Abstract
Background
Chronic subdural hematoma (cSDH) is caused by rupture of bridging intracranial veins located in the subdural space. Predominantly, cSDH is a disease of older adults and other predisposing risk factors include minor head trauma, cerebral atrophy, antiplatelet and anticoagulation therapies, arterial hypertension, cerebrovascular atherosclerosis, diabetes mellitus, cerebrospinal fluid loss, and excessive alcohol consumption. The incidence of cSDH is rising worldwide due to an aging population and the increased use of anticoagulant or antiplatelet medications. Clinical manifestations are varied, with altered mental status and focal neurologic deficits among the most common.
Methodological innovations
Treatment strategies depend on the symptomatology and extent of hematoma. In general, conservative treatment is sought for patients who are asymptomatic or have only mild symptoms, whereas symptomatic patients are often treated surgically. However, the recurrence rate of cSDH may be as high as 30%. In recent years, middle meningeal artery embolization has emerged as a complementary option to surgery aimed at decreasing the recurrence rate after excision as well as an alternative therapeutic approach to surgical therapy in certain circumstances.
Conclusion
Embolization of the middle meningeal artery appears to be a promising treatment for patients with cSDH, both before and after surgical excision.
Zusammenfassung
Hintergrund
Ein chronisches Subduralhämatom (cSDH) wird durch die Ruptur von intrakranialen Brückenvenen im Subduralraum verursacht. In erster Linie ist das cSDH eine Erkrankung älterer Erwachsener; zu den prädisponierenden Risikofaktoren gehören leichtgradige Kopftraumata, zerebrale Atrophie, Thrombozytenaggregationshemmer- und Antikoagulationstherapie, arterielle Hypertonie, zerebrovaskuläre Arteriosklerose, Diabetes mellitus, Verlust von Liquor cerebrospinalis und exzessiver Alkoholkonsum. Weltweit gibt es einen Anstieg der Inzidenz des cSDH aufgrund einer alternden Bevölkerung und der vermehrten therapeutischen Anwendung von Thrombozytenaggregationshemmern oder Antikoagulanzien. Das klinische Bild variiert, dabei zählen ein veränderter Geisteszustand und fokale neurologische Defizite zu den häufigsten Symptomen.
Methodische Innovationen
Die Behandlungsstrategie wird in Abhängigkeit von der Symptomatik und dem Ausmaß des Hämatoms festgelegt. Im Allgemeinen wird eine konservative Therapie für Patienten angestrebt, die asymptomatisch sind oder nur leichte Symptome aufweisen, während symptomatische Patienten häufig chirurgisch behandelt werden. Allerdings kann die Rezidivrate des cSDH bis zu 30% betragen. In den letzten Jahren stellte sich die Embolisation der A. meningea media als ergänzende Option zur Operation heraus, die darauf abzielt, die Rezidivrate nach Exzision zu senken, sowie in bestimmten Fällen als alternativer Therapieansatz zur chirurgischen Therapie.
Schlussfolgerung
Die Embolisation der A. meningea media scheint eine vielversprechende Behandlung für Patienten mit cSDH zu sein, sowohl vor als auch nach der chirurgischen Exzision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chronic subdural hematoma (cSDH) is defined as a collection of blood between the dura mater and the arachnoid in the subdural space that appears as either hypodense or isodense to the brain parenchyma on computed tomography (CT) examination. In many cases, cSDH contains fresh blood that is hyperdense on CT within static components and thus there are dynamic processes occurring within the hematoma. Bilateral cSDH occurs in up to 20% of cases. At the time of the initial diagnosis, the interval for formation of the cSDH often remains unclear; the process is thought to last up to 3 weeks or more [1, 17, 22].
Predominantly, cSDH occurs in older adults and the incidence of this disease is rising due to an aging population and the increasing use of antithrombotic drugs. For this reason, it is one of the most common diseases in neurosurgical practice. Risk factors include minor head trauma, long-term use of antiplatelet or anticoagulant medications, and excessive alcohol consumption. Predisposing conditions include arterial hypertension, cerebrovascular atherosclerosis, cerebral atrophy, epilepsy, and diabetes mellitus. In addition, conditions that lead to low intracranial pressure, such therapeutic intervention with a ventricular shunt or leakage of cerebrospinal fluid, can also lead to cSDH [1, 18].
The clinical presentation of cSDH is nonspecific and ranges from no symptoms to headache, seizures, memory impairment, confusion, and coma. The most common symptomology of cSDH in older adults is an altered mental state (50–70%) such as behavioral changes and somnolence. Transient or progressive neurological deficits, such as hemiparesis of the contralateral limb, sensory disturbances, or aphasia, are also represented in the clinical presentation [8].
The pathophysiology of cSDH is not fully understood. It is believed that cSDH is related to inflammatory, fibrinolytic, and angiogenesis processes. After liquefaction of an acute subdural hematoma, blood degradation products induce inflammation leading to a new membrane in the inner dural layer, followed by neovascularization of an immature capillary network in this new membrane. These vessels arise from distal branches of dura-supplying arteries, usually the middle meningeal artery (MMA). The immature vessels are fragile and chronically cause microbleeds, which lead to a progressive increase in fluid accumulation in the subdural space and an increase in fibrinolytic activity, inflammation, and further angiogenesis. These processes initiate a positive feedback cycle that results in persistence or increase in cSDH volume over time [6, 9, 12].
Treatment strategies for cSDH
Treatment strategies are dependent on the patient’s clinical presentation and the size of the hematoma. To date, there are no guidelines for the treatment of patients with cSDH [14].
Conservative treatment
Asymptomatic patients or patients with small cSDHs without brain compression are usually treated conservatively with close clinical monitoring and serial imaging. In addition, various drug therapies have been introduced, including steroids, platelet-activating factor antagonists, and statins. There are few published data on success rates after conservative treatment. Conservative treatment requires an extended observational strategy and therefore hospitalization tends to be longer [9, 19].
Surgical treatment
In symptomatic patients, surgical treatment aimed at evacuating the cSDH is standard practice; techniques include twist drilling, burr hole, and craniotomy. The results of these techniques are predominantly described as favorable. However, to date, there is no consensus among neurosurgeons regarding the optimal surgical technique [4, 11].
Despite low complication rates and good outcomes after surgery, re-accumulation of the hematoma is the most common postoperative problem. Depending on the technique, post-surgery recurrence rates range from 8 to 37% and are more frequently observed in bilateral cSDHs [1, 5, 15, 21]. The 1‑year mortality rate is reported to be as high as 32% in older patients [13].
Endovascular embolization of the MMA
The objective of MMA embolization (MMAE) is to devascularize the fragile, immature capillary network within the subdural membranes in order to stop rebleeding and the inflammatory cascade. Several studies have reported enlargement of the MMA and the de novo vascular networks supplying the cSDHs, making the MMA a good target for embolization to achieve a reduction in the size and recurrence of these hematomas [7, 16, 20]. Generally, MMAE can be performed for ipsilateral and bilateral cSDHs as a sole or supportive preoperative or postoperative procedure.
Techniques of MMAE
Various embolic agents (e.g., microparticles, coils, gelatin sponge, acrylates, and other liquid embolic agents) have been used for MMAE. Waquas et al. performed a systematic review of the small number of studies that have evaluated the safety and efficacy of MMAE and concluded that the procedures for treating cSDH were similarly safe and effective when different agents were used [23]. However, comparative studies have not yet been conducted, and level-one evidence for the use of MMAE is lacking [3].
The vast majority of MMAE case series have been reported for microparticles [23]. Ban and coworkers compared the outcomes of 72 patients who underwent MMAE with microparticles against 469 conventionally treated patients with cSDH and found complication rates were similar in both groups. Rates of treatment failure (residual or recurrent hematoma) and additional surgical rescue therapy were lower with MMAE (1.4 vs. 27.5% and 1.4 vs. 18.8%, respectively; [2]).
Recently, several liquid embolic agents (Onyx®, Medtronic, Dublin, Ireland; PHIL, MicroVention, Tustin, CA, USA; Squid, Montmorency, France) have gained popularity for embolization. Fiorella et al. discussed the potential advantages of liquid embolics over microparticles: greater depth of penetration, larger embolization volume, increased durability, better visualization, and faster procedures [7]. In a multicenter study, Kan et al. demonstrated > 50% improvement in cSDH volume in 70.8% of patients treated with different embolic agents [10]. However, there are no comparative studies to indicate the most effective embolic agent for MMAE.
Example cases
Case 1
A 67-year-old man presented with progressive hemiparesis caused by cSDH with midline shift after minor trauma (Fig. 1a, b). The patient was on anticoagulant therapy because of atrial fibrillation. Following surgery, improvement of the symptoms and the hematoma (Fig. 1c, d) was noted. Additional MMAE with Onyx 18 was performed (Fig. 2). At the 90-day follow-up CT examination, the cSDH had completely resolved.
Axial (a) and coronal (b) computed tomography (CT) scans of the head showing left-sided chronic subdural hematoma (cSDH) collections of mixed density leading to contralateral midline shift. After craniotomy, improvement of the cSDH and regression of the midline shift are noted on follow-up CT (c, d)
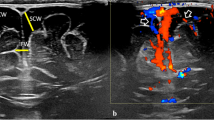
Subtracted (a) and roadmap (b) angiogram of the external carotid artery showing the anatomy of the middle meningeal artery (black arrowheads). The distal marker of the microcatheter (white arrowhead) is positioned in the anterior meningeal branch. Unsubtracted image shows Onyx18 (black arrowhead) within the middle meningeal artery after embolization (c). Follow-up angiogram (d) confirms successful occlusion of the target vessel
Case 2
A 73-year-old woman presented with progressive headache and development of aggressive behavior within 3 weeks of space-occupying bilateral cSDHs (Fig. 3a) without any history of trauma. The patient was being treated with antiplatelet monotherapy for a history of lacunar infarction. Surgical excision of the hematoma was performed twice within 4 weeks. However, incomplete resolution was still observed after the second hematoma evacuation (Fig. 3b). Because of the slow recurrence of cSDH (Fig. 3c) within 1 week of the second surgery, bilateral MMAE with Squid 18 was performed (Fig. 4a–c). Complete regression was observed at the 3‑month follow-up CT examination (Fig. 4d).
Axial computed tomography (CT) scans of the head. Initial CT (a) shows bilateral chronic subdural hematoma (cSDH) collections of mixed density leading to compression of both cerebral hemispheres. On the follow-up CT (b), incomplete resolution of the cSDHs was observed after bilateral burr hole drainage. One week after the second surgery, a slow recurrence of the cSDHs was noted, which were more pronounced on the left side (c)
Superselective angiograms from a catheter position within a frontal branch of the right (a) and left (b) middle meningeal artery demonstrates the anatomy of the branches with a capillary blush of the dura. Unsubtracted image (c) shows Squid 18 within the middle meningeal artery of both sides after embolization. Follow-up computed tomography (d) examination 3 months after embolization, showing complete resolution of cSDHs
Practical conclusion
-
Embolization of middle meningeal artery (MMA) appears to be a promising treatment for patients with Chronic subdural hematoma (cSDH), both before and after surgical excision.
-
Embolization is less invasive than open surgery. This is particularly important for cSDH patients with concomitant disease and patients who require sustained anticoagulation or antiaggregation therapies.
-
Potential advantages of liquid embolic agents compared to microparticles for therapeutic treatment of MMA embolization include greater depth of penetration, greater embolic volume, longer shelf life, better visualization, and faster procedures.
References
Adhiyaman V (2002) Chronic subdural haematoma in the elderly. Postgrad Med J 78:71–75. https://doi.org/10.1136/pmj.78.916.71
Ban SP, Hwang G, Byoun HS et al (2018) Middle meningeal artery embolization for chronic subdural hematoma. Radiology 286:992–999. https://doi.org/10.1148/radiol.2017170053
Catapano JS, Nguyen CL, Wakim AA et al (2020) Middle meningeal artery embolization for chronic subdural hematoma. Front Neurol 11:557233. https://doi.org/10.3389/fneur.2020.557233
Cenic A, Bhandari M, Reddy K (2005) Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci 32:501–506. https://doi.org/10.1017/S0317167100004510
Chon K‑H, Lee J‑M, Koh E‑J, Choi H‑Y (2012) Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir 154:1541–1548. https://doi.org/10.1007/s00701-012-1399-9
Edlmann E, Giorgi-Coll S, Whitfield PC et al (2017) Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation 14:108. https://doi.org/10.1186/s12974-017-0881-y
Fiorella D, Arthur AS (2019) Middle meningeal artery embolization for the management of chronic subdural hematoma. J NeuroIntervent Surg 11:912–915. https://doi.org/10.1136/neurintsurg-2019-014730
Fogelholm R, Heiskanen O, Waltimo O (1975) Chronic subdural hematoma in adults: influence of patient’s age on symptoms, signs, and thickness of hematoma. J Neurosurg 42:43–46. https://doi.org/10.3171/jns.1975.42.1.0043
Holl DC, Volovici V, Dirven CMF et al (2018) Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World Neurosurg 116:402–411.e2. https://doi.org/10.1016/j.wneu.2018.05.037
Kan P, Maragkos GA, Srivatsan A et al (2021) Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery 88:268–277. https://doi.org/10.1093/neuros/nyaa379
Matsumoto H, Hanayama H, Okada T et al (2018) Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci 49:40–47. https://doi.org/10.1016/j.jocn.2017.11.009
Mureb MC, Kondziolka D, Shapiro M et al (2020) DynaCT enhancement of subdural membranes after middle meningeal artery embolization: insights into pathophysiology. World Neurosurg 139:e265–e270. https://doi.org/10.1016/j.wneu.2020.03.188
Nia AM, Srinivasan VM, Siddiq F et al (2022) Trends and outcomes of primary, rescue, and adjunct middle meningeal artery embolization for chronic subdural hematomas. World Neurosurg. https://doi.org/10.1016/j.wneu.2022.05.011
Nouri A, Gondar R, Schaller K, Meling T (2021) Chronic subdural hematoma (cSDH): a review of the current state of the art. Brain Spine. https://doi.org/10.1016/j.bas.2021.100300
Ohba S, Kinoshita Y, Nakagawa T, Murakami H (2013) The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev 36:145–150. https://doi.org/10.1007/s10143-012-0396-z
Pouvelle A, Pouliquen G, Premat K et al (2020) Larger middle meningeal arteries on computed tomography angiography in patients with chronic subdural hematomas as compared with matched controls. J Neurotrauma 37:2703–2708. https://doi.org/10.1089/neu.2020.7168
Sahyouni R, Goshtasbi K, Mahmoodi A et al (2017) Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg 108:948–953. https://doi.org/10.1016/j.wneu.2017.09.064
Sim Y‑W, Min K‑S, Lee M‑S et al (2012) Recent changes in risk factors of chronic subdural hematoma. J Korean Neurosurg Soc 52:234. https://doi.org/10.3340/jkns.2012.52.3.234
Soleman J, Nocera F, Mariani L (2017) The conservative and pharmacological management of chronic subdural haematoma. Swiss Med Wkly 147:w14398. https://doi.org/10.4414/smw.2017.14398
Tanaka T, Fujimoto S, Saitoh K et al (1998) Superselective angiographic findings of ipsilateral middle meningeal artery of chronic subdural hematoma in adults. No Shinkei Geka 26:339–347
Torihashi K, Sadamasa N, Yoshida K et al (2008) Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery 63:1125–1129. https://doi.org/10.1227/01.NEU.0000335782.60059.17
Tsai T‑H, Lieu A‑S, Hwang S‑L et al (2010) A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma 68:571–575. https://doi.org/10.1097/TA.0b013e3181a5f31c
Waqas M, Vakhari K, Weimer PV et al (2019) Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World Neurosurg 126:228–236. https://doi.org/10.1016/j.wneu.2019.02.208
Acknowledgements
I would like to thank Prof. Wolfgang Reith and Frederik Fries from the Department of Diagnostic and Interventional Neuroradiology at Saarland University for their support with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Mühl-Benninghaus declares that he has no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case. Additional written informed consent was obtained from all individual participants or their legal representatives for whom identifying information is included in this article.
The supplement containing this article is not sponsored by industry.
Additional information

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Mühl-Benninghaus, R. Middle meningeal artery embolization for treatment of chronic subdural hematoma. Radiologie 62 (Suppl 1), 17–21 (2022). https://doi.org/10.1007/s00117-022-01074-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00117-022-01074-8