Abstract
Key message
Genotyping-by-sequencing (GBS)-derived molecular markers reveal the distinct genetic population structure and relatively narrow genetic diversity of Chinese hulless oat landraces. Four markers linked to the naked grain gene (N1) are identified by genome-wide association study (GWAS).
Abstract
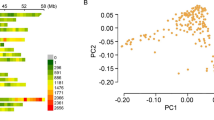
Interest in hulless oat (Avena sativa ssp. nuda), a variant of common oat (A. sativa) domesticated in Western Asia, has increased in recent years due to its free-threshing attribute and its domestication history. However, the genetic diversity and population structure of hulless oat, as well as the genetic mechanism of hullessness, are poorly understood. In this study, the genetic diversity and population structure of a worldwide sample of 805 oat lines including 186 hulless oats were investigated using genotyping-by-sequencing. Population structure analyses showed a strong genetic differentiation between hulless landraces vs other oat lines, including the modern hulless cultivars. The distinct subpopulation stratification of hulless landraces and their low genetic diversity suggests that a domestication bottleneck existed in hulless landraces. Additionally, low genetic diversity within European oats and strong differentiation between the spring oats and southern origin oat lines revealed by previous studies were also observed in this study. Genomic regions contributing to these genetic differentiations suggest that genetic loci related to growth habit and stress resistance may have been under intense selection, rather than the hulless-related genomic regions. Genome-wide association analysis detected four markers that were highly associated with hullessness. Three of these were mapped on linkage group Mrg21 at a genetic position between 195.7 and 212.1 cM, providing robust evidence that the dominant N1 locus located on Mrg21 is the single major factor controlling this trait.









Similar content being viewed by others
References
Achleitner A, Tinker NA, Zechner E, Buerstmayr H (2008) Genetic diversity among oat varieties of worldwide origin and associations of AFLP markers with quantitative traits. Theor Appl Genet 117:1041–1053
Al-Hajaj N, Peterson GW, Horbach C, Al-Shamaa K, Tinker NA, Fu Y-B (2018) Genotyping-by-sequencing empowered genetic diversity analysis of Jordanian oat wild relative Avena sterilis. Genet Resour Crop Evol 65:2069–2082
Aulchenko YS, Ripke S, Isaacs A, Van Duijn CM (2007) GenABEL: an R library for genome-wide association analysis. Bioinformatics 23:1294–1296
Baohong G, Zhou X, Murphy J (2003) Genetic variation within Chinese and western cultivated oat accessions. Cereal Res Commun 31:339–346
Beissinger TM, Wang L, Crosby K, Durvasula A, Hufford MB, Ross-Ibarra J (2016) Recent demography drives changes in linked selection across the maize genome. Nat Plants 2(7):1–7
Bekele WA, Itaya A, Boyle B, Yan W, Fetch JM, Tinker NA (2019) A targeted genotyping-by-sequencing tool (Rapture) for genomics-assisted breeding in oat. Theor Appl Genet 133:653–664
Bekele WA, Wight CP, Chao S, Howarth CJ, Tinker NA (2018) Haplotype-based genotyping-by-sequencing in oat genome research. Plant Biotechnol J 16:1452–1463
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B 57:289–300
Biel W, Bobko K, Maciorowski R (2009) Chemical composition and nutritive value of husked and naked oats grain. J Cereal Sci 49:413–418
Biel W, Jacyno E, Kawęcka M (2014) Chemical composition of hulled, dehulled and naked oat grains. S Afr J Anim Sci 44:189–197
Boczkowska M, Onyśk A (2016) Unused genetic resources: a case study of Polish common oat germplasm. Ann Appl Biol 169:155–165
Boland P, Lawes DA (1973) The inheritance of the naked grain character in oats studied in a cross between the naked variety Caesar and the husked variety Bo 1/11. Euphytica 22:582–591
Breseghello F, Sorrells ME (2006) Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 172:1165–1177
Burrows V, Molnar S, Tinker N, Marder T, Butler G, Lybaert A (2001) Groat yield of naked and covered oat. Can J Plant Sci 81:727–729
Cabral CB, Milach SCK, Federizzi LC, Bothona CA, Taderka I, Tisian LM, Limberger E (2000) Genetics of naked grain oats in crosses with Brazilian genotypes. Genet Mol Biol 23:851–854
Cavanagh CR et al (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci USA 110:8057–8062
Chaffin AS et al (2016) A consensus map in cultivated hexaploid oat reveals conserved grass synteny with substantial subgenome rearrangement. Plant Genome US 9:1–21
Chew P et al (2016) A study on the genetic relationships of Avena taxa and the origins of hexaploid oat. Theor Appl Genet 129:1405–1415
Coffman FA (1977) Oat history, identification and classification. USDA-ARS Tech Bull No 1516, Washington
Dempewolf H, Baute G, Anderson J, Kilian B, Smith C, Guarino L (2017) Past and future use of wild relatives in crop breeding. Crop Sci 57(3):1070–1082
De Koeyer DL et al (2004) A molecular linkage map with associated QTLs from a hulless x covered spring oat population. Theor Appl Genet 108:1285–1298
Diederichsen A (2008) Assessments of genetic diversity within a world collection of cultivated hexaploid oat (Avena sativa L.) based on qualitative morphological characters. Genet Resour Crop Evol 55:419–440
Dwivedi SL, Ceccarelli S, Blair MW, Upadhyaya HD, Are AK, Ortiz R (2016) Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci 21:31–42
Earl DA, VonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Esvelt Klos K, Huang Y-F, Bekele WA, Obert DE, Babiker E, Beattie AD, Bjørnstad Å, Bonman JM, Carson ML, Chao S (2016) Population genomics related to adaptation in elite oat germplasm. Plant Genome US 9:1–12
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Eyre-Walker A, Gaut RL, Hilton H, Feldman DL, Gaut BS (1998) Investigation of the bottleneck leading to the domestication of maize. Proc Natl Acad Sci USA 95:4441–4446
Fu Y-B, Peterson GW, Scoles G, Rossnagel B, Schoen DJ, Richards KW (2003) Allelic diversity changes in 96 Canadian oat cultivars released from 1886 to 2001. Crop Sci 43:1989–1995
Fu Y-B, Kibite S, Richards KW (2004) Amplified fragment length polymorphism analysis of 96 Canadian oat cultivars released between 1886 and 2001. Can J Plant Sci 84:23–30
Fu Y-B, Peterson GW, Williams D, Richards KW, Fetch JM (2005) Patterns of AFLP variation in a core subset of cultivated hexaploid oat germplasm. Theor Appl Genet 111:530–539
Givens D, Davies T, Laverick R (2004) Effect of variety, nitrogen fertiliser and various agronomic factors on the nutritive value of husked and naked oats grain. Anim Feed Sci Technol 113:169–181
Gnanesh B, Fetch JM, Menzies J, Beattie A, Eckstein P, McCartney C (2013) Chromosome location and allele-specific PCR markers for marker-assisted selection of the oat crown rust resistance gene Pc91. Mol Breed 32:679–686
Goudet J (2005) Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol Ecol Notes 5:184–186
Hamblin MT et al (2010) Population structure and linkage disequilibrium in US barley germplasm: implications for association mapping. Crop Sci 50:556–566
Huang Y-F, Poland JA, Wight CP, Jackson EW, Tinker NA (2014) Using genotyping-by-sequencing (GBS) for genomic discovery in cultivated oat. PLoS ONE 9:e102448
Jellen E, Beard J (2000) Geographical distribution of a chromosome 7C and 17 intergenomic translocation in cultivated oat. Crop Sci 40:256–263
Jenkins G, Hanson P (1976) The genetics of naked oats (Avena nuda L.). Euphytica 25:167–174
Kebede AZ et al (2019) Mapping of the stem rust resistance gene Pg13 in cultivated oat. Theor Appl Genet 133(1):259–270
Lawes DA, Boland P (1974) Effect of temperature on the expression of the naked grain character in oats. Euphytica 23:101–104
Li T, Cao Y, Wu X, Chen S, Wang H, Li K, Shen L (2015) First report on race and virulence characterization of Puccinia graminis f. sp. avenae and resistance of oat cultivars in China. Eur J Plant Pathol 142:85–91
Lopes MS et al (2015) Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J Exp Bot 66:3477–3486
Loskutov IG (2008) On evolutionary pathways of Avena species. Genet Resour Crop Evol 55:211–220
Lu F et al (2013) Switchgrass genomic diversity, ploidy, and evolution: novel insights from a network-based SNP discovery protocol. PLoS Genet 9:e1003215
Mangin BA, Siberchicot S, Nicolas A, Doligez P (2012) Novel measures of linkage disequilibrium that correct the bias due to population structure and relatedness. Heredity 108:285–291
Marshall A, Cowan S, Edwards S, Griffiths I, Howarth C, Langdon T, White E (2013) Crops that feed the world 9. Oats-a cereal crop for human and livestock feed with industrial applications. Food Sec 5:13–33
Money D, Gardner K, Migicovsky Z, Schwaninger H, Zhong GY, Myles S (2015) LinkImpute: fast and accurate genotype imputation for nonmodel organisms. G3 5(11):2383–2390
Montilla-Bascón G et al (2013) Genetic diversity and population structure among oat cultivars and landraces. Plant Mol Biol Rep 31:1305–1314
Murphy JP, Phillips T (1993) Isozyme variation in cultivated oat and its progenitor species Avena sterilis L. Crop Sci 33:1366–1372
Morris GP, Ramu P, Deshpande SP, Hash CT, Shah T, Upadhyaya HD, Riera-Lizarazu O, Brown PJ, Acharya CB, Mitchell SE, Harriman J, Glaubitz JC, Buckler ES, Kresovich S (2013) Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc Natl Acad Sci USA 110:453–458
Newell M, Cook D, Tinker N, Jannink J-L (2011) Population structure and linkage disequilibrium in oat (Avena sativa L.): implications for genome-wide association studies. Theor Appl Genet 122(3):623–632
Oliver RE et al (2013) SNP discovery and chromosome anchoring provide the first physically-anchored hexaploid oat map and reveal synteny with mdel species. PLoS one 8(3):e58068
Ougham HJ, Latipova G, Valentine J (1996) Morphological and biochemical characterization of spikelet development in naked oats (Avena sativa). New Phytol 134:5–12
Pasam RK, Sharma R, Malosetti M, Eeuwijk FA, Haseneyer G, Kilian B, Graner A (2012) Genome-wide association studies for agronomical traits in a world wide spring barley collection. BMC Plant Biol 12:16
Peltonen-Sainio P, Kontturi M, Rajala AJA, Science F (2004) Impact dehulling oat grain to improve quality of on-farm produced feed: 1. Hullability and associated changes in nutritive value and energy content. Agric Food Sci 13:29–38
Perrier X, Flori A, Bonnot F (2003) DARwin software: Dissimilarity analysis and representation for windows. In: Hamon P, Seguin M, Perrier X, Glaszmann JC (eds) Genetic diversity of cultivated tropical plants. Enfield Science Publishers, Montpellier, pp 43–76
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Qian L, Qian W, Snowdon RJ (2014) Sub-genomic selection patterns as a signature of breeding in the allopolyploid Brassica napus genome. BMC Genom 15(1):1170
Remington DL, Thornsberry JM, Matsuoka Y, Wilson LM, Whitt SR, Doebley J, Kresovich S, Goodman MM, Buckler ES (2001) Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc Natl Acad Sci USA 175(2):805–817
Ren C, Yang C (2018) Oat cultivars in China. China Agriculture Press, Beijing (in Chinese)
Sormacheva I, Golovnina K, Vavilova V, Kosuge K, Watanabe N, Blinov A, Goncharov NP (2015) Q gene variability in wheat species with different spike. Genet Resour Crop Evol 62:837–852
Stanton TR (1955) Oat identification and classification. USDA, Washington
Stracke S, Presterl T, Stein N, Perovic D, Ordon F, Graner A (2007) Effects of introgression and recombination on haplotype structure and linkage disequilibrium surrounding a locus encoding Bymovirus resistance in barley. Genetics 168(1):435–446
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Thomas H (1995) Oats. Avena spp. (Gramineae-Aveneae). In: Smartt J, Simmonds NW (eds) Evolution of crop plants, 2nd edn. Longman, New York
Tinker NA, Bekele WA, Hattori J (2016) Haplotag: software for haplotype-based genotyping-by-sequencing analysis. G3-Genes Genom Genet 6:857–863
Tinker NA et al (2014) A SNP genotyping array for hexaploid oat. Plant Genome-US 7:1–8
Tinker NA, Deyl JK (2005) A curated internet database of oat pedigrees. Crop Sci 45:2269–2272
Tumino G et al (2016) Population structure and genome-wide association analysis for frost tolerance in oat using continuous SNP array signal intensity ratios. Theor Appl Genet 129:1711–1724
Ubert IP, Zimmer CM, Pellizzaro K, Federizzi LC, Nava IC (2017) Genetics and molecular mapping of the naked grains in hexaploid oat. Euphytica 213:41
Valentini APF, Pellizzaro K, Pacheco MT, Federizzi LC (2014) Genetic analysis of the naked trait in panicles of hexaploid oat. Crop Breed Appl Biot 14:116–123
Vavilov N (1926) Centres of origin of cultivated plants. Works Appl Bot Breed 17:9–107 (in Russian)
Winkler LR, Michael Bonman J, Chao S, Admassu Yimer B, Bockelman H, Esvelt Klos K (2016) Population structure and genotype-phenotype associations in a collection of oat landraces and historic cultivars. Front Plant Sci 7:1077
Xu W, Zhang Z, W, Wu B, Cui L, (2009) Genetic diversity in naked oat (Avena nuda) germplasm revealed by AFLP markers. Acta Agron Sin 35:2205–2212
Yan H et al (2016) High-density marker profiling confirms ancestral genomes of Avena species and identifies D-genome chromosomes of hexaploid oat. Theor Appl Genet 129:2133–2149
Zheng DS, Zhang ZW (2011) Discussion on the origin and taxonomy of naked oat (Avena nuda L). J Plant Genet Resour 5:667–670 (in Chinese with English abstract)
Acknowledgements
We gratefully acknowledge the professional and technical assistance provided by Charlene Wight.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31571739, 31801430) and the Sichuan International (Hong Kong/Macao/Taiwan) Innovation Cooperation in Science and Technology (Grant No. 2019YFH0125).
Author information
Authors and Affiliations
Contributions
YYP and NAT conceived and designed the experiments. HY and PZ conducted experiments and analyzed the data, and HY drafted the manuscript. YP contributed to preparation of reagents and materials. RC contributed to interpretation of results. WAB, NAT and YYP assisted with the interpretation of results and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Andreas Graner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2020_3674_MOESM2_ESM.tif
Fig. S1 Number of haplotypes called by discovery (a) and production (b) modes after population-based filtering and the percentage of distribution of minor allele frequency (MAF) of these haplotypes in the panel of 158 Chinese hulless oat lines (CN Panel). Haplotypes with MAF < 0.05 were filtered out (TIF 686 kb)
122_2020_3674_MOESM4_ESM.tif
Fig. S3 Percentage of distribution of minor allele frequency (MAF) of 8675 haplotypes called from 728 oat lines in the full panel. Haplotypes with MAF < 0.05 were filtered out (TIF 163 kb)
122_2020_3674_MOESM5_ESM.tif
Fig. S4 Grouping results of 158 Chinese hulless oats at K = 6. Oat lines with membership probability less than 0.6 of either subgroup were grouped into admixture group (AD) (TIF 830 kb)
Fig. S5 Neighbor-joining (NJ) tree of 158 Chinese hulless oats based on 3093 haplotypes (TIF 185 kb)
122_2020_3674_MOESM7_ESM.tif
Fig. S6 Plot of the eigenvalues of the first 15 principal coordinates (PCs) in an analysis of the full panel (TIF 54 kb)
122_2020_3674_MOESM8_ESM.pdf
Fig. S7 Heatmaps for linkage disequilibrium (LD) by using uncorrected value of r2 as well as corrected value of r2 by population structure (rs2), kinship (rk2), and both (rsk2) (PDF 1496 kb)
122_2020_3674_MOESM9_ESM.pdf
Fig. S8 Linkage disequilibrium (LD) decay rate in each individual chromosomes (linkage groups).Critical LD value is shown as horizontal broken lines at 0.167 (PDF 1592 kb)
Rights and permissions
About this article
Cite this article
Yan, H., Zhou, P., Peng, Y. et al. Genetic diversity and genome-wide association analysis in Chinese hulless oat germplasm. Theor Appl Genet 133, 3365–3380 (2020). https://doi.org/10.1007/s00122-020-03674-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03674-1