Abstract
Background
The introduction of 3D printing in the medical field led to new possibilities in the planning of complex procedures, as well as new ways of training junior physicians. Especially in the field of vascular interventions, 3D printing has a wide range of applications.
Methodological innovations
3D-printed models of aortic aneurysms can be used for procedural training of endovascular aortic repair (EVAR), which can help boost the physician’s confidence in the procedure, leading to a better outcome for the patient. Furthermore, it allows for a better understanding of complex anatomies and pathologies. In addition to teaching applications, the field of pre-interventional planning benefits greatly from the addition of 3D printing. Especially in the preparation for a complex endovascular aortic repair, prior orientation and test implantation of the stent grafts can further improve outcomes and reduce complications. For both teaching and planning applications, high-quality imaging datasets are required that can be transferred into a digital 3D model and subsequently printed in 3D. Thick slice thickness or suboptimal contrast agent phase can reduce the overall detail of the digital model, possibly concealing crucial anatomical details.
Conclusion
Based on the digital 3D model created for 3D printing, another new visualization technique might see future applications in the field of vascular interventions: virtual reality (VR). It enables the physician to quickly visualize a digital 3D model of the patient’s anatomy in order to assess possible complications during endovascular repair. Due to the short transfer time from the radiological dataset into the VR, this technique might see use in emergency situations, where there is no time to wait for a printed model.
Zusammenfassung
Hintergrund
Die Einführung des 3‑D-Drucks im medizinischen Bereich führt zu neuen Möglichkeiten bei der Planung komplexer Eingriffe sowie zu neuen Wegen der Ausbildung junger Ärzte. Besonders im Bereich der Gefäßchirurgie ist das Anwendungsspektrum des 3‑D-Drucks sehr breit.
Methodische Innovationen
3‑D-gedruckte Modelle von Aortenaneurysmen können für das Verfahrenstraining der endovaskulären Aortenreparatur (EVAR) verwendet werden, was dazu beitragen kann, das Vertrauen des Arztes in das Verfahren zu stärken. Zusätzlich kann es zu einem besseren Ergebnis für den Patienten führen. 3‑D-Modelle ermöglichen außerdem ein besseres Verständnis komplexer Anatomien und Pathologien. Neben Lehranwendungen profitiert der Bereich der präinterventionellen Planung stark von der Integration des 3‑D-Drucks. Insbesondere bei der Vorbereitung einer komplexen EVAR kann eine vorherige Orientierung und Testimplantation der Stentgrafts das Ergebnis weiter verbessern und Komplikationen reduzieren. Sowohl für Lehr- als auch Planungsanwendungen ist es erforderlich, adäquate Datensätze der Bildgebung zu generieren, die in ein digitales 3‑D-Modell überführt und anschließend 3‑D-gedruckt werden können. Eine inadäquate Schichtdicke oder die suboptimale Kontrastmittelphase können die Gesamtdetails des digitalen Modells reduzieren und möglicherweise wichtige anatomische Details verbergen.
Schlussfolgerung
Basierend auf dem für den 3‑D-Druck erstellten digitalen 3‑D-Modell könnte eine weitere neue Visualisierungstechnik künftig im Bereich der Gefäßinterventionen Anwendung finden; die virtuelle Realität (VR). Sie ermöglicht dem Arzt, ohne Verzögerung ein digitales 3‑D-Modell der Anatomie des Patienten zu visualisieren, um mögliche Komplikationen während der endovaskulären Reparatur zu beurteilen. Aufgrund der kurzen Übertragungszeit vom radiologischen Datensatz in die VR kann dies in Notfallsituationen sinnvoll sein, in denen nicht auf ein gedrucktes Modell gewartet werden kann.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
With the introduction of new computer-based technologies, striking innovations have found their way in many different fields of medicine. The ongoing advances in the field of radiology have contributed significantly to the development of modern therapeutic strategies [1]. With the available imaging modalities, the physician can obtain a clear understanding of the anatomy, which is crucial for procedural planning. Preoperative planning has been traditionally based on 2D images and reconstructions, which means that physicians need to mentally reconstruct images, observed on flat monitors, to 3D structures. This can prove complicated for junior surgeons who lack experience or in cases of complex anatomy [2].
In vascular medicine, endovascular treatment is becoming the first-choice therapy for an increasing number of indications. Endovascular treatment of aortic aneurysms, in particular, has become the first-line therapy for the abdominal and thoracoabdominal aorta steadily replacing the gold standard of open repair [3, 4]. Today, complex thoraco-abdominal and aortic arch pathologies can be treated with sophisticated endografts [5, 6]. The physicians who perform these complex procedures need to be highly skilled in endovascular techniques. One powerful tool that offers a better 3D understanding of the patient-specific anatomy is a 3D working station. Procedures planned with multiplanar reconstruction and central line analysis have better postoperative results with lower re-intervention rates [7]. The main drawback is that these are still 2D images projected on screens with lack of tactile experience, which can lead to anticipated difficulties for both experienced and inexperienced surgeons.
To overcome these shortcomings, 3D printing technologies have been introduced in the field of medicine in recent years. The concept of 3D printing, also referred to as “additive manufacturing” (AM), “rapid prototyping” (RP), or “solid-freeform technology” (SFF), was developed by Charles Hull over 30 years ago [8]. Today, 3D technology in medicine is not used only for planning of surgical procedures and education, but also for cell bioprinting [9], metallic internal implants [10], scaffolds [11] etc.
A recently published systemic review showed that 3D printing is well integrated in surgical planning and research. The majority of the publications stem from the field of orthopedics, maxillofacial surgery, and spine surgery. The cardiovascular field accounts for only 3.5% of the scientific papers. The main advantages described in the selected papers are reduced surgical time, improved medical outcome, and decreased radiation exposure, with greater advantages when used to handle complex cases and with less experienced surgeons [12].
Because this technology enables the creation of patient-specific models at a relatively low cost, it is especially interesting in planning endovascular surgical procedures [13, 14], education and training [15, 16], and vascular device and tissue engineering [17, 18].
From CT and MRI datasets to 3D-printable models
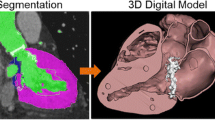
To create accurate, patient-specific digital models, the workflow always starts with a large image dataset (Fig. 1). Different modalities can be used to create a 3D model including mainly computed tomography (CT) scans, magnetic resonance imaging (MRI) scans but also occasionally x‑ray and even ultrasound imaging [19, 20]. Both CT and MRI are the most common imaging modalities used for the 3D modeling of the vascular system, and different parameters can impact the quality of the final model. It is of course essential to acquire contrast-enhanced images to enable visualization of the blood flow and lumen in the vessels [20]. This is especially helpful in the modeling of aneurysms and aortic dissections. Additionally, the slices of the dataset should be as thin as possible. The 3D modeling software used to create the digital anatomical model will interpolate the areas between individual slices, leading to very crude models, lacking detail if the slice thickness is inappropriate (>1 mm).
Design workflow to create a 3D-printed model from a CT dataset. a Starting at a contrast-enhanced CT dataset, masks based on Hounsfield units can be drawn to select the desired anatomy. b Based on these masks, 3D models can be created and manipulated to create a hollow model of the aortic arch (Step 2). c The created model can then be transferred to a 3D printer and subsequently printed
The range of modeling software that creates 3D models from radiological datasets has steadily increased in the last decade, offering medically licensed modeling software (i.e., Materialise Mimics Innovation Suite, Materialise NV, Leuven, Belgium) or even open-source freeware (i.e., 3DSlicer, www.slicer.org, ultimaker.com). While every software offers a different range of additional features, the underlying principle in creating a 3D model from a radiological dataset remains the same. Based on Hounsfield unit (HU) values, masks can be created, marking specific areas with similar HU values in every slice of the dataset. From these masks, 3D models can be rendered by interpolation. Additionally, most modeling software offer editing tools to remove artifacts and unwanted anatomical structures from the final model. Commercial software suites like the Mimics Innovation Suite offer additionally functionality, i.e., hollowing of the model, adjustment of the model’s surface.
Depending on the individual requirements of every case, different printing materials are available, varying in color, elasticity, chemistry, weight, and costs. Production prices of 3D-printed models can range from just $3.50 to $700 [20, 21]. Total printing time, resolution, and material composition vary greatly with the size, complexity, and printing technology with a flexible real-size aortic arch printed using a Polyjet printer taking approximately 12–18 h (Fig. 2).
3D-printed models in education and teaching
In the field of vascular interventions there is a lack of literature about the use of 3D-printed models for educational purposes [22]. Nevertheless, 3D models for education and training can help young surgeons gain skills and increase their confidence level before performing a procedure on a patient ([23]; Fig. 3). The complexity of these training simulators can vary widely, from simple setups that enable the learning of basic guidewire skills [23], to patient-specific models with a pulsatile flow loop attached [24]. Kärkkäinen et al. fabricated an abdominal aortic (AAA) phantom to simulate an endovascular aortic repair (EVAR) for young trainees. In a total of 22 EVAR simulations, the experienced trainees could significantly lower the procedural and fluoroscopy time (p < 0.05). The group concluded that EVAR simulation was feasible and simulated all procedural steps with high fidelity [25]. Another study with 3D-printed aortic models for EVAR simulation of AAA came to similar conclusions [23].
3D-printed flexible model for fenestrated endovascular aortic repair (FEVAR) planning and training.; a Implantation of the FEVAR using fluoroscopic imaging. b Fluoroscopic image of the graft deployment. c 3D-printed model of an abdominal aortic aneurysm with implanted FEVAR. d Distal view of the implanted graft inside the model
The main advantage of 3D-printed models compared to computer-based models is the opportunity to use all materials (guidewires, sheaths, catheters, implants, and the interventional devices) that are used in real-life scenarios. Moreover, the effect of blood pressure on the behavior of guidewires and catheters and on the implant deployment can be imitated [25]. Additionally, the price of a 3D-printed model is significantly lower than computer-based simulators [26], which have been considered the most important educational tool for endovascular procedures for some time. Due to the complex nature of endovascular interventions, constant training could potentially help surgeons improve the handling of the devices and overall confidence. Especially in lengthy, complex procedures, extensive prior training might help reduce radiation exposure for both the patient and the physician [23]. The described 3D-printed models can be upgraded to simulate more complex procedures like thoraco-abdominal pathologies, as well as iliac aneurysms, to train less experienced surgeons before they perform these procedures on a patient [25].
Training of young residents and the education of medical students is another field in which 3D-printed models offer various advantages. Especially complex anatomy and pathologies can be simulated better with 3D models and can provide an alternative to cadaver models, thus resolving ethical issues that often exist with use of cadavers for medical training. Some studies have found that 3D-printed simulations increased student test scores when studying physiologic and anatomy [27].
3D models for interventional planning
In the planning of endovascular repair, Winder et al. in 2002 were the first to describe the use of a 3D-printed aneurysm model to determine the usability of a fenestrated endograft [28]. While in the beginning most studies focused on the use of rigid 3D-printed models, newer studies use flexible silicone-like models to mimic the behavior of the aorta more accurately during pre-interventional implantation. This is especially useful in the selection of correct endograft oversizing, landing zones, access sites and sides in complex aortic aneurysm repairs. The use of such models offers a new perspective in complex cases and can help evaluate the surgeon’s decision regarding the approach.
With the steady increase in endovascular procedures and the parallel rise of more complex cases, endograft manufacturers have also adapted the advantages of 3D-printed models in planning. One company producing fenestrated endografts for complex abdominal aortic aneurysms (Terumo Aortic, Inchinnan, Scotland, UK) offers a patient-specific 3D model with every fenestrated endograft so that surgeons can more accurately plan their approach. A study in which prototype testing for 60 fenestrated grafts in a patient-specific aortic model was carried out revealed that in 21% of the cases, modifications to the implantation procedure were required and that in 5% of cases, a new endograft was ordered [13]. One of the main drawbacks of this study was the rigid material used to create the 3D models, which did not realistically simulate the in vivo behavior of the aorta. Nevertheless, this confirms that there can be a discrepancy between the measurements performed on a 3D working station and the fenestration position after deployment of a graft in a 3D model. Koleilat et al. described the same phenomena in a smaller study and concluded that an average of six independent measurements could improve the overall results [29]. While more experienced planners are not expected to have such a high rate of deviation from graft to graft planning, it is obvious that 3D modeling could serve as a quality assurance tool before implanting a complex aortic endograft in the patient, especially in the learning curve of centers practicing the techniques.
The use of a presurgical patient-specific phantom allows the physicians to rehearse and refine their planned approach, with the possibility of avoiding periprocedural complications and extra time spent on device learning during the patient procedure [30]. With further refinements of the technology, and a focus on the material used for printing, this could present a valid option for standardized use in presurgical planning.
Besides the use of 3D-printed models for the planning of an endovascular implantation, the models can also be used in the planning of physician-modified stent grafts. If the time to order a patient-specific fenestrated endograft is not available, as occurs in emergencies, many physicians could modify a standard tube endograft with fenestrations for the reno-visceral vessels [31]. In these cases, the 3D-printed model can be used as surgical templates to accurately suture fenestrations into the endograft. The standard thoracic endograft is deployed in the model of the paravisceral aneurysm and the fenestrations are marked and then created directly at the origins of the vessels. In a series of 12 patients, 3D-printed templates were successfully used to create a physician-modified graft for patients with symptomatic thoraco-abdominal aortic aneurysms [32]. An even bigger series of 34 patient with a thoraco-abdominal aneurysm (19 patients with dissection and 15 patients with degenerative aneurysm) has also proven that 3D-printed models are feasible for creating fenestrated stent grafts for the treatment of thoraco-abdominal aortic diseases. The printing process took approximately 5 h (1 h for image reconstruction, 3 h for printing, and 1 h for postprocessing) and the modification of the graft another 1.26 ± 0.35 h [33]. 3D printing can also be used to create graft modifications in the aortic arch, which is especially challenging because of the curvature of the vessel. In a case series of 17 patients, 3D printing was used to create templates for modifications of endografts to treat pathologies of the aortic arch. The technical success rate of the treatment was 94.8%, with 0% early and mid-term mortality and neurological complications [34].
In the field of cardiovascular medicine, 3D printing can be also used for planning prior of the treatment of congenital heart disease, as well as planning of transcatheter aortic valve replacement (TAVR; [34, 35]). Innovative approaches to benchtop testing and multi-material printing used particularly in the planning of TAVR procedures can also be implemented in the field of complex endovascular aortic surgery to further improve the existing 3D models.
Future perspectives
3D printing offers a multitude of possibilities to improve patient care, especially in surgical disciplines. Nevertheless, the technology in its current state has some limitations when approaching an integration into clinical day-to-day practice. The most significant limitation is the time required from CT scan to the final printed model. Depending on the scale of the model, it can take up to 48 h until the physician can inspect the final model. In elective, complex surgical cases, the time factor is not as relevant as in emergencies. Secondly, 3D-printed models are still relatively expensive, and currently there is no standardized reimbursement from medical insurance for the use of a planning model in routine surgery. This will also hinder the regular integration of the technology into clinical routine.
A technology that might be able to help with both limitations is virtual reality (VR). Coming from the video game industry, VR goggles offer the possibility of transferring the user into a virtual world, where they can interact with digital objects. The digital anatomical models created for the printing process can be easily imported into a VR development platform and subsequently sent to a VR device. Several companies already offer this technology for use in the medical field (i.e., Materialise NV, Holoeyes Inc., Tokyo, Japan). The transfer from the design software to the VR platform takes minutes, allowing for a faster integration into the planning process prior to an intervention. Overall, the implementation of VR might further improve patient care, especially in complex endovascular aortic repair. To achieve a smooth workflow with excellent outcome for the patient, a close interdisciplinary cooperation between vascular surgeons, radiologists, and engineers will be of great importance.
Summary
3D printing technology is continuously gaining ground in the field of cardiovascular interventions for training of less-experienced users and adequate pre-procedural preparation in complex interventions. While a number of limitations, such as the quality of materials, production time, and cost issues, require significant improvement before the technology can be widely used, the perspectives for 3D printing in vascular interventions are promising.
Practical conclusion
-
3D printing can improve planning of endovascular interventions.
-
3D printing is a valuable tool in the training of young residents.
-
Accurate 3D-printed models require excellent quality contrast-enhanced fine sliced imaging.
References
Doi K (2006) Diagnostic imaging over the last 50 years: research and development in medical imaging science and technology. Phys Med Biol 51(13):R5–R27. https://doi.org/10.1088/0031-9155/51/13/r02
Pugliese L, Marconi S, Negrello E, Mauri V, Peri A, Gallo V et al (2018) The clinical use of 3D printing in surgery. Updates Surg 70(3):381–388. https://doi.org/10.1007/s13304-018-0586-5
Antoniou GA, Antoniou SA, Torella F (2020) Editor’s choice—endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long term data of Randomised controlled trials. Eur J Vasc Endovasc Surg 59(3):385–397. https://doi.org/10.1016/j.ejvs.2019.11.030
Wiseman JT, Fernandes-Taylor S, Saha S, Havlena J, Rathouz PJ, Smith MA et al (2017) Endovascular versus open revascularization for peripheral arterial disease. Ann Surg 265(2):424–430. https://doi.org/10.1097/sla.0000000000001676
Schanzer A, Simons JP, Flahive J, Durgin J, Aiello FA, Doucet D et al (2017) Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg 66(3):687–694. https://doi.org/10.1016/j.jvs.2016.12.111
Tsilimparis N, Detter C, Law Y, Rohlffs F, Heidemann F, Brickwedel J et al (2019) Single-center experience with an inner branched arch endograft. J Vasc Surg 69(4):977–985.e1. https://doi.org/10.1016/j.jvs.2018.07.076
Sobocinski J, Chenorhokian H, Maurel B, Midulla M, Hertault A, Le Roux M et al (2013) The benefits of EVAR planning using a 3D workstation. Eur J Vasc Endovasc Surg 46(4):418–423. https://doi.org/10.1016/j.ejvs.2013.07.018
Hull CWA (1986) inventorpparatus for production of three-dimensional objects by stereolithography. U.S. Patent
Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T (2019) 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci 6(11):1900344. https://doi.org/10.1002/advs.201900344
Jardini AL, Larosa MA, Filho MR, Zavaglia CA, Bernardes LF, Lambert CS et al (2014) Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J Craniomaxillofac Surg 42(8):1877–1884. https://doi.org/10.1016/j.jcms.2014.07.006
Buj-Corral I, Bagheri A, Petit-Rojo O (2018) 3D printing of porous scaffolds with controlled porosity and pore size values. materials. https://doi.org/10.3390/ma11091532
Tack P, Victor J, Gemmel P, Annemans L (2016) 3D-printing techniques in a medical setting: a systematic literature review. BioMed Eng OnLine 15(1):115. https://doi.org/10.1186/s12938-016-0236-4
Taher F, Falkensammer J, McCarte J, Strassegger J, Uhlmann M, Schuch P et al (2017) The influence of prototype testing in three-dimensional aortic models on fenestrated endograft design. J Vasc Surg 65(6):1591–1597. https://doi.org/10.1016/j.jvs.2016.10.108
Tam MD, Laycock SD, Brown JR, Jakeways M (2013) 3D printing of an aortic aneurysm to facilitate decision making and device selection for endovascular aneurysm repair in complex neck anatomy. J Endovasc Ther 20(6):863–867. https://doi.org/10.1583/13-4450mr.1
Mafeld S, Nesbitt C, McCaslin J, Bagnall A, Davey P, Bose P et al (2017) Three-dimensional (3D) printed endovascular simulation models: a feasibility study. Ann Transl Med 5(3):42. https://doi.org/10.21037/atm.2017.01.16
Andolfi C, Plana A, Kania P, Banerjee PP, Small S (2017) Usefulness of three-dimensional modeling in surgical planning, resident training, and patient education. J Laparoendosc Adv Surg Tech A 27(5):512–515. https://doi.org/10.1089/lap.2016.0421
Langer R, Vacanti J (2016) Advances in tissue engineering. J Pediatr Surg 51(1):8–12. https://doi.org/10.1016/j.jpedsurg.2015.10.022
Treasure T (2013) Personalized external aortic root support. Tex Heart Inst J 40(5):549–552
Sodian R, Kruttschnitt M, Hitschrich N, Mumm B, Schnell C, Hagl C et al (2021) 3‑dimensional printing for the diagnosis of left ventricular outflow tract obstruction after mitral valve replacement. Interact CardioVasc Thorac Surg 32(5):724–726. https://doi.org/10.1093/icvts/ivaa319
Grab M, Hopfner C, Gesenhues A, Konig F, Haas NA, Hagl C et al (2021) Development and evaluation of 3D-printed cardiovascular phantoms for Interventional planning and training. J Vis Exp. https://doi.org/10.3791/62063
Bangeas P, Voulalas G, Ktenidis K (2016) Rapid prototyping in aortic surgery. Interact CardioVasc Thorac Surg 22(4):513–514. https://doi.org/10.1093/icvts/ivv395
Tenewitz C, Le RT, Hernandez M, Baig S, Meyer TE (2021) Systematic review of three-dimensional printing for simulation training of interventional radiology trainees. 3d Print Med 7(1):10. https://doi.org/10.1186/s41205-021-00102-y
Torres IO, De Luccia N (2017) A simulator for training in endovascular aneurysm repair: the use of three dimensional printers. Eur J Vasc Endovasc Surg 54(2):247–253. https://doi.org/10.1016/j.ejvs.2017.05.011
Kaschwich M, Sieren M, Matysiak F, Bouchagiar J, Dell A, Bayer A et al (2020) Feasibility of an endovascular training and research environment with exchangeable patient specific 3D printed vascular anatomy: simulator with exchangeable patient-specific 3D-printed vascular anatomy for endovascular training and research. Ann Anat. https://doi.org/10.1016/j.aanat.2020.151519
Kärkkäinen JM, Sandri G, Tenorio ER, Alexander A, Bjellum K, Matsumoto J et al (2019) Simulation of endovascular aortic repair using 3D printed abdominal aortic aneurysm model and fluid pump. Cardiovasc Intervent Radiol 42(11):1627–1634. https://doi.org/10.1007/s00270-019-02257-y
Davis GR, Illig KA, Yang G, Nguyen TH, Shames ML (2014) An approach to EVAR simulation using patient specific modeling. Ann Vasc Surg 28(7):1769–1774. https://doi.org/10.1016/j.avsg.2014.05.007
Marconi S, Pugliese L, Botti M, Peri A, Cavazzi E, Latteri S et al (2017) Value of 3D printing for the comprehension of surgical anatomy. Surg Endosc 31(10):4102–4110. https://doi.org/10.1007/s00464-017-5457-5
Winder RJ, Sun Z, Kelly B, Ellis PK, Hirst D (2002) Abdominal aortic aneurysm and stent graft phantom manufactured by medical rapid prototyping. J Med Eng Technol 26(2):75–78. https://doi.org/10.1080/03091900210124404
Koleilat I, Jaeggli M, Ewing JA, Androes M, Simionescu DT, Eidt J (2016) Interobserver variability in physician-modified endograft planning by comparison with a three-dimensional printed aortic model. J Vasc Surg 64(6):1789–1796. https://doi.org/10.1016/j.jvs.2015.09.044
Meess KM, Izzo RL, Dryjski ML, Curl RE, Harris LM, Springer M et al (2017) 3D printed abdominal aortic aneurysm phantom for image guided surgical planning with a patient specific fenestrated endovascular graft system. Proc SPIE Int Soc Opt Eng. https://doi.org/10.1117/12.2253902
Tsilimparis N, Heidemann F, Rohlffs F, Diener H, Wipper S, Debus ES et al (2017) Outcome of surgeon-modified fenestrated/branched stent-grafts for symptomatic complex aortic pathologies or contained rupture. J Endovasc Ther 24(6):825–832. https://doi.org/10.1177/1526602817729673
Branzan D, Geisler A, Grunert R, Steiner S, Bausback Y, Gockel I et al (2021) The influence of 3D printed aortic models on the evolution of physician modified stent grafts for the urgent treatment of thoraco-abdominal and pararenal aortic pathologies. Eur J Vasc Endovasc Surg 61(3):407–412. https://doi.org/10.1016/j.ejvs.2020.10.023
Tong YH, Yu T, Zhou MJ, Liu C, Zhou M, Jiang Q et al (2020) Use of 3D printing to guide creation of fenestrations in physician-modified stent-grafts for treatment of thoracoabdominal aortic disease. J Endovasc Ther 27(3):385–393. https://doi.org/10.1177/1526602820917960
Lau I, Sun Z (2018) Three-dimensional printing in congenital heart disease: A systematic review. J Med Radiat Sci 65(3):226–236. https://doi.org/10.1002/jmrs.268
Levin D, Mackensen GB, Reisman M, McCabe JM, Dvir D, Ripley B (2020) 3D printing applications for transcatheter aortic valve replacement. Curr Cardiol Rep 22(4):23. https://doi.org/10.1007/s11886-020-1276-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Stana, M. Grab, R. Kargl and N. Tsilimparis declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors. Planning and 3D modeling were conducted using an anonymized CT-dataset.
The supplement containing this article is not sponsored by industry.
Additional information

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Stana, J., Grab, M., Kargl, R. et al. 3D printing in the planning and teaching of endovascular procedures. Radiologie 62 (Suppl 1), 28–33 (2022). https://doi.org/10.1007/s00117-022-01047-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00117-022-01047-x