified into five families, PHT1-5 (Wang et al. 2017), from which the PHT1 is known to be mainly involved in root-mediated Pi uptake from soil. PHT1 genes are systemically induced in Arabidopsis under Pi-limiting conditions (Raghothama and Karthikeyan 2005; Thibaud et al. 2010). PHT1 transporters have been characterized in Arabidopsis and several crops such as maize, rice, wheat, soybean, tomato (Solanum lycopersicum), and barley (for review see Wang et al. 2017). In the case of Arabidopsis, AtPHT1;1 and AtPHT1;4 account for up to 75% of total plant Pi uptake (Shin et al. 2004). In rice, OsPHT1;4 is the main transporter involved in root-mediated Pi uptake and its overexpression leads to Pi over-accumulation in roots (Ye et al. 2015). Moreover, maize ZmPHT1 genes are induced by Pi starvation and mediate the symbiotic association with arbuscular mycorrhizal fungi (AMF). Phosphate transporters upregulated by AMF in rice (OsPT11/13) and soybean (GmPT7/10/11) support a role for plant Pi-uptake at this symbiotic interface (Tamura et al. 2012; Yang et al. 2012).
The root cap, which stands at the forefront of soil exploration, has been described to enable up to 20% of the total plant Pi uptake. Interestingly, AtPHT1;4 is predominantly expressed in this region of the root, and root-cap-mediated Pi-uptake was also reported to occur in rice and lotus (Kanno et al. 2016), indicating that this Pi-uptake mechanism might be generally present in plants. Proteins that belong to the PHT1-5 families of Pi transporters play additional roles involved in maintaining Pi homeostasis, either by facilitating Pi-uptake or by enabling remobilization of internal Pi among different tissues or organelles. This is particularly important because most of the Pi in the cell is stored in the vacuole, while only 1–5% is present in the cytoplasm (Wang et al. 2017; Versaw and Garcia 2017). Once Pi is taken up by the root cells, root-to-shoot translocation of Pi is enabled by PHOSPHATE1 (PHO1), AtPHO1 in Arabidopsis, a protein involved in loading Pi into the xylem (Poirier et al. 1991; Hamburger et al. 2002). Orthologs for PHO1 have been identified and characterized to have similar functions in soybean, rice, and maize (Secco et al. 2010; Salazar-Vidal et al. 2016; Wang et al. 2019c).
Phosphate scavenging and remobilization
Besides the induction of high affinity Pi transport and translocation in response to low-Pi, systemic responses in plants also comprehend the activation of Pi remobilization, scavenging and recycling mechanisms. Plants recycle Pi from the hydrolysis of phospholipids which leads to an increase in internal Pi availability (Plaxton and Tran 2011); non-Pi lipids like galactolipids and sulfolipids replace phospholipids to maintain the functionality and structure of the plasma membrane. Enzymes in Arabidopsis that are involved in this process include PHOSPHOLIPASE D ZETA 2 (AtPLDZ2) and MONOGALACTOSYL DIACYLGLICEROL SYNTHASE 1–2 (AtMGDG1-2) and DIACYLGLICEROL SYNTHASE 1–2 (AtDGDG1-2) and SULFOQUINOVOSYL DIACYLGLYCEROL SYNTHASE 1 (AtSQD1) (Essigmann et al. 1998; Awai et al. 2001; Kelly et al. 2003; Cruz-Ramírez et al. 2006; Plaxton and Tran 2011). As indicated by the names of these enzymes, they play roles in phospholipid breakdown and the synthesis of substitute monogalactosyl-, digalactosyl-, sulfoquinovosyl diacylglycerol-, and glucuronosyl diacylglycerol-lipids (Okazaki et al. 2013). Phospholipid hydrolysis and Pi-recycling from nucleic acids, through the induction of nuclease-coding genes, contribute to buffering the cytosolic Pi pools when systemic levels of Pi are limiting (Plaxton and Tran 2011; Jeong et al. 2017). Maintaining adequate cytosolic Pi levels is crucial because low Pi levels have a negative effect on photosynthesis which eventually leads to the inhibition of plant growth and development (Ajmera et al. 2019). Interestingly, plants belonging to the Proteaceae family predominantly use galactolipids and sulfolipids instead of phospholipids in mature photosynthetic leaves allowing them to maintain high photosynthetic Pi-use efficiency and thrive on the sands of southwestern Australia (Hayes et al. 2018).
Pi scavenging in plants is promoted through the upregulation of genes encoding acid phosphatases (AP) which hydrolyze Pi from organic Pi-esters present in a wide variety of organic compounds including nucleic acids, ATP, 3-phosphoglycerate, and various hexose-Pi compounds (Hurley et al. 2010). A large group of these proteins, named purple acid phosphatases (PAPs), is induced as part of the systemic response to Pi-starvation and help the plant in Pi-solubilization from organic Pi sources (Hurley et al. 2010; Thibaud et al. 2010). In Arabidopsis, AtPAP26 is the predominantly secreted and intracellular phosphatase (Li et al. 2002; Hurley et al. 2010). AtPAP10 and AtPAP12 are not secreted, but associated with the root surface, increasing the availability of Pi where it is useful for the plant (Tran et al. 2010; Wang et al. 2011). Vacuolar or other intercellular PAPs are expressed in stage-specific and tissue-specific fashion to mobilize Pi from storage organelles or from senescent leaves (Gao et al. 2017). Putative PAPs have been identified in maize (González-Muñoz et al. 2015), soybean (Li et al. 2012), and rice (Oryza sativa, (Zhang et al. 2011). Enhanced levels of PAPs remains an interesting perspective to boost Pi uptake in crops.
Local monitoring of external phosphate
Local responses to low-Pi levels include modifications in root development and the subsequent remodeling of root system morphology and architecture in response to the concentrations of external Pi. These responses become important because plant Pi-uptake capacity is greatly increased by an enhancement in the root’s ability to explore the soil which is accompanied by higher expression of Pi-transporter and phosphatase genes and an increase in exudation of organic acids from root tips (Sánchez-Calderón et al. 2005; Ruiz Herrera et al. 2015). Earliest evidence of these type of responses was reported in common bean and subsequently in Arabidopsis (Lynch and Brown 2001; López-Bucio et al. 2002).
Root architecture modifications are oriented to the development of a shallow root architecture in order to more efficiently explore the top layers of the soil where Pi tends to accumulate. This adaptation is known as topsoil Pi foraging (Lynch and Brown 2001) and is common to multiple plant species, including cereals that do not form a main root growth axis (Lynch and Brown 2001; Zhu et al. 2005; see Péret et al. 2014 for review). Depending on the species, modifications of root architecture and morphology also include an increase in the density and length of root hairs (Bates and Lynch 1996), an increase in the emergence of lateral roots with a shallower root angle (Lynch and Brown 2001; López-Bucio et al. 2002), the formation of adventitious roots (Ochoa et al. 2006), and the inhibition of primary root growth (Gutiérrez-Alanís et al. 2018). Additionally, there are less common but important root morphological adaptations that are triggered in response to limited Pi-availability, such as the formation of dense interspaced clusters of roots called ‘cluster roots’. These structures are associated with plants adapted to environments with extreme Pi-scarcity like the Proteaceae and the Cyperaceae (Lambers et al. 2012; Ruiz Herrera et al. 2015). Characteristically, carboxylates, mainly citrate, are profusely secreted through these structures to solubilize Pi from both inorganic and organic Pi-compounds present in the soil (Lambers et al. 2012; Ruiz Herrera et al. 2015).
These responses are regulated by local signals, at least at the transcriptional level, independently of the systemic Pi homeostasis status of the plant (Thibaud et al. 2010; Péret et al. 2011) and are determined by the dynamics of different hormones. Low external Pi increases the root sensitivity to auxins which has been described as a key component regulating lateral root growth (López-Bucio et al. 2002). An increase in lateral root formation due to increased mitotic activity is observed during Pi-deprivation in Arabidopsis (Ajmera et al. 2019), which is regulated by the auxin receptor TIR1 and the transcription factors ARF7/19 (Auxin Response Factor 7 and 19) (Pérez-Torres et al. 2008).
Genetic regulation of low-phosphate adaptations
Regulons controlling phosphate homeostasis
Systemic transcriptional responses to Pi-deprivation are largely controlled by the MYB transcription factor PHOSPHATE STARVATION RESPONSE 1 (PHR1) which was first discovered in Arabidopsis (Rubio et al. 2001). Pi deprived phr1 seedlings do not accumulate anthocyanins, which is characteristic of Pi-deprived plants, and present low expression of phosphate starvation responsive (PSR) genes (Rubio et al. 2001; Bustos et al. 2010). Interestingly, phr1 mutants do not present defects in the root developmental response to low-Pi, which provides evidence of the dissection between local and systemic responses to Pi-deprivation (Rubio et al. 2001). AtPHR1 regulates the expression of PSR genes by binding to the imperfect palindromic sequence GNATATNC that is known as PHR1-Binding Site (P1BS), which is present in the promoter sequences of its target genes (Bustos et al. 2010). AtPHR1 has orthologs that play a similar function in several crop species including maize ZmPHR1 (Calderón-Vázquez et al. 2011), rice OsPHR2 (Zhou et al. 2008), common bean PvPHR1 (Valdés-López et al. 2008) and wheat TaPHR1 (Wang et al. 2013).
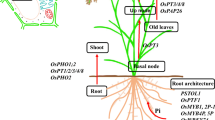
When Pi levels are limiting, PHR1 transcription factors upregulate the expression of high affinity PHT1-like genes (Nussaume et al. 2011). Pi transport is followed by root-to-shoot translocation enabled by PHO1. However, OsPHO1;2 expression is not responsive to Pi-deprivation which suggests post-transcriptional activation mechanisms (Secco et al. 2010). Interestingly, the cis-Natural Antisense Transcript (cis-NAT) cis-NATPHO1;2 present in the OsPHO1;2 locus is induced in response to Pi-deficiency and stimulates PHO1;2 accumulation by enhancing its translation independently of PHO1;2 mRNA levels (Jabnoune et al. 2013). The cis-NATPHO1;2a was also found out to be present in one of the four PHO1 orthologs in maize, ZmPHO1;2a, and responsive to Pi-starvation (Salazar-Vidal et al. 2016), suggesting that its upregulation might play a similar role. cis-NAT regulation of ZmPHO1 translation (Fig. 3a) emerges as an interesting perspective for studies, engineering, and breeding of Pi homeostasis and Pi translocation in crops.
Regulons controlling plant phosphate (Pi) homeostasis. Schematic summary of a cis-NAT mediated control of Pi translocation; b miRNA-mediated control of PHO2 transcript levels; and c ubiquitin-mediated turnover of Pi-transporter proteins in plants. Explanation and discovery of the regulons for Arabidopsis and crops is presented throughout the text
PHOSPHATE2 (PHO2), a ubiquitin E2-ligase, plays also a key role in Pi homeostasis because it regulates the ubiquitin-mediated degradation of Pi-transporter proteins PHT1-4 and the phosphate translocator PHO1, thus preventing Pi shoot toxicity (Liu et al. 2012; Huang et al. 2013). Under Pi-starvation conditions, PHR1 upregulates the expression of the microRNA Atmir399 which triggers the downregulation of AtPHO2 in Arabidopsis (Bari et al. 2006; Hsieh et al. 2009; Castrillo et al. 2017). Atmir399-output is also modulated by AtIPS1, a long noncoding RNA (lncRNA) also induced by AtPHR1, which sequesters Atmir399 to prevent cleavage of PHO2 mRNA (Franco-Zorrilla et al. 2007). The discovery of this regulon revealed target mimicry as a biological mechanism to inhibit miRNA activity (Franco-Zorrilla et al. 2007). There is conservation of the PHR1-mir399-IPS1 regulon in various crops including maize (ZmPHR1-Zmmir399- PILNCR19), rice (OsPHR2-Osmir399-OsIPS1/2), and common bean (PvPHR1-Pvmir399-PvIPS1) (Valdés-López et al. 2008; Hu et al. 2011; Du et al. 2018). A schematic summary of miRNA-mediated control of PHO2 transcript levels in plants is presented in Fig. 3b.
Another ubiquitin-ligase that controls Pi homeostasis in plants is NITROGEN LIMITATION ADAPTATION 1 (NLA) which activates the degradation of PHT1 Pi-transporters via ubiquitin-mediated endocytosis in Arabidopsis (Lin et al. 2013) and rice (Yue et al. 2017). AtNLA1 is negatively regulated by both Atmir399 and Atmir827 microRNAs which are induced upon Pi-starvation (Kant et al. 2011). Even though both NLA1 and PHO2 regulate turnover of Pi-transporters, they have been reported to act independently as they do not interact with each other (Lin et al. 2013; Yue et al. 2017). Both NLA1 and PHO2 prevent Pi-toxicity when Pi levels are high. A schematic summary of ubiquitin-mediated turnover of Pi-transporter proteins in plants is presented in Fig. 3c.
Regulation mechanisms by inositol polyphosphate sensing
Because PHR1 transcription factors play a central role in activating the expression of multiple genes that enable plant adaptation to Pi-scarcity, an interesting question regarding Pi sensing is how PHR1 transcription factors are regulated. This process is known to be under control of SPX (SIG1-Pho81-XPR1) regulatory proteins in Arabidopsis and rice (Puga et al. 2014; Wang et al. 2014). Earliest evidence demonstrated that SPX1 binds to AtPHR1 in plants grown under optimal Pi conditions inhibiting its activity as a transcription factor (Puga et al. 2014). Interestingly, AtPHR1 induces AtSPX1 expression when Pi levels are limiting, forming a negative regulatory loop that was proposed to enable the plant to rapidly turn off PHR1 activity upon Pi refeeding or a sudden increase in cellular Pi levels (Fig. 4a). The PHR1-SPX1 regulon was simultaneously reported in rice in which OsSPX1 and OsSPX2 were shown to interact with OsPHR2 and inhibit its binding to P1BS sites in a Pi-supply dependent fashion (Wang et al. 2014). AtPHR1-SPX1 and OsPHR2-SPX1-2 regulons provided the first example of a Pi-sensing mechanism in plants. Further investigation of SPX-domain proteins in rice and Arabidopsis demonstrated that they are cellular sensors of inositol-polyphosphate (IP) levels (Fig. 4a). SPX-domain proteins selectively interact with target transcription factors in the presence of IP molecules in the µm concentration range (~ 20 µM) and with high affinity, i.e., Kd of 50 and 7 µM for IP6 and IP7, respectively (Wild et al. 2016).
Control of plant phosphate (Pi) homeostasis by inositol (IP)-signaling and IP-sensing domains. a Schematic representation of SPX-domain regulation on plant PHR transcription factors. See main text for complete explanation. Because SPX-domains are IP-sensing domains conserved among eukaryotes (Wild et al. 2016), the crystal structure of IP6-bound SPX domain from the fungi Chaetomium thermophilum is presented as an example. b Representation of IP synthesis in plants. Dual phosphatase/pyrophosphate kinases AtVIH1 and AtVIH2 phosphorylate InsP7 to produce InsP8 and dephosphorylate InsP7 to InsP6. It is currently unknown which enzyme catalyzes the formation of InsP7. AtIPK1 and AtITPK1 kinases catalyze, respectively, the subsequent phosphorylation of IP4 to IP5 and to further IP6
Besides their role in regulating transcription factor activity, SPX-regulatory proteins play additional roles in the regulation of Pi homeostasis. Examples of these proteins include the previously mentioned Pi translocator PHO1 and NLA1. AtNLA1 and OsNLA1 interact with Pi transporters via their SPX domain (Lin et al. 2013; Yue et al. 2017). In the case of AtPHO1, the function of the SPX-domain is still unknown, but it is involved in regulating AtPHO1 Pi-translocation activity (Wege et al. 2016) (Fig. 3c). Because SPX-domain containing proteins have been identified and functionally validated in some crops in addition to rice (see Liu et al. 2018b for review), their study and manipulation open an interesting perspective for breeding approaches to generate low-Pi tolerant crop varieties.
Recent studies have focused on the nature of the IP molecule that regulates Pi homeostasis through the SPX-PHR regulon. Two independent reports proposed that the enzymes AtVIH1 and AtVIH2 play a key role in controlling plant Pi homeostasis by regulating the turnover of IP8 inside the plant cell (Zhu et al. 2019; Dong et al. 2019). Atvih1vih2 double mutants have undetectable levels of IP8 and constitutive activation of PSR genes under high-Pi conditions (Zhu et al. 2019; Dong et al. 2019). IP8 levels were shown to correlate with the intracellular concentration of Pi, supporting the role of IP8 as a Pi-homeostasis signaling molecule in Arabidopsis (Zhu et al. 2019; Dong et al. 2019). Interestingly, Arabidopsis Atipk1 and Atitpk1 mutants which are defective in the synthesis of IP5 and IP6 are also altered in Pi homeostasis (Kuo et al. 2018). These mutants present higher shoot Pi content, higher expression of Pi-transporter genes and other transcripts regulated by AtPHR1, and higher Pi-uptake relative to wild-type plants under Pi-sufficiency conditions (Kuo et al. 2018). These data suggest that specific species of IP might play a specific role in the regulation of plant Pi homeostasis and that it is not solely controlled by IP8. In support of this notion, a recent report of novel alleles of Atvih1 and Atvih2 double mutants showed that IP8 levels are downregulated in these mutants; however, the expression of AtPHR1-targets remains responsive to Pi-deprivation (Land et al. 2021). Because IP6, IP7, and IP8 can bind the SPX domain (Wild et al. 2016), further in planta studies on the specific roles of IPs individual species are required. A schematic summary of IP synthesis in Arabidopsis is presented in Fig. 4b.
Epigenetic regulation mechanisms activated under phosphate starvation
Epigenetic regulation is important because changes in chromatin structure and DNA modifications influence plant adaptation to stress conditions (Grimanelli and Roudier 2013; Zhang et al. 2018a; Séré and Martin 2019). Whole genome cytosine DNA methylation (mC) dynamics respond to Pi-availability in multiple plant species including tomato, soybean, rice and Arabidopsis (Secco et al. 2015; Yong-Villalobos et al. 2015; Chu et al. 2020; Tian et al. 2021). Arabidopsis mutants defective in mC present disrupted responses to low-Pi availability including increased lateral root density, lower phosphatase activity and reduced total P content (Yong-Villalobos et al. 2015). Moreover, global mC changes in Arabidopsis and soybean correlate with positive and negative changes in gene expression depending on the mC pattern (Yong-Villalobos et al. 2015; Chu et al. 2020). However, in the case of tomato and rice, Pi-deprivation induced changes in global mC patterns do not correlate with global changes in gene expression (Secco et al. 2015; Tian et al. 2021). Therefore, a clear role for global mC dynamics on the genome-wide gene expression regulation during plant Pi-starvation has not been clearly established, rather it seems that methylation occurs only for a specific set of Pi-starvation responsive genes and that this might take place in tissue-specific fashion (Secco et al. 2015; Yong-Villalobos et al. 2015). Interestingly, data indicate that transposable elements are enriched in mC in response to Pi-limitation, which has been suggested to protect genome stability (Secco et al. 2015; Yong-Villalobos et al. 2015; Chu et al. 2020; Tian et al. 2021). Thus, methylation-directed transposable element silencing seems to be a conserved mechanism among multiple plant species. Nonetheless, more studies are required to further understand additional roles of mC in plant adaptation to low-Pi conditions.
Another epigenetic regulatory mechanism of gene expression is the modification of chromatin accessibility (Kornberg and Lorch 2003; Mellor 2005). Pi-deprived root cells undergo genome-wide changes of chromatin accessibility in Arabidopsis and AtPHR1 plays a key role in this process (Barragán-Rosillo et al. 2021), providing yet another role for PHR1. Furthermore, up to 40% of rice genes undergo chromatin state changes in response to low-Pi stress which positively correlates with differentially expressed genes (Foroozani et al. 2020). Another chromatin modification, histone deacetylation, represses the transcriptional activation of genes related to the modification of root architecture in response to low-Pi in Arabidopsis (Xu et al. 2020). Altogether, recent evidence highlights the importance of epigenetic regulation on plant adaptation to low-Pi availability and its value as the foundation for the development of innovative genome manipulation tools that might enable breeders to produce low-Pi tolerant crops.
Control of root changes
The regulation of primary root growth in response to external Pi availability in Arabidopsis is one of the best characterized developmental programs (for reviews see Abel 2017; Gutiérrez-Alanís et al. 2018). Upon contact of the root tip with low-Pi medium, a determinate developmental program known as meristematic exhaustion is activated in the root apical meristem (Sánchez-Calderón et al. 2005; Svistoonoff et al. 2007). Meristematic exhaustion consists in a cease in cellular proliferation and the premature differentiation of the cells that comprise the root meristem (Sánchez-Calderón et al. 2005). Studies have highlighted a central role for the transcription factor SENSITIVE TO PROTON RHIZOTOXICITY 1 (AtSTOP1) and iron (Fe) signaling in this developmental program (Fig. 5a, b). Pi and Fe antagonistically interact to limit each other’s availability, and this external Pi/Fe ratio influences meristematic activity in the root (Müller et al. 2015). The levels of STOP1 increase in response to low Pi/Fe ratio which induces its target gene ALUMINUM ACTIVATED MALATE TRANSPORTER 1 (AtALMT1) (Balzergue et al. 2017; Mora-Macías et al. 2017; Godon et al. 2019). The AtALMT1 protein facilitates malate efflux into the root apoplast which together with the activities of the multicopper oxidase LOW PHOSPHATE ROOT 1 (AtLPR1) and the PHOSPHATE DEFICIENT ROOT 2 (AtPDR2) P5-type ATPase, adjust root meristem activity in response to external Pi (Ticconi et al. 2009; Müller et al. 2015). AtALMT1, AtPDR2 and AtLPR1 activities contribute to changes in zone-specific Fe accumulation in response to low-Pi availability, referred to as Fe remobilization (Fig. 5c). Fe remobilization activates the expression of CLAVATA3/EMBRYO SURROUNDING REGION 14 (AtCLE14) signaling peptide which is perceived by AtCLAVATA2/AtPEPR2 receptors, then triggering the downregulation of auxin and other signaling pathways related to meristem maintenance and ultimately leading to the inhibition of primary root growth (Gutiérrez-Alanís et al. 2017). A mitogen-activated protein kinase (MPK), AtMPK6, has been recently linked to the determination of primary root growth by integrating Pi/Fe signals to adjust cellular divisions through auxin signaling in the meristem (López-Bucio et al. 2019). However, the molecular components that are targets of MPK6-dependent regulation remain unknown, and it is yet to be determined whether MPK6 interacts with some of the components that adjust root growth in response to low-Pi availability.
Local phosphate (Pi) sensing in the Arabidopsis root tip. a STOP1 accumulates in response to root tip contact with low Pi/Fe ratio and pH < 6; it regulates its own turnover via activation of RAE1/RAH1. MEDIATOR subunit MED16 promotes the STOP1-activation of ALMT1 expression under low-Pi conditions. b STOP1 activates ALMT1 expression which results in the increase of malate exudation to the root apoplast contributing to Pi solubilization, Al chelation, and Fe accumulation/remobilization in the root apical meristem. STOP1-ALMT1 and PDR2-LPR1 modules are essential and cooperate independently for this phenomenon. The tonoplast transporter complex ALS3/STAR1 is involved in the negative regulation of Fe accumulation. MPK6 kinase negatively regulates meristem maintenance in response to the low Pi/Fe ratio. Fe remobilization activates CLE14 expression which leads to the downregulation of meristem maintenance pathways. c Schematic of Fe remobilization in the root meristem (see text for explanation)
The molecular nature of the mechanism that activates the initial spike in STOP1 accumulation leading to determinate root growth remains unknown. A recent report showed that the activation of ALMT1 expression by STOP1 is facilitated by a subunit of the MEDIATOR complex, AtMEDIATOR16, which also acts as a transcriptional co-activator of several low-Pi responsive genes, including AtALMT1 (Raya-González et al. 2021). Following its accumulation, STOP1 turnover is controlled by ubiquitin ligases REGULATION OF ALMT1 EXPRESSION 1 (RAE1) and its homolog RAE1 HOMOLOG 1 (RAH1) that tag STOP1 for 26S proteasomal degradation (Zhang et al. 2019; Fang et al. 2021). Because RAE1/RAH1 are targets of STOP1, this constitutes a regulation loop by which STOP1 controls its own turnover (Fig. 5b). Other components involved in the regulation of primary root growth include ALUMINUM SENSITIVE 3 (ALS3) and SENSITIVE TO ALUMINUM RHIZOTOXICITY (STAR1) (Dong et al. 2017). A genetic dissection of responses to low-Pi proposed that the ALS3/STAR1 complex functions upstream of STOP1 because STOP1 accumulates more in als3 mutant than in wild-type seedlings under high and low-Pi conditions, whreas als3stop1 mutants continue primary root growth under Pi-starvation (Wang et al. 2019a, b, c). However, because there is evidence that ALS3 is a STOP1 target (Sawaki et al. 2009; O’Malley et al. 2016; Ojeda-Rivera et al. 2020), at least two negative regulation feedback loops (RAE1/STOP1, ALS3/STOP1) control STOP1 turnover in response to abiotic stress factors.
AtSTOP1 and AtALMT1 were first described to contribute to Arabidopsis tolerance to acidic pH and increased Al levels that prevails in acidic soils by activating and mediating, respectively, organic acid exudation (Hoekenga et al. 2006; Iuchi et al. 2007). STOP1-mediated organic acid exudation plays multiple beneficial roles including the prevention of detrimental effects of Al in root development, the release of Pi from complexes with cations for root uptake and the modification of root growth. Al-resistance related loci might be of the outmost interest for breeding crop varieties with enhanced root morphology for topsoil exploration in acidic soils. A recent transcriptional dissection of root responses under this condition pinpoints some common regulatory hubs for tolerance to acidic pH, Al and Pi-deficiency in Arabidopsis (Ojeda-Rivera et al. 2020). Finding orthologs for these regulatory genes in crops might prove a good starting point toward new targets for breeding and genetic engineering strategies.
Major QTLs associated with low phosphate tolerance
Quantitative trait loci (QTLs) associated with enhanced Pi acquisition and utilization efficiencies have been described in several crop species including rice, maize, bean, rapeseed and soybean (Maharajan et al. 2018). The traits related to these QTLs include root length, lateral root number, root surface area, adventitious root number, lateral root diameter, and root and shoot ratio, among others (Maharajan et al. 2018). Only a few studies have been able to identify and validate the genetic features underlying QTLs related to root architectures that are better adapted to low-Pi soils. The QTL named Phosphorus Uptake 1 (Pup1) confers Pi-starvation tolerance in rice variety Kasalath (Wissuwa et al. 2002). Further studies identified a protein kinase underlying Pup1 QTL; this gene was named OsPSTOL1 (PHOSPHORUS STARVATION TOLERANCE 1) (Gamuyao et al. 2012). The expression pattern of OsPSTOL1 in low-Pi intolerant rice varieties indicates that it acts as an enhancer of early root growth by increasing the plant capacity to acquire more nutrients including Pi (Gamuyao et al. 2012). Interestingly, when introduced in Pi-starvation intolerant rice varieties, OsPSTOL1 significantly enhanced grain yield in Pi-deficient soils (Gamuyao et al. 2012). However, although the PSTOL1 homolog in wheat contributes to agronomically important traits like flowering time and grain size, its manipulation did not show an increase in Pi use efficiency in this species (Milner et al. 2018). Moreover, Pup1-specific markers are not present in the rice landrace Wazuhophek, recently reported as low-Pi tolerant, indicating the potential use of this material as donor of novel loci for breeding programs (Swamy et al. 2019). Another approach for improvement of low Pi-tolerance in crops includes introgression. Wheat lines carrying wheat-rye chromosomal translocations showed a significant positive correlation between root biomass and Pi uptake (Ehdaie et al. 2010; Jung and Seo 2014; Moskal et al. 2021). Thus, suggesting this could be a promising tool for breeding low-Pi tolerance in other crops. We summarized major QTLs related to Pi-use efficiency and Pi-acquisition recently discovered in crops in Table 1 and encourage the reader to get further details for rice (Mahender et al. 2018), soybean (Kumawat et al. 2016; Zogli et al. 2017), maize (Wang et al. 2019a) and wheat (Colasuonno et al. 2021) studies.
Strategies to produce low phosphate-tolerant plants
Potential routes to accelerate the development of low-Pi tolerant crop varieties include favoring changes in root system architecture, improvement of Pi uptake and remobilization, remodeling of rhizosphere-microbial associations, and fine-tuning of organic acids secretion. In this section we discuss on the potential of some of these routes and propose novel strategies (Fig. 6).
Overview of traits and strategies to develop low-phosphate (Pi) tolerance in crops. Several traits and strategies have potential to develop low Phosphorus (P) tolerant crops. These include strategies related to the deployment and engineering of plant associated microorganisms, the use of alternative P sources like phosphite (Phi), and the improvement of the plant genetics based on current knowledge of the molecular regulation of Pi starvation responses. This latter considers the fine-tuning of different traits such us Pi recycling and remobilization, phytohormones and inositol polyphosphate molecules (IP) levels and modulation of organic acids exudation. The use of the CRISPR/Cas9 technology together with more integrative synthetic biology approaches assisted by biosensor molecules could help speed up this process. Phospholipids (P-lipids), galactolipids (G-lipids), sulfolipid (S-lipids). Some elements in this figure were created with BioRender
Genes for the modulation of phosphate metabolism
In the context of genetic engineering, the heterologous expression of bacterial genes to improve Pi-metabolism from alternative Pi sources seems very promising. Transgenic Arabidopsis plants expressing bacterial phytases (PaPhyC and 168phA) can use phytic acid as a Pi source without any negative physiological effects on plant development and germination (Valeeva et al. 2018). Furthermore, reduction of phytic acid content in grain has been also proposed as a solution that might contribute to reduce Pi-based fertilizer use by preventing excessive removal of Pi from the soil (Perera et al. 2018). This is the case of the SULTR-like Pi DISTRIBUTION TRANSPORTER (SPDT), a sulfur transporter (SULTR)-like, that is expressed in the rice nodes and controls Pi allocation to the grain. Rice spdt knockout lines resulted in reduced phytate content in grain without compromising yield and seed germination (Yamaji et al. 2017). Likewise, knocking out IPK1 (Inositol pentakisphosphate 2) and ITPK1 (Inositol tetrakisphosphate 1) kinase-coding genes in crops such as maize, rice and wheat resulted in plants with reduced accumulation of phytic acid in the seed (Shi et al. 2003; Shukla et al. 2009; Ali et al. 2013; Aggarwal et al. 2018).
Engineered plants that express the ptxD gene from Pseudomonas stutzeri, which codes for an oxidoreductase that oxidizes phosphite into Pi, can use this compound as a P-source, requiring 30–50% less P input than when fertilized with the traditional P source under greenhouse (López-Arredondo and Herrera-Estrella 2012). This technology has been applied in rice, maize, and cotton plants that can be fertilized with phosphite as the sole source of P (Nahampun et al. 2016; Manna et al. 2016; Pandeya et al. 2018; Lopez-Arredondo et al. 2021). One of the main advantages of phosphite-fertilization technology is that it suppresses weed growth and enables transgenic crops to outcompete aggressive and glyphosate-resistant weeds while, at the same time, phosphite provides Pi-fertilization that can only be harnessed by ptxD-expressing plants (López-Arredondo and Herrera-Estrella 2012; Pandeya et al. 2018). Although these strategies seem feasible, applicable to several crops, and have proven effective under field conditions, as is the case of the ptxD/Phi technology, a long path for their optimization and implementation at large-scale still remains to be completed.
Deployment of symbionts to enhance phosphate uptake
Plant roots host a diversity of bacterial communities which include Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria. These microorganisms provide protection from pathogen attack and enable nutrient foraging through solubilization (for review see Bulgarelli et al. 2013). Endophytic fungi are microorganisms that live within the plant and have been object of study for their possible role in the enhancement of plan nutrient acquisition (for review see García-Latorre et al. 2021). Root colonization by microbiota and endophytic fungi have proven beneficial roles in improving plant fitness under Pi-starvation in plants (Hiruma et al. 2016; Castrillo et al. 2017). In Arabidopsis, this process is mediated by AtPHR1 (Hiruma et al. 2016; Castrillo et al. 2017). Thus, fine-tuning of PHR1-mediated regulation in crops also emerges as a tool to enhance root colonization by beneficial microbial consortiums. Inhibition of suberin deposition by microbiota facilitates plant nutrient uptake (Salas-González et al. 2021). This process occurs via the inhibition of abscisic acid signaling (Salas-González et al. 2021), thus suggesting the downregulation of synthesis-related genes in crops to prevent suberization and promote association with beneficial consortiums of rhizosphere microbiota. Inoculation of root crops with Pi-solubilizing microorganisms is also an interesting solution to enhance Pi availability from organic matter in soil (Alori et al. 2017).
Root symbiosis with AMF is another type of beneficial symbiotic association. It may benefit Pi uptake for the plant in exchange for carbohydrates for the fungi and contribute to improve plant Pi nutrition (Willmann et al. 2013; Liu et al. 2018a; Chen et al. 2018). AMF are effective in foraging Pi for the plant as these symbionts can spread beyond the rhizosphere Pi-depleted zones and provide Pi that otherwise would be inaccessible for the root system (Chen and Liao 2017). The majority of plant species (~ 70%) form symbiotic relationships with AMF including crops of major economic importance like maize and rice. Nonetheless, there are some exceptions which include the Chenopodiaceae, Carophyllaceae, and Brassicaceae plant families (Cosme et al. 2018). The latter includes some crops of agricultural relevance like cabbage and broccoli and the model plant Arabidopsis. In the case of maize, for instance, AMF-symbiotic Pi uptake accounts for up to one third of grain yield in rain-fed sub-tropical fields (Ramírez-Flores et al. 2020). Up to 70% of Pi-uptake in rice is symbiotically acquired, corroborating the relevance of AMF in crops (Yang et al. 2012). Pi-transporters induced by AMF in rice (OsPT11/13) and soybean (GmPT7/10/11) are evidence of plant Pi-uptake at the symbiotic interface (Chen and Liao 2017). Interestingly, high Pi fertilizer application can reduce the percentage of root colonization and decrease AMF biomass (Smith et al. 2011; López-Arredondo et al. 2014) which indicates that AMF might only play a Pi-uptake enhancing role when Pi conditions are limiting. Further investigation on the role of AMF in “high Pi” soils is required to sort out this controversy. Moreover, knowing the genetic elements that regulate the activation/deactivation of the symbiotic Pi-uptake pathways could lead to the development of plant varieties that could maintain AMF symbiosis independently of the Pi status in the plant. Undoubtedly, because limited Pi-availability is a major constrain in most croplands, deployment of AMF remains a valuable source to enhance crop Pi uptake.
The potential of genome editing-approaches
CRISPR/Cas (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein) has emerged as one of the most promising systems to facilitate crop improvement by altering in a precise way the genome of any organism (Khatodia et al. 2016; Wang et al. 2019d; Zhao et al. 2019; Jiang and Doudna 2017). It offers a series of advantages over traditional approaches; for example, in contrast to the transgenic approach in which DNA expression cassettes are randomly inserted in the genome and may cause pleiotropic phenotypes, genome editing approaches allow a precise design of the desired trait. Moreover, since edited varieties may be generated via selectable marker-independent methods, they might be subject to less regulatory issues compared to transgenics. Thus, the resulting events may be directly incorporated into breeding programs and commercialized as feed and food.
The CRISPR/Cas system has been adapted to allow simultaneous editing of multiple sites of both coding and regulatory sequences. Furthermore, the availability of multiple Cas proteins (e.g., Cas9, Cas12, Cpf1, CasΦ) and Cas9 variants [nickase Cas9 (nCas9), deactivated Cas9 (dCas9)] expands the application of the CRISPR/Cas system beyond editing (Jaganathan et al. 2018). Besides the “off-targets” mutations that it may induce and some technical hurdles for its successful implementation (see Montecillo et al. 2020), the CRISPR/Cas system has boosted the study of plant responses to diverse abiotic stresses including drought, and K and N deficiencies and to a lesser extent P starvation. Most of the studies have been performed specially on rice, maize, and tomato and are limited to the functional validation of already reported genes in which knockouts are described, thus providing the proof-of-concept for future crop improvement (Jaganathan et al. 2018). Using CRISPR/Cas, deletion mutants of the AtPHO1 homolog in tomato, SlPHO1;1 were generated, which displayed typical characteristics of Pi-starvation, shorter and redder leaves than the wild-type control as previously reported for Arabidopsis (Zhao et al. 2019). Allelic variants of HvITPK1 in barley were also generated with the purpose of lowering phytic acid levels (Vlčko and Ohnoutková 2020). Interestingly, barley ITPK1 allelic variants showed salinity stress tolerance, thus posing new questions on the role of these enzymes on general abiotic stress signaling. A similar approach was implemented using TALEN and CRISPR/Cas9 to lower phytic acid synthesis by impairing ZmIPK1 in maize (Shukla et al. 2009; Liang et al. 2014). In a recent report, the role of OsACS1 (1-aminocyclopropane-1-carboxylic acid synthase) and OsACS2, involved in ethylene biosynthesis, was investigated in rice by taking advantage of CRISPR/Cas9 (Lee et al. 2019). Both enzymes were found involved in remodeling Arabidopsis root system architecture, transcriptional regulation of Pi-starvation responsive genes, and Pi homeostasis.
Evidence on N metabolism provide clear examples of the utility and effectiveness of this technology, thus suggesting it is a plausible strategy to speed up targeted crop breeding for low-Pi tolerance. An interesting case study is the NRT1.1 nitrate transporter in rice. Previously, a single nucleotide polymorphism consisting of C/T (Thr327Met) change was associated with N-use efficiency improvement (Hu et al. 2015). Therefore, by using and optimizing the CRISPR/Cas9 system, the precise base-editing to generate C/T replacement at this location (Thr327Met) in NRT1.1B was successfully done (Lu and Zhu 2017). Furthermore, Li and co-workers (2018), using CRISPR/Cas9 replaced the complete japonica NRT1.1B allele by the high N-use efficiency indica allele in only one generation (Li et al. 2018), which by traditional introgression would take between 4 and 6 generations. These examples prove the versatility and power of this technology to improve agricultural traits.
Transcriptional engineering
Transcription factors represent promising candidates for improving resistance to multiple environmental stresses. Through controlling growth and development, transcription factors orchestrate plant responses to various abiotic stresses and present overlapping functions (Patikoglou and Burley 1997; Lindemose et al. 2013). Several transcription factors belonging to different families have been described to play crucial roles in modulating low-Pi starvation responses and crosstalk with hormones in crops including MYB62 (regulating the crosstalk between Pi and gibberellic acid) (Devaiah et al. 2009), ETC1 (important in favoring higher root hair density under low-P conditions) (Savage et al. 2013), and MYB2 (involved in mediating Pi-starvation responses through miR399f) (Baek et al. 2013; Jyoti et al. 2019). Thus, opening new avenues to apply transcriptional engineering approaches by means of engineering multiple transcription factors simultaneously.
In recent years, regulatory networks composed of key transcription factors controlling plant responses to Pi-starvation have been identified in rice which include a network of 266 transcription factors underlying low-Pi responses in the context of AMF symbiosis (Shi et al. 2021). The network is centered in PHR transcription factors, widely known to control Pi-starvation adaptive responses (Fig. 4a), and is enriched in Pi-starvation, N-metabolism, and chromatin remodeling related transcription factors. Moreover, it captured transcription factors controlling mycorrhizal symbiosis and hormone signaling pathways (i.e., ethylene, jasmonic acid, auxins) reported previously such as ARF12, ARF25, MYC2, among others (Shi et al. 2021). These findings represent emerging opportunities to modulate and remodel in a precise way the activity of specific nodes of regulation orchestrating low-Pi responses at specific organs or cell types, and at specific developmental stages when the plant requires Pi the most. Such level of regulation involving a crosstalk between Pi metabolism, mycorrhizal symbiosis, and hormone signaling, suggests that highly complex and intricate regulatory mechanisms underlie Pi-nutrition in the context of biotic interactions. This complexity can be studied by applying CRISPR/Cas multiplexed approaches. These studies should allow genome-wide transcriptional reprogramming in crop plants for breeding purposes. Gene regulons composed of key transcription factors, protein regulators, and microRNAs controlling Pi homeostasis in plants are presented in Fig. 3, which can serve as the basis to remodel Pi-starvation responses in crops.
An interesting candidate is the transcription factor PHR1. Three residues (K325, H328, R335) in the coiled-coil (CC) domain of AtPHR1 are essential for binding AtSPX1 (Ried et al. 2021). Interestingly, mutation of these residues disrupts AtSPX-binding without affecting AtPHR1’s ability to bind its target sequence which results in constitutive activation of Pi-starvation responses (Ried et al. 2021). Now, with the possibility of doing specific nucleotide base replacements with CRISPR/Cas9, strategies to study these three PHR1KHR residues can be implemented. Although this task might be technically challenging, it could be interesting to assess how the replacement of these residues impacts the low-Pi stress responses in crops. Likewise, IP molecules are essential to stabilize the structure of SPX proteins, enabling them to interact with regulators of Pi homeostasis in eukaryotes (Wild et al. 2016). The possibility of fine-tuning IP-molecules biosynthesis, IP6, IP7 and IP8 for instance, remains an interesting perspective for future breeding. Given the essential role of IP molecules as cofactors of auxin and jasmonic acid sensing complexes and the crosstalk with plant Pi status, a possibility remains that by modifying the balance of IP-signaling molecules such as IP8, IP5, and IP6, stress responses can be finely modulated at a system level as well.
Fine-tuning of plant hormones and organic acids
Due to the crosstalk between hormone signaling and the regulation of Pi-starvation responses, a tight modulation of these responses can be achieved by fine-tuning both internal and secreted hormone levels through CRISPR/Cas9. Strigolactones are hormones involved in regulating the growth of primary and lateral roots; upregulation of strigolactone synthesis and root-exudation under Pi-limiting conditions have been shown to favor AMF symbiosis (Mayzlish-Gati et al. 2012; Sun et al. 2014; Santoro et al. 2020). However, high levels of strigolactones can also promote the germination of weeds, for example Striga, which threatens crop productivity (Khosla and Nelson 2016; Yacoubou et al. 2021). Therefore, by fine-tuning strigolactone levels, through generating CRISPR/Cas9 allelic variants, one would be able to improve crop fitness by modulating root traits and AMF symbiosis in benefit of low-Pi tolerance while avoiding weeds germination. CRISPR/Cas9 edited rice lines to disrupt CCD7 (CAROTENOID CLEAVAGE DIOXYGENASE 7), an enzyme involved in a limiting step in strigolactone biosynthesis, have already been reported (Butt et al. 2018). Interestingly, CCD7-edited lines showed reduced height, increased tiller production, and reduced exudation of strigolactones compared to the wild-type. However, total yield, plant Pi-starvation responses, and association with AMF remain to be studied in these plants. Further studies are required to test the effect of strigolactone downregulation in AMF formation and Pi nutrition in soils with Pi limitation.
The transcription factor STOP1 controls an interesting network that modulates root adaptation to acidic soils, which present low pH, Al toxicity and low-Pi availability (Sawaki et al. 2009; Ojeda-Rivera et al. 2020). Some of the STOP1-targets include organic acid transporters that mediate Pi solubilization and, in the particular case of ALMT1, mediate root growth in response to low-Pi availability (Balzergue et al. 2017; Mora-Macías et al. 2017). Because the STOP1-binding site has been identified and the activation of STOP1-targets correlates with its nuclear accumulation (Ojeda-Rivera et al. 2020), the CRISPR/Cas9 system could be used to edit the promoter sequence of STOP1-target genes to modulate and re-wire root organic-acid synthesis and exudation. This strategy might contribute to designing crops that are able to thrive and have a root system architecture better adapted to acidic soils with low-Pi availability. In this context, another interesting candidate gene is the root specific HRS1 (hypersensitivity to low Pi-elicited primary root shortening 1) transcription factor, involved in modulating primary root growth in response to Pi-starvation only in presence of nitrate (Liu et al. 2009; Medici et al. 2015). Therefore, as this transcription factor helps integrate signaling responses to both nitrate and Pi signals in the root tip (Medici et al. 2015), it provides an interesting perspective to achieve the coordinated modulation of plant responses to both stresses.
Fine-tuning of Pi recycling and remobilization has been proposed as a key breeding target for producing Pi-use efficient genotypes because it could enable plants to release and mobilize Pi from phospholipids without compromising photosynthesis (Heuer et al. 2017). Genomic and transcriptomic characterization of gene-regulation networks in the Proteaceae family, which adapted to environments with extreme Pi-limitation, should provide insights into the re-wiring of phospholipid metabolism (Hayes et al. 2018). Editing of promoter sequences of genes related to phospholipid substitution without compromising photosynthesis in crops, seems an interesting strategy.
Perspectives
Current agricultural systems are built on improved crop varieties and the extensive use of agrochemicals. For years, breeders have taken advantage of natural or induced genetic diversity in order to generate improved varieties. Clear examples of these efforts can be seen in improved rice varieties that have undergone drastic changes in plant architecture and grain yield and, thus, have sustained the Green Revolution. However, this process is slow and its application on crop low-Pi tolerance has not yet produced the varieties needed to significantly reduce the application of P-fertilizers. By exploiting the promises of novel approaches such as CRISPR/Cas9 and synthetic biology it would be possible to speed up the creation of the genetic diversity that is required for breeding purposes, and even to enrich this diversity beyond what we naturally though possible.
Now that complex networks of regulation controlling Pi-starvation responses are being elucidated and versatile CRISPR/Cas-based gene editing strategies can be implemented with the help of the latest and more sophisticated on-development computational resources, we might think on crop improvement based on more rational designs that can be systematically generated and evaluated (Liu and Stewart 2015). In this context, synthetic biology approaches that integrate and take advantage of multiple disciplines for the redesign and rewiring of genetic connections might be a useful asset (Kassaw et al. 2018; Pouvreau et al. 2018). This will help removing inefficiencies naturally associated with these processes while optimizing the essential components toward the desired trait. As stated by Medford and Prasad (2014), this type of approaches will help “not only uncover the natural genetic circuits behind complex gene regulations in plants, but we could also design traits that are new to evolution and beneficial to humanity” (Medford and Prasad 2014).
The design of biosensor molecules might also contribute to achieve these types of strategies by adding an external predictable and controllable switch to the system. The infiltration into plant cells of nanosensors wrapped into single-walled carbon nanotubes has already been used for different purposes including detection of external nitroaromatic compounds (Wong et al. 2017), arsenic (Lew et al. 2021), auxins (Ang et al. 2021), and H2O2 in stressed plants (Lew et al. 2020). Furthermore, a genetically encoded auxin biosensor was recently reported that allows the direct, rapid and real-time monitoring of auxin levels in individual cells and within cell compartments during the plant´s life cycle (Herud-Sikimic et al. 2021). This sensor is based on the rational engineering of a tryptophan repressor from E. coli and although originally designed for in planta visualization of auxin concentration, it can be easily incorporated for the design of biotechnological applications.
The previously mentioned perspectives can be accomplished by thoroughly harnessing all the power and potential from emerging technologies. Short- and long-read sequencing technologies should coordinately aid in deciphering the genomes and transcriptomes of species adapted to extreme Pi-scarcity and provide new gene pools and gene regulatory networks for low-Pi tolerance. Single-cell technologies will be crucial to decipher the hierarchical role of molecular regulators in such gene networks. Developments in proteomics, like the recent application of nanopores to determine protein fingerprinting (Lucas et al. 2021), together with high-throughput metabolomic characterization should aid defining the phenotypic profile of the Pi-efficient crop varieties. The development of efficient and standardized tissue-culture-independent gene editing systems will be key to speed up gene replacement and genome editing of crop plants which will ultimately help plant scientists and breeders to fine-tune plant Pi metabolism and root systems to boost Pi-foraging and Pi-use efficiency. Artificial intelligence approaches, like machine learning (Esposito et al. 2020), could be applied to detect and predict low-Pi tolerance-related genes and traits. Close collaboration of the scientific community and plant breeders with industry, policy and decision makers and government agencies must be promoted to guarantee technology transfer, a favorable public perception and fair distribution of crop materials to ultimately help us achieve sustainable crop Pi-nutrition.
References
Abel S (2017) Phosphate scouting by root tips. Curr Opin Plant Biol 39:168–177
Aggarwal S, Kumar A, Bhati KK et al (2018) RNAi-mediated downregulation of inositol pentakisphosphate kinase (IPK1) in wheat grains decreases phytic acid levels and increases Fe and Zn accumulation. Front Plant Sci. https://doi.org/10.3389/FPLS.2018.00259
Ågren GI, Wetterstedt JÅM, Billberger MFK (2012) Nutrient limitation on terrestrial plant growth–modeling the interaction between nitrogen and phosphorus. New Phytol 194:953–960. https://doi.org/10.1111/J.1469-8137.2012.04116.X
Ajmera I, Charlie Hodgman T, Lu C (2019) An integrative systems perspective on plant phosphate research. Genes (basel) 10:139
Alewell C, Ringeval B, Ballabio C et al (2020) Global phosphorus shortage will be aggravated by soil erosion. Nat Commun 11:1–12. https://doi.org/10.1038/s41467-020-18326-7
Ali N, Paul S, Gayen D et al (2013) Development of low phytate rice by rnai mediated seed-specific silencing of inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene (IPK1). PLoS ONE 8:e68161. https://doi.org/10.1371/JOURNAL.PONE.0068161
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00971
Ang MC-Y, Dhar N, Khong DT et al (2021) Nanosensor detection of synthetic auxins in planta using corona phase molecular recognition. ACS Sensors 6:3032–3046. https://doi.org/10.1021/ACSSENSORS.1C01022
Awai K, Maréchal E, Block MA et al (2001) Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc Natl Acad Sci U S A 98:10960–10965. https://doi.org/10.1073/pnas.181331498
Azevedo GC, Cheavegatti-Gianotto A, Negri BF et al (2015) Multiple interval QTL mapping and searching for PSTOL1 homologs associated with root morphology, biomass accumulation and phosphorus content in maize seedlings under low-P. BMC Plant Biol 151(15):1–17. https://doi.org/10.1186/S12870-015-0561-Y
Baek D, Kim MC, Chun HJ et al (2013) Regulation of miR399f transcription by AtMYB2 affects phosphate starvation responses in Arabidopsis. Plant Physiol 161:362–373. https://doi.org/10.1104/pp.112.205922
Balzergue C, Dartevelle T, Godon C et al (2017) Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat Commun 8:15300. https://doi.org/10.1038/ncomms15300
Bari R, Pant BD, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141:988–999. https://doi.org/10.1104/pp.106.079707
Barragán-Rosillo AC, Peralta-Alvarez CA, Ojeda-Rivera JO et al (2021) Genome accessibility dynamics in response to phosphate limitation is controlled by the PHR1 family of transcription factors in Arabidopsis. Proc Natl Acad Sci. https://doi.org/10.1073/PNAS.2107558118
Bates TR, Lynch JP (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell Environ 19:529–538. https://doi.org/10.1111/j.1365-3040.1996.tb00386.x
Bernardino KC, Pastina MM, Menezes CB et al (2019) The genetic architecture of phosphorus efficiency in sorghum involves pleiotropic QTL for root morphology and grain yield under low phosphorus availability in the soil. BMC Plant Biol 191(19):1–15. https://doi.org/10.1186/S12870-019-1689-Y
Bulgarelli D, Schlaeppi K, Spaepen S et al (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Bustos R, Castrillo G, Linhares F et al (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6:e1001102. https://doi.org/10.1371/journal.pgen.1001102
Butt H, Jamil M, Wang JY et al (2018) Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol 181(18):1–9. https://doi.org/10.1186/S12870-018-1387-1
Cai H, Chu Q, Yuan L et al (2012a) Identification of quantitative trait loci for leaf area and chlorophyll content in maize (Zea mays) under low nitrogen and low phosphorus supply. Mol Breed 301(30):251–266. https://doi.org/10.1007/S11032-011-9615-5
Cai H, Chen F, Mi G et al (2012) Mapping QTLs for root system architecture of maize (Zea mays L.) in the field at different developmental stages. Theor Appl Genet 1256(125):1313–1324. https://doi.org/10.1007/S00122-012-1915-6
Calderón-Vázquez C, Sawers RJH, Herrera-Estrella L (2011) Phosphate deprivation in maize: genetics and genomics. Plant Physiol 156:1067–1077. https://doi.org/10.1104/pp.111.174987
Castrillo G, Teixeira PJPL, Paredes SH et al (2017) Root microbiota drive direct integration of phosphate stress and immunity. Nat 5437646(543):513–518. https://doi.org/10.1038/nature21417
Chen L, Liao H (2017) Engineering crop nutrient efficiency for sustainable agriculture. J Integr Plant Biol 59:710–735
Chen M, Arato M, Borghi L et al (2018) Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01270
Chen J, Xu L (2011) Comparative mapping of QTLs for H+ secretion of root in maize (Zea mays L.) and cross phosphorus levels on two growth stages. Front Agric China 53(5):284–290. https://doi.org/10.1007/S11703-011-1075-5
Chu S, Zhang X, Yu K et al (2020) Genome-wide analysis reveals dynamic epigenomic differences in soybean response to low-phosphorus stress. Int J Mol Sci 6817(21):6817. https://doi.org/10.3390/IJMS21186817
Colasuonno P, Marcotuli I, Gadaleta A, Soriano JM (2021) From genetic maps to qtl cloning: an overview for durum wheat. Plants 10:1–25
Cordell D, White S (2015) Tracking phosphorus security: indicators of phosphorus vulnerability in the global food system. Food Sec 7:337–350. https://doi.org/10.1007/s12571-015-0442-0
Cordell D, White S (2014) Life’s bottleneck: sustaining the world’s phosphorus for a food secure future. Annu Rev Environ Resour 39:161–188. https://doi.org/10.1146/annurev-environ-010213-113300
Cordell D, Turner A, Chong J (2015) The hidden cost of phosphate fertilizers: mapping multi-stakeholder supply chain risks and impacts from mine to fork. Glob Chang Peace Secur 27:323–343. https://doi.org/10.1080/14781158.2015.1083540
Cosme M, Fernández I, Van der Heijden MGA, Pieterse CMJ (2018) Non-mycorrhizal plants: the exceptions that prove the rule. Trends Plant Sci 23:577–587. https://doi.org/10.1016/j.tplants.2018.04.004
Cruz-Ramírez A, Oropeza-Aburto A, Razo-Hernández F et al (2006) Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc Natl Acad Sci USA 103:6765–6770. https://doi.org/10.1073/pnas.0600863103
Delhaize E, Rathjen TM, Cavanagh CR (2015) The genetics of rhizosheath size in a multiparent mapping population of wheat. J Exp Bot 66:4527–4536. https://doi.org/10.1093/JXB/ERV223
Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2:43–58. https://doi.org/10.1093/mp/ssn081
Diaz LM, Ricaurte J, Cajiao C et al (2017) Phenotypic evaluation and QTL analysis of yield and symbiotic nitrogen fixation in a common bean population grown with two levels of phosphorus supply. Mol Breed 376(37):1–16. https://doi.org/10.1007/S11032-017-0673-1
Dong J, Piñeros MA, Li X et al (2017) An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol Plant 10:244–259. https://doi.org/10.1016/j.molp.2016.11.001
Dong J, Ma G, Sui L et al (2019) Inositol Pyrophosphate InsP8 acts as an intracellular phosphate signal in Arabidopsis. Mol Plant 12:1463–1473. https://doi.org/10.1016/j.molp.2019.08.002
Du Q, Wang K, Zou C et al (2018) The PILNCR1-miR399 regulatory module is important for low phosphate tolerance in maize. Plant Physiol 177:1743–1753. https://doi.org/10.1104/pp.18.00034
Ehdaie B, Merhaut DJ, Ahmadian S et al (2010) Root system size influences water-nutrient uptake and nitrate leaching potential in wheat. J Agron Crop Sci 196:455–466. https://doi.org/10.1111/J.1439-037X.2010.00433.X
Esposito S, Carputo D, Cardi T, Tripodi P (2020) Applications and trends of machine learning in genomics and phenomics for next-generation breeding. Plants 9:34
Essigmann B, Güler S, Narang RA et al (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1 a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A 95:1950–1955. https://doi.org/10.1073/pnas.95.4.1950
Fang Q, Zhou F, Zhang Y et al (2021) Degradation of STOP1 mediated by the F-box proteins RAH1 and RAE1 balances aluminum resistance and plant growth in Arabidopsis thaliana. Plant J 106:493–506. https://doi.org/10.1111/TPJ.15181
FAO (2020) World fertilizer trends and outlook to 2022. World Fertil Trends Outlook to 2022:40. https://doi.org/10.4060/ca6746en
FAO, IFAD, UNICEF, WFP, WHO (2021) The State of Food Security and Nutrition in the World 2021. Transforming food 10.1007/s00122-022-04095-y systems for food security, improved nutrition and affordable healthy diets for all. Rome, FAO
Foroozani M, Zahraeifard S, Oh D-H et al (2020) Low-phosphate chromatin dynamics predict a cell wall remodeling network in rice shoots. Plant Physiol 182:1494–1509. https://doi.org/10.1104/PP.19.01153
Franco-Zorrilla JM, Valli A, Todesco M et al (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39:1033–1037. https://doi.org/10.1038/ng2079
Gamuyao R, Chin JH, Pariasca-Tanaka J et al (2012) The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nat 4887412(488):535–539. https://doi.org/10.1038/nature11346
Gao W, Lu L, Qiu W et al (2017) OsPAP26 encodes a major purple acid phosphatase and regulates phosphate remobilization in rice. Plant Cell Physiol 58:885–892. https://doi.org/10.1093/pcp/pcx041
Gao SQ, Chen GD, Hu DY et al (2018) A major quantitative trait locus controlling phosphorus utilization efficiency under different phytate-P conditions at vegetative stage in barley. J Integr Agric 17:285–295. https://doi.org/10.1016/S2095-3119(17)61713-1
Gao S, Xia J, Yuan S et al (2020) Novel QTL conferring phosphorus acquisition and utilization efficiencies in barley. Front Genet. https://doi.org/10.3389/FGENE.2020.580452
García-Latorre C, Rodrigo S, Santamaría O (2021) Endophytes as Plant Nutrient Uptake-Promoter in Plants. 247–265. https://doi.org/10.1007/978-3-030-65447-4_11
Godon C, Mercier C, Wang X et al (2019) Under phosphate starvation conditions, Fe and Al trigger accumulation of the transcription factor STOP1 in the nucleus of Arabidopsis root cells. Plant J 99:937–949. https://doi.org/10.1111/tpj.14374
González-Muñoz E, Avendaño AOV, Chávez Montes RA et al (2015) The maize (Zea mays ssp. mays var. B73) genome encodes 33 members of the purple acid phosphatase family. Front Plant Sci 6:1–12. https://doi.org/10.3389/fpls.2015.00341
Grimanelli D, Roudier F (2013) Epigenetics and development in plants: green light to convergent innovations. Curr Top Dev Biol 104:189–222. https://doi.org/10.1016/B978-0-12-416027-9.00006-1
Gu R, Chen F, Long L et al (2016a) Enhancing phosphorus uptake efficiency through QTL-based selection for root system architecture in maize. J Genet Genomics 43:663–672. https://doi.org/10.1016/J.JGG.2016.11.002
Gu Z, Eils R, Schlesner M (2016b) Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32:2847–2849. https://doi.org/10.1093/bioinformatics/btw313
Guo J, Chen G, Zhang X et al (2018) Quantitative trait locus analysis of adventitious and lateral root morphology of barley grown at low and high P. Funct Plant Biol 45:957–967. https://doi.org/10.1071/FP17271
Gutiérrez-Alanís D, Yong-Villalobos L, Jiménez-Sandoval P et al (2017) Phosphate starvation-dependent iron mobilization induces CLE14 expression to trigger root meristem differentiation through CLV2/PEPR2 signaling. Dev Cell 41:555-570.e3. https://doi.org/10.1016/j.devcel.2017.05.009
Gutiérrez-Alanís D, Ojeda-Rivera JO, Yong-Villalobos L et al (2018) Adaptation to phosphate scarcity: tips from Arabidopsis roots. Trends Plant Sci 23:721–730. https://doi.org/10.1016/j.tplants.2018.04.006
Hacisalihoglu G, Burton AL, Gustin JL et al (2018) Quantitative trait loci associated with soybean seed weight and composition under different phosphorus levels. J Integr Plant Biol 60:232–241. https://doi.org/10.1111/JIPB.12612
Hamburger D, Rezzonico E, Petétot JMDC et al (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14:889–902. https://doi.org/10.1105/tpc.000745
Hasan MM, Hasan MM, Teixeira Da Silva JA, Li X (2016) Regulation of phosphorus uptake and utilization: transitioning from current knowledge to practical strategies. https://doi.org/10.1186/s11658-016-0008-y
Hayes PE, Clode PL, Oliveira RS, Lambers H (2018) Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: an adaptation improving phosphorus-use efficiency. Plant Cell Environ 41:605–619. https://doi.org/10.1111/PCE.13124
Herud-Sikimić O, Stiel AC, Kolb M et al (2021) A biosensor for the direct visualization of auxin. Nat 5927856(592):768–772. https://doi.org/10.1038/s41586-021-03425-2
Heuer S, Gaxiola R, Schilling R et al (2017) Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J 90:868–885. https://doi.org/10.1111/tpj.13423
Hiruma K, Gerlach N, Sacristán S et al (2016) Root endophyte colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165:464–474. https://doi.org/10.1016/J.CELL.2016.02.028
Hoekenga OA, Maron LG, Piñeros MA et al (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci U S A 103:9738–9743. https://doi.org/10.1073/pnas.0602868103
Hou E, Luo Y, Kuang Y et al (2020) Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat Commun 111(11):1–9. https://doi.org/10.1038/s41467-020-14492-w
Hsieh L-C, Lin S-I, Shih AC-C et al (2009) Uncovering small RNA-Mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151:2120–2132. https://doi.org/10.1104/PP.109.147280
Hu B, Zhu C, Li F et al (2011) LEAF TIP NECROSIS1 plays a pivotal role in the regulation of multiple phosphate starvation responses in rice. Plant Physiol 156:1101–1115. https://doi.org/10.1104/PP.110.170209
Hu B, Wang W, Ou S et al (2015) Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat Genet 477(47):834–838. https://doi.org/10.1038/ng.3337
Huang TK, Han CL, Lin SI et al (2013) Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 25:4044–4060. https://doi.org/10.1105/tpc.113.115998
Hunter MC, Smith RG, Schipanski ME et al (2017) Agriculture in 2050: recalibrating targets for sustainable intensification. Bioscience 67:386–391
Hurley B, Tran HT, Plaxton WC (2010) Feeding hungry plants: The role of purple acid phosphatases in phosphate nutrition. Plant Sci 179:14–27
Iuchi S, Koyama H, Iuchi A et al (2007) Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci U S A 104:9900–9905. https://doi.org/10.1073/pnas.0700117104
Jabnoune M, Secco D, Lecampion C et al (2013) A rice cis-Natural antisense RNA acts as a translational enhancer for its cognate mRNA and contributes to phosphate homeostasis and plant fitness. Plant Cell 25:4166–4182. https://doi.org/10.1105/TPC.113.116251
Jaganathan D, Ramasamy K, Sellamuthu G et al (2018) CRISPR for crop improvement: an update review. Front Plant Sci 9:985
James RA, Weligama C, Verbyla K et al (2016) Rhizosheaths on wheat grown in acid soils: phosphorus acquisition efficiency and genetic control. J Exp Bot 67:3709–3718. https://doi.org/10.1093/JXB/ERW035
Jasinski S (2021) Phosphate rock statistics and information. In: USGS Natl. Miner. Inf. Cent. https://www.usgs.gov/centers/nmic/phosphate-rock-statistics-and-information. Accessed 25 Oct 2021
Jeong K, Baten A, Waters DLE et al (2017) Phosphorus remobilization from rice flag leaves during grain filling: an RNA-seq study. Plant Biotechnol J 15:15–26. https://doi.org/10.1111/PBI.12586
Jiang F, Doudna JA (2017) CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys 46:505–529
John DA, Babu GR (2021) Lessons from the aftermaths of green revolution on food system and health. Front Sustain Food Syst. https://doi.org/10.3389/FSUFS.2021.644559
Jung WJ, Seo YW (2014) Employment of wheat-rye translocation in wheat improvement and broadening its genetic basis. J Crop Sci Biotechnol 174(17):305–313. https://doi.org/10.1007/S12892-014-0086-1
Jyoti A, Kaushik S, Srivastava VK et al (2019) The potential application of genome editing by using CRISPR/Cas9, and its engineered and ortholog variants for studying the transcription factors involved in the maintenance of phosphate homeostasis in model plants. Semin Cell Dev Biol 96:77–90
Kale RR, Rani CVD, Anila M et al (2021) Novel major QTLs associated with low soil phosphorus tolerance identified from the Indian rice landrace. Wazuhophek Plos One 16:e0254526. https://doi.org/10.1371/JOURNAL.PONE.0254526
Kanno S, Arrighi JF, Chiarenza S et al (2016) A novel role for the root cap in phosphate uptake and homeostasis. Elife. https://doi.org/10.7554/ELIFE.14577
Kant S, Peng M, Rothstein SJ (2011) Genetic regulation by NLA and MicroRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLOS Genet 7:e1002021. https://doi.org/10.1371/JOURNAL.PGEN.1002021
Kassaw TK, Donayre-Torres AJ, Antunes MS et al (2018) Engineering synthetic regulatory circuits in plants. Plant Sci 273:13–22. https://doi.org/10.1016/j.plantsci.2018.04.005
Kelly AA, Froehlich JE, Dörmann P (2003) Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. Plant Cell 15:2694–2706. https://doi.org/10.1105/tpc.016675
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganisms in sustainable agriculture—a review. Agron Sustain Dev 271(27):29–43. https://doi.org/10.1051/AGRO:2006011
Khatodia S, Bhatotia K, Passricha N et al (2016) The CRISPR/Cas genome-editing tool: application in Improvement of Crops. Front Plant Sci. https://doi.org/10.3389/FPLS.2016.00506
Khosla A, Nelson DC (2016) Strigolactones, super hormones in the fight against Striga. Curr Opin Plant Biol 33:57–63. https://doi.org/10.1016/J.PBI.2016.06.001
Kornberg RD, Lorch Y (2003) Chromatin structure and transcription. 8:563–587. https://doi.org/10.1146/annurev.cb.08.110192.003023
Kumawat G, Gupta S, Ratnaparkhe MB et al (2016) QTLomics in soybean: a way forward for translational genomics and breeding. Front Plant Sci. https://doi.org/10.3389/FPLS.2016.01852
Kuo H-F, Hsu Y-Y, Lin W-C et al (2018) Arabidopsis inositol phosphate kinases IPK1 and ITPK1 constitute a metabolic pathway in maintaining phosphate homeostasis. Plant J 95:613–630. https://doi.org/10.1111/TPJ.13974
Lambers H, Cawthray GR, Giavalisco P et al (2012) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol 196:1098–1108. https://doi.org/10.1111/j.1469-8137.2012.04285.x
Land ES, Cridland CA, Craige B et al (2021) A role for inositol pyrophosphates in the metabolic adaptations to low phosphate in arabidopsis. Metab 601(11):601. https://doi.org/10.3390/METABO11090601
Lee HY, Chen Z, Zhang C, Yoon GM (2019) Editing of the OsACS locus alters phosphate deficiency-induced adaptive responses in rice seedlings. J Exp Bot 70:1927–1940. https://doi.org/10.1093/jxb/erz074
Lew TTS, Koman VB, Silmore KS et al (2020) Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat Plants 64(6):404–415. https://doi.org/10.1038/s41477-020-0632-4
Lew TTS, Park M, Cui J, Strano MS (2021) Plant nanobionic sensors for arsenic detection. Adv Mater 33:2005683. https://doi.org/10.1002/ADMA.202005683
Li C, Gui S, Yang T et al (2012) Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann Bot 109:275–285. https://doi.org/10.1093/aob/mcr246
Li J, Zhang X, Sun Y et al (2018) Efficient allelic replacement in rice by gene editing: a case study of the NRT1.1B gene. J Integr Plant Biol 60:536–540
Li D, Zhu H, Liu K, et al (2002) Purple acid phosphatases of Arabidopsis thaliana COMPARATIVE ANALYSIS AND DIFFERENTIAL REGULATION BY PHOSPHATE DEPRIVATION*. https://doi.org/10.1074/jbc.M204183200
Liang Z, Zhang K, Chen K, Gao C (2014) Targeted mutagenesis in Zea mays using TALENs and the CRISPR/Cas system. J Genet Genomics 41:63–68. https://doi.org/10.1016/j.jgg.2013.12.001
Lin W-Y, Huang T-K, Chiou T-J (2013) NITROGEN LIMITATION ADAPTATION, a target of MicroRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25:4061–4074. https://doi.org/10.1105/TPC.113.116012
Lindemose S, O’Shea C, Jensen MK, Skriver K (2013) Structure, function and networks of transcription factors involved in abiotic stress responses. Int J Mol Sci 14:5842–5878
Liu W, Stewart CN (2015) Plant synthetic biology. Trends Plant Sci 20:309–317
Liu H, Yang H, Wu C et al (2009) Overexpressing HRS1 confers hypersensitivity to low phosphate-elicited inhibition of primary root growth in Arabidopsis thaliana. J Integr Plant Biol 51:382–392. https://doi.org/10.1111/j.1744-7909.2009.00819.x
Liu TY, Huang TK, Tseng CY et al (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24:2168–2183. https://doi.org/10.1105/tpc.112.096636
Liu F, Xu Y, Han G et al (2018a) Identification and functional characterization of a maize phosphate transporter induced by mycorrhiza formation. Plant Cell Physiol 59:1683–1694. https://doi.org/10.1093/pcp/pcy094
Liu N, Shang W, Li C, et al (2018b) Evolution of the SPX gene family in plants and its role in the response mechanism to phosphorus stress. Open Biol. 8
López-Arredondo DL, Herrera-Estrella L (2012) Engineering phosphorus metabolism in plants to produce a dual fertilization and weed control system. Nat Biotechnol 30:889–893. https://doi.org/10.1038/nbt.2346
López-Arredondo DL, Leyva-González MA, González-Morales SI et al (2014) Phosphate nutrition: improving low-phosphate tolerance in crops. Annu Rev Plant Biol 65:95–123. https://doi.org/10.1146/annurev-arplant-050213-035949
López-Arredondo D, Fan X, Jiao Y (2021) Recent advancement in molecular breeding for improving nutrient-use efficiency in maize. In: Molecular breeding in wheat, maize and sorghum: strategies for improving abiotic stress tolerance and yield. CABI, pp 340–359
López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L et al (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129:244–256. https://doi.org/10.1104/pp.010934
López-Bucio JS, Salmerón-Barrera GJ, Ravelo-Ortega G et al (2019) Mitogen-activated protein kinase 6 integrates phosphate and iron responses for indeterminate root growth in Arabidopsis thaliana. Planta 250:1177–1189. https://doi.org/10.1007/s00425-019-03212-4
Lu Y, Zhu JK (2017) Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol Plant 10:523–525. https://doi.org/10.1016/J.MOLP.2016.11.013
Lucas FLR, Versloot RCA, Yakovlieva L et al (2021) Protein identification by nanopore peptide profiling. Nat Commun 12:1–9. https://doi.org/10.1038/s41467-021-26046-9
Lynch JP, Brown KM (2001) Topsoil foraging–an architectural adaptation of plants to low phosphorus availability. In: Plant and soil. Springer, pp 225–237
Mackey KRM, Paytan A (2009) Phosphorus Cycle. In: Encyclopedia of microbiology. Academic Press, pp 322–334
Maharajan T, Ceasar SA, Krishna TPA et al (2018) Utilization of molecular markers for improving the phosphorus efficiency in crop plants. Plant Breed 137:10–26. https://doi.org/10.1111/PBR.12537
Mahender A, Anandan A, Pradhan SK, Singh ON (2018) Traits-related QTLs and genes and their potential applications in rice improvement under low phosphorus condition. Arch Agron Soil Sci 64:449–464
Mai NTP, Mai CD, Van NH et al (2021) Discovery of new genetic determinants of morphological plasticity in rice roots and shoots under phosphate starvation using GWAS. J Plant Physiol 257:153340. https://doi.org/10.1016/J.JPLPH.2020.153340
Malhotra H, Vandana SS, Pandey R (2018) Phosphorus nutrition: plant growth in response to deficiency and excess. Plant Nutr Abiotic Stress Toler. https://doi.org/10.1007/978-981-10-9044-8_7
Manna M, Achary VMM, Islam T et al (2016) The development of a phosphite-mediated fertilization and weed control system for rice. Sci Rep 6:1–12. https://doi.org/10.1038/srep24941
Marschner H (2011) Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier Science
Mayzlish-Gati E, De-Cuyper C, Goormachtig S et al (2012) Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol 160:1329–1341. https://doi.org/10.1104/pp.112.202358
Medford JI, Prasad A (2014) Plant synthetic biology takes root. Science 346:162–163. https://doi.org/10.1126/SCIENCE.1261140
Medici A, Marshall-Colon A, Ronzier E et al (2015) AtNIGT1/HRS1 integrates nitrate and phosphate signals at the Arabidopsis root tip. Nat Commun 6:1–11. https://doi.org/10.1038/ncomms7274
Mellor J (2005) The dynamics of chromatin remodeling at promoters. Mol Cell 19:147–157. https://doi.org/10.1016/J.MOLCEL.2005.06.023
Milner MJ, Howells RM, Craze M et al (2018) A PSTOL-like gene, TaPSTOL, controls a number of agronomically important traits in wheat. BMC Plant Biol. https://doi.org/10.1186/S12870-018-1331-4
Mogollón JM, Bouwman AF, Beusen AHW et al (2021) More efficient phosphorus use can avoid cropland expansion. Nat Food 27(2):509–518. https://doi.org/10.1038/s43016-021-00303-y
Mohr S, Evans G (2013) Projections of future phosphorus production. Philicacom 380:1–47
Montecillo JAV, Chu LL, Bae H (2020) CRISPR-Cas9 system for plant genome editing: current approaches and emerging developments. Agron 1033(10):1033. https://doi.org/10.3390/AGRONOMY10071033
Mora-Macías J, Ojeda-Rivera JO, Gutiérrez-Alanís D et al (2017) Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc Natl Acad Sci U S A 114:E3563–E3572. https://doi.org/10.1073/pnas.1701952114
Moskal K, Kowalik S, Podyma W et al (2021) The pros and cons of rye chromatin introgression into wheat genome. Agron 456(11):456. https://doi.org/10.3390/AGRONOMY11030456
Müller J, Toev T, Heisters M et al (2015) Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Dev Cell 33:216–230. https://doi.org/10.1016/j.devcel.2015.02.007
Nahampun HN, López-Arredondo D, Xu X et al (2016) Assessment of ptxD gene as an alternative selectable marker for Agrobacterium-mediated maize transformation. Plant Cell Rep 355(35):1121–1132. https://doi.org/10.1007/S00299-016-1942-X
Nussaume L, Kanno S, Javot H et al (2011) Phosphate import in plants: focus on the PHT1 transporters. Front Plant Sci. https://doi.org/10.3389/FPLS.2011.00083
O’Malley RC, Huang SSC, Song L et al (2016) Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 165:1280–1292. https://doi.org/10.1016/j.cell.2016.04.038
Ochoa IE, Blair MW, Lynch JP (2006) QTL analysis of adventitious root formation in common bean under contrasting phosphorus availability. Crop Sci 46:1609–1621. https://doi.org/10.2135/CROPSCI2005.12-0446
Ojeda-Rivera JO, Oropeza-Aburto A, Herrera-Estrella L (2020) Dissection of root transcriptional responses to Low pH, aluminum toxicity and iron excess under pi-limiting conditions in Arabidopsis wild-type and stop1 seedlings. Front Plant Sci 11:1200. https://doi.org/10.3389/fpls.2020.01200
Okazaki Y, Otsuki H, Narisawa T et al (2013) A new class of plant lipid is essential for protection against phosphorus depletion. Nat Commun 41(4):1–10. https://doi.org/10.1038/ncomms2512
Pandeya D, López-Arredondo DL, Janga MR et al (2018) Selective fertilization with phosphite allows unhindered growth of cotton plants expressing the ptxD gene while suppressing weeds. Proc Natl Acad Sci 115:E6946–E6955. https://doi.org/10.1073/PNAS.1804862115
Patikoglou G, Burley SK (1997) Eukaryotic transcription factor-DNA complexes. Annu Rev Biophys Biomol Struct 26:289–325
Pavinato PS, Cherubin MR, Soltangheisi A et al (2020) Revealing soil legacy phosphorus to promote sustainable agriculture in Brazil. Sci Reports 101(10):1–11. https://doi.org/10.1038/s41598-020-72302-1
Perera I, Seneweera S, Hirotsu N (2018) Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability. Rice. https://doi.org/10.1186/S12284-018-0200-Y
Péret B, Clément M, Nussaume L, Desnos T (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16:442–450
Péret B, Desnos T, Jost R et al (2014) Root architecture responses: in search of phosphate. Plant Physiol 166:1713–1723. https://doi.org/10.1104/pp.114.244541
Pérez-Torres C-A, López-Bucio J, Cruz-Ramírez A et al (2008) Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20:3258. https://doi.org/10.1105/TPC.108.058719
Pingali PL (2012) Green revolution: impacts, limits, and the path ahead. Proc Natl Acad Sci 109:12302–12308. https://doi.org/10.1073/PNAS.0912953109
Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiol 156:1006–1015. https://doi.org/10.1104/pp.111.175281
Poirier Y, Thoma S, Somerville C, Schiefelbein J (1991) A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol 97:1087–1093. https://doi.org/10.1104/pp.97.3.1087
Pouvreau B, Vanhercke T, Singh S (2018) From plant metabolic engineering to plant synthetic biology: the evolution of the design/build/test/learn cycle. Plant Sci 273:3–12. https://doi.org/10.1016/J.PLANTSCI.2018.03.035
Puga MI, Mateos I, Charukesi R et al (2014) SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc Natl Acad Sci U S A 111:14947–14952. https://doi.org/10.1073/pnas.1404654111
Qiu H, Liu C, Yu T et al (2014) Identification of QTL for acid phosphatase activity in root and rhizosphere soil of maize under low phosphorus stress. Euphytica 1971(197):133–143. https://doi.org/10.1007/S10681-013-1058-0
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Biol 50:665–693. https://doi.org/10.1146/annurev.arplant.50.1.665
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49. https://doi.org/10.1007/s11104-004-2005-6
Ramírez-Flores MR, Perez-Limon S, Li M et al (2020) The genetic architecture of host response reveals the importance of arbuscular mycorrhizae to maize cultivation. Elife 9:1–18. https://doi.org/10.7554/ELIFE.61701
Ray DK, Foley JA (2013) Increasing global crop harvest frequency: recent trends and future directions. Environ Res Lett 8:044041. https://doi.org/10.1088/1748-9326/8/4/044041
Raya-González J, Ojeda-Rivera JO, Mora-Macias J et al (2021) MEDIATOR16 orchestrates local and systemic responses to phosphate scarcity in Arabidopsis roots. New Phytol 229:1278–1288. https://doi.org/10.1111/NPH.16989
Ren Y, Qian Y, Xu Y et al (2017) Characterization of QTLs for root traits of wheat grown under different nitrogen and phosphorus supply levels. Front Plant Sci. https://doi.org/10.3389/FPLS.2017.02096
Ried MK, Wild R, Zhu J et al (2021) Inositol pyrophosphates promote the interaction of SPX domains with the coiled-coil motif of PHR transcription factors to regulate plant phosphate homeostasis. Nat Commun 121(12):1–13. https://doi.org/10.1038/s41467-020-20681-4
Rubio V, Linhares F, Solano R et al (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133. https://doi.org/10.1101/gad.204401
Ruiz Herrera LF, Shane MW, López-Bucio J (2015) Nutritional regulation of root development. Wiley Interdiscip Rev Dev Biol 4:431–443. https://doi.org/10.1002/wdev.183
Ryan PR, Liao M, Delhaize E et al (2015) Early vigour improves phosphate uptake in wheat. J Exp Bot 66:7089–7100. https://doi.org/10.1093/JXB/ERV403
Salas-González I, Reyt G, Flis P et al (2021) Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science. https://doi.org/10.1126/SCIENCE.ABD0695
Salazar-Vidal MN, Acosta-Segovia E, Sánchez-León N et al (2016) Characterization and transposon mutagenesis of the Maize (Zea mays) Pho1 gene family. PLoS ONE 11:e0161882. https://doi.org/10.1371/journal.pone.0161882
Sánchez-Calderón L, López-Bucio J, Chacón-López A et al (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46:174–184. https://doi.org/10.1093/pcp/pci011
Santoro V, Schiavon M, Gresta F et al (2020) Strigolactones control root system architecture and tip anatomy in solanum lycopersicum l. Plants under p starvation. Plants 9:612. https://doi.org/10.3390/plants9050612
Sattari SZ, Bouwman AF, Giller KE, van Ittersum MK (2012) Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc Natl Acad Sci 109:6348–6353. https://doi.org/10.1073/PNAS.1113675109
Savage N, Yang TJW, Chen CY et al (2013) Positional signaling and expression of ENHANCER OF TRY AND CPC1 are tuned to increase root hair density in response to phosphate deficiency in Arabidopsis thaliana. PLoS ONE 8:e75452. https://doi.org/10.1371/journal.pone.0075452
Sawaki Y, Iuchi S, Kobayashi Y et al (2009) Stop1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol 150:281–294. https://doi.org/10.1104/pp.108.134700
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453. https://doi.org/10.1104/PP.116.2.447
Secco D, Baumann A, Poirier Y (2010) Characterization of the rice PHO1 gene family reveals a key role for OsPHO1;2 in phosphate homeostasis and the evolution of a distinct clade in dicotyledons. Plant Physiol 152:1693–1704. https://doi.org/10.1104/pp.109.149872
Secco D, Wang C, Shou H et al (2015) Stress induced gene expression drives transient DNA methylation changes at adjacent repetitive elements. Elife. https://doi.org/10.7554/ELIFE.09343
Séré D, Martin A (2019) Epigenetic regulation: another layer in plant nutrition. https://doi.org/10.1080/15592324.2019.1686236
Sharpley A, Jarvie HP, Buda A et al (2013) Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. J Environ Qual 42:1308–1326. https://doi.org/10.2134/jeq2013.03.0098
Shi J, Wang H, Wu Y et al (2003) The maize low-phytic acid mutant lpa2 is caused by mutation in an inositol phosphate kinase gene. Plant Physiol 131:507–515. https://doi.org/10.1104/PP.014258
Shi J, Zhao B, Zheng S et al (2021) A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell. https://doi.org/10.1016/J.CELL.2021.09.030
Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642. https://doi.org/10.1111/j.1365-313X.2004.02161.x
Shukla VK, Doyon Y, Miller JC et al (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nat 4597245(459):437–441. https://doi.org/10.1038/nature07992
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057. https://doi.org/10.1104/PP.111.174581
Sun H, Tao J, Liu S et al (2014) Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J Exp Bot 65:6735–6746. https://doi.org/10.1093/jxb/eru029
Svistoonoff S, Creff A, Reymond M et al (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39:792–796. https://doi.org/10.1038/ng2041
Swamy HKM, Anila M, Kale RR et al (2019) Phenotypic and molecular characterization of rice germplasm lines and identification of novel source for low soil phosphorus tolerance in rice. Euphytica 2157(215):1–15. https://doi.org/10.1007/S10681-019-2443-0
Tamura Y, Kobae Y, Mizuno T, Hata S (2012) Identification and expression analysis of arbuscular mycorrhiza-inducible phosphate transporter genes of soybean. Biosci Biotechnol Biochem 76:309–313. https://doi.org/10.1271/bbb.110684
Thibaud MC, Arrighi JF, Bayle V et al (2010) Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J 64:775–789. https://doi.org/10.1111/j.1365-313X.2010.04375.x
Tian P, Lin Z, Lin D et al (2021) The pattern of DNA methylation alteration, and its association with the changes of gene expression and alternative splicing during phosphate starvation in tomato. Plant J. https://doi.org/10.1111/TPJ.15486
Ticconi CA, Lucero RD, Sakhonwasee S et al (2009) ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proc Natl Acad Sci U S A 106:14174–14179. https://doi.org/10.1073/pnas.0901778106
Tiessen H (2008) Phosphorus in the global environment. Springer, Dordrecht, pp 1–7
To HTM, Le KQ, Van Nguyen H et al (2020) A genome-wide association study reveals the quantitative trait locus and candidate genes that regulate phosphate efficiency in a Vietnamese rice collection. Physiol Mol Biol Plants 2611(26):2267–2281. https://doi.org/10.1007/S12298-020-00902-2
Tran HT, Qian W, Hurley BA, et al (2010) Biochemical and molecular characterization of AtPAP12 and AtPAP26: the predominant purple acid phosphatase isozymes secreted by phosphate-starved. https://doi.org/10.1111/j.1365-3040.2010.02184.x
UN Department of Economics and Social Affairs (2015) World Population Prospects - Population Division—United Nations. In: Int. J. Logist. Manag. https://population.un.org/wpp/. Accessed 25 Oct 2021
Valdés-López O, Arenas-Huertero C, Ramírez M et al (2008) Essential role of MYB transcription factor: PvPHR1 and microRNA: PvmiR399 in phosphorus-deficiency signalling in common bean roots. Plant, Cell Environ 31:1834–1843. https://doi.org/10.1111/j.1365-3040.2008.01883.x
Valeeva LR, Nyamsuren C, Sharipova MR, Shakirov EV (2018) Heterologous expression of secreted bacterial BPP and HAP phytases in plants stimulates Arabidopsis thaliana growth on phytate. Front Plant Sci. https://doi.org/10.3389/FPLS.2018.00186
Van Vuuren DP, Bouwman AF, Beusen AHW (2010) Phosphorus demand for the 1970–2100 period: a scenario analysis of resource depletion. Glob Environ Chang 20:428–439. https://doi.org/10.1016/J.GLOENVCHA.2010.04.004
Versaw WK, Garcia LR (2017) Intracellular transport and compartmentation of phosphate in plants. Curr Opin Plant Biol 39:25–30. https://doi.org/10.1016/J.PBI.2017.04.015
Vlčko T, Ohnoutková L (2020) Allelic variants of CRISPR/Cas9 induced mutation in an inositol trisphosphate 5/6 kinase gene manifest different phenotypes in Barley. Plants. https://doi.org/10.3390/plants9020195
Wang X, Shen J, Liao H (2010) Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci 179:302–306. https://doi.org/10.1016/J.PLANTSCI.2010.06.007
Wang L, Li Z, Qian W et al (2011) The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation. Plant Physiol 157:1283–1299. https://doi.org/10.1104/pp.111.183723
Wang J, Sun J, Miao J et al (2013) A phosphate starvation response regulator Ta-PHR1 is involved in phosphate signalling and increases grain yield in wheat. Ann Bot 111:1139–1153. https://doi.org/10.1093/AOB/MCT080
Wang Z, Ruan W, Shi J et al (2014) Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc Natl Acad Sci U S A 111:14953–14958. https://doi.org/10.1073/pnas.1404680111
Wang W, Ding G-D, White PJ et al (2018) Mapping and cloning of quantitative trait loci for phosphorus efficiency in crops: opportunities and challenges. Plant Soil 4391(439):91–112. https://doi.org/10.1007/S11104-018-3706-6
Wang H, Wei J, Li P et al (2019a) Integrating GWAS and gene expression analysis identifies candidate genes for root morphology traits in Maize at the seedling stage. Genes (basel) 10:773. https://doi.org/10.3390/genes10100773
Wang X, Wang Z, Zheng Z et al (2019b) Genetic dissection of Fe-Dependent signaling in root developmental responses to phosphate deficiency. Plant Physiol 179:300–316. https://doi.org/10.1104/pp.18.00907
Wang Y, Gao H, He L et al (2019c) The PHOSPHATE1 genes participate in salt and Pi signaling pathways and play adaptive roles during soybean evolution. BMC Plant Biol 191(19):1–19. https://doi.org/10.1186/S12870-019-1959-8
Wang T, Zhang H Zhu H (2019d) CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic Res 61(6):1–13. https://doi.org/10.1038/s41438-019-0159-x
Wang D, Lv S, Jiang P, Li Y (2017) Roles, regulation, and agricultural application of plant phosphate transporters. Front. Plant Sci. 8
Wege S, Khan GA, Jung J-Y et al (2016) The EXS domain of PHO1 participates in the response of shoots to phosphate deficiency via a Root-to-Shoot Signal. Plant Physiol 170:385. https://doi.org/10.1104/PP.15.00975
Wild R, Gerasimaite R, Jung JY et al (2016) Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352:986–990. https://doi.org/10.1126/science.aad9858
Willmann M, Gerlach N, Buer B et al (2013) Mycorrhizal phosphate uptake pathway in maize: vital for growth and cob development on nutrient poor agricultural and greenhouse soils. Front Plant Sci. https://doi.org/10.3389/FPLS.2013.00533
Wissuwa M, Wegner J, Ae N, Yano M (2002) Substitution mapping of Pup1: a major QTL increasing phosphorus uptake of rice from a phosphorus-deficient soil. Theor Appl Genet 105:890–897. https://doi.org/10.1007/s00122-002-1051-9
Wong MH, Giraldo JP, Kwak SY et al (2017) Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat Mater 16:264–272. https://doi.org/10.1038/nmat4771
Wu D, Li C, Jing Y et al (2020) Identification of quantitative trait loci underlying soybean (Glycine max) 100-seed weight under different levels of phosphorus fertilizer application. Plant Breed 139:959–968. https://doi.org/10.1111/PBR.12850
Xu Y, Wang R, Tong Y et al (2014) Mapping QTLs for yield and nitrogen-related traits in wheat: influence of nitrogen and phosphorus fertilization on QTL expression. Theor Appl Genet 127:59–72. https://doi.org/10.1007/s00122-013-2201-y
Xu JM, Wang ZQ, Wang JY et al (2020) Low phosphate represses histone deacetylase complex1 to regulate root system architecture remodeling in Arabidopsis. New Phytol 225:1732–1745. https://doi.org/10.1111/NPH.16264
Yacoubou A-M, Wallis NZ, Menkir A et al (2021) Breeding maize (Zea mays) for Striga resistance: past, current and prospects in sub-saharan africa. Plant Breed 140:195–210. https://doi.org/10.1111/PBR.12896
Yadav RS, Meena SC, Patel SI et al (2012) Bioavailability of soil P for plant nutrition. Springer, Dordrecht, pp 177–200
Yamaji N, Takemoto Y, Miyaji T et al (2017) Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 541:92–95. https://doi.org/10.1038/nature20610
Yang S-Y, Grønlund M, Jakobsen I et al (2012) Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the phosphate transporter1 gene family. Plant Cell 24:4236–4251. https://doi.org/10.1105/TPC.112.104901
Yang M, Wang C, Hassan MA et al (2021) QTL mapping of root traits in wheat under different phosphorus levels using hydroponic culture. BMC Genomics 221(22):1–12. https://doi.org/10.1186/S12864-021-07425-4
Ye Y, Yuan J, Chang X, et al (2015) The phosphate transporter gene OsPht1;4 is involved in phosphate homeostasis in rice. https://doi.org/10.1371/journal.pone.0126186
Yong-Villalobos L, González-Morales SI, Wrobel K et al (2015) Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proc Natl Acad Sci 112:E7293–E7302. https://doi.org/10.1073/PNAS.1522301112
Yu X, Keitel C, Dijkstra FA (2021) Global analysis of phosphorus fertilizer use efficiency in cereal crops. Glob Food Sec 29:100545. https://doi.org/10.1016/J.GFS.2021.100545
Yuan Y, Gao M, Zhang M et al (2017) QTL mapping for phosphorus efficiency and morphological traits at seedling and maturity stages in wheat. Front Plant Sci. https://doi.org/10.3389/FPLS.2017.00614
Yue W, Ying Y, Wang C et al (2017) OsNLA1, a RING-type ubiquitin ligase, maintains phosphate homeostasis in Oryza sativa via degradation of phosphate transporters. Plant J 90:1040–1051. https://doi.org/10.1111/TPJ.13516
Zhang H, Wang H (2014) QTL mapping for traits related to P-deficient tolerance using three related RIL populations in wheat. Euphytica 2033(203):505–520. https://doi.org/10.1007/S10681-014-1248-4
Zhang H, Lang Z, Zhu J-K (2018a) Dynamics and function of DNA methylation in plants. Nat Rev Mol Cell Biol 198(19):489–506. https://doi.org/10.1038/s41580-018-0016-z
Zhang Y, Anis GB, Wang R et al (2018b) Genetic dissection of QTL against phosphate deficiency in the hybrid rice ‘Xieyou9308.’ Plant Growth Regul 841(84):123–133. https://doi.org/10.1007/S10725-017-0326-8
Zhang Q, Wang C, Tian J et al (2011) Identification of rice purple acid phosphatases related to posphate starvation signalling. Plant Biol 13:7–15. https://doi.org/10.1111/j.1438-8677.2010.00346.x
Zhang D, Li H, Wang J et al (2016) High-density genetic mapping identifies new major loci for tolerance to low-phosphorus stress in soybean. Front Plant Sci. https://doi.org/10.3389/FPLS.2016.00372
Zhang Y, Zhang J, Guo J et al (2019) F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc Natl Acad Sci U S A 116:319–327. https://doi.org/10.1073/pnas.1814426116
Zhao P, You Q, Lei M (2019) A CRISPR/Cas9 deletion into the phosphate transporter SlPHO1;1 reveals its role in phosphate nutrition of tomato seedlings. Physiol Plant 167:556–563. https://doi.org/10.1111/ppl.12897
Zhou J, Jiao F, Wu Z et al (2008) OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol 146:1673–1686. https://doi.org/10.1104/PP.107.111443
Zhu J, Kaeppler SM, Lynch JP (2005) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays). Funct Plant Biol 32:749–762. https://doi.org/10.1071/FP05005
Zhu J, Lau K, Puschmann R et al (2019) Two bifunctional inositol pyrophosphate kinases/phosphatases control plant phosphate homeostasis. Elife. https://doi.org/10.7554/eLife.43582
Zogli P, Pingault L, Libault M (2017) Physiological and molecular mechanisms and adaptation strategies in soybean (Glycine max) under phosphate deficiency. Legume nitrogen fixation in soils with low phosphorus availability: adaptation and regulatory implication. Springer, Cham, pp 219–242
Acknowledgements
We sincerely thank Dr. Luis Herrera-Estrella at the Institute of Genomics for Crop Abiotic Stress Tolerance (IGCAST), Texas Tech University for valuable comments on the manuscript. We also thank the two reviewers who assessed our manuscript for all comments and recommendations. This work was supported partially by USDA-NIFA 20196702929912, USDA-ARS 21A546, and Cotton Incorporated 21-844 grants. We are grateful to the State of Texas’ Governor’s University Research Initiative (GURI)/Texas Tech University support grant 5-2018.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
DL-A defined the concept, outlined the contents and supervised the entire work. All authors conducted literature review and wrote different sections of the manuscript. JOO-R generated the first draft of the manuscript. DLL-A and JOO-R designed figures; GA-J and H-RN-G curated data and prepared table. DLL-A critically revised the work and finalized the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no financial interests.
Additional information
Communicated by Hermann Buerstmayr.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ojeda-Rivera, J.O., Alejo-Jacuinde, G., Nájera-González, HR. et al. Prospects of genetics and breeding for low-phosphate tolerance: an integrated approach from soil to cell. Theor Appl Genet 135, 4125–4150 (2022). https://doi.org/10.1007/s00122-022-04095-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-022-04095-y