Abstract
Multiparametric magnetic resonance imaging (mpMRI) of the prostate and mpMRI-guided biopsy have proved to be a valuable part of the diagnostic pathway for prostate cancer. This review reports on the current results in terms of clinical performance of these diagnostic tools and their role in clinical decision-making.
Zusammenfassung
Die multiparametrische Magnetresonanztomographie (mpMRT) der Prostata und die mpMRT-gesteuerte Biopsie sind ein wichtiger Bestandteil der Diagnostik des Prostatakarzinoms. In dieser Übersichtsarbeit berichten wir über die aktuelle Studienlage zur klinischen Anwendung dieser diagnostischen Mittel und bewerten deren Stellenwert in der klinischen Entscheidungsfindung.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Prostate cancer is the most prevalent type of cancer and the second most prevalent cause of cancer-related death among men in Germany [1]. Approximately 60,700 men were diagnosed with this disease in 2018 [1]. The average age of diagnosis is around 72 years and the average age of cancer-related death is 79 years [1]. Survival rates for prostate cancer are among the highest of all forms of cancer: 5‑year and 10-year survival rates are 91% and 90%, respectively [1]. However, these numbers hide the heterogeneity of this disease. Patients diagnosed with high-risk prostate cancer have a high chance of recurrence after initial treatment and development of metastases [2]. These patients are likely to require multimodal therapy involving surgery, radiation therapy and chemotherapy. A randomized controlled trial by Bill-Axelson et al. that assigned patients with localized prostate cancer to either radical prostatectomy or watchful waiting showed that, after a follow-up of 29 years, patients with extracapsular extension in the specimen of radical prostatectomy had a five times higher chance of death from prostate cancer compared to patients without extracapsular extension. Patients with a Gleason grade higher than 7 had a 10 times higher chance of death from prostate cancer than patients with Gleason grade 6 [3]. On the other hand, the current literature shows that, in consideration of the average age of diagnosis, the life expectancy of patients with low-risk prostate cancer is often unaltered and that these patients are often unlikely to experience symptoms during the process of the disease. Bill-Axelson et al. also showed that, after a follow-up of 29 years, patients benefited from radical prostatectomy with a mean of 2.9 years of lifetime gained compared to patients under watchful waiting. The recently published update results of the PROTECT trial by Hamdy et al., who randomly assigned patients to either radical prostatectomy, radiation therapy or prostate-specific antigen (PSA) monitoring, showed that patients with localized Gleason 6 prostate cancer had a cancer-specific mortality of less than 1% after 10-year follow-up. The incidence of metastases was only 3.5% [4]. Treating patients with the same form of curative therapy, e.g. radical prostatectomy or radiation therapy regardless of their risk classification would result in overtreatment of a large cohort of patients with unnecessary side effects such as erectile dysfunction and urinary incontinence. Therefore, clinicians tend to distinguish between clinically significant prostate cancer, with a need for further treatment, and clinically insignificant prostate cancer, eligible for surveillance. However, there is no general definition of clinically significant cancer as yet. While some studies define it as prostate cancer including Gleason pattern 4 or 5, others classify high-volume Gleason score 6 as clinically significant [5, 6]. Nevertheless, in order to distinguish clinically significant from insignificant prostate cancer, safe diagnostic and risk stratification tools are needed.
Many associations have published models for risk stratifications of prostate cancer. In most cases these rely on the same clinical parameters such as PSA value, Gleason Score of the prostate biopsy and local tumor extension. The Guidelines of the European Association of Urology (EAU) as well as the American Urological Association (AUA) use the risk stratification system by d’Amico et al. [7]. This group was the first to define the risk of biochemical recurrence after radical prostatectomy by PSA, Gleason score and T stage according to the TNM classification [2].
In recent years, other diagnostic tools have contributed to the diagnostics and risk stratification of prostate cancer. In addition to other risk stratification nomogramms or biomarkers, multiparametric magnetic resonance imaging (mpMRI) has probably had the biggest influence on the diagnosis of prostate cancer.
Multiparametric magnetic resonance imaging of the prostate
From its introduction in the 1980s with 0.3‑T systems, slice thicknesses of around 10 mm and an examination time of more than 1 h, experts have been continuously evolving the use of MRI sequences and standards of interpretation [8]. To date, functional sequences such as diffusion-weighted imaging (DWI), dynamic contrast-enhanced imaging (DCE) and standardized interpretation according to the Prostate Imaging Reporting and Data System (PI-RADS) have contributed to the diagnostic value of mpMRI of the prostate.
T1-weighted and T2-weighted imaging
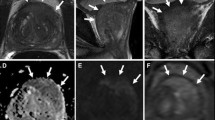
T1-weighted and T2-weighted imaging provide essential information on the anatomical zones of the prostate. The prostate gland can be divided into the peripheral zone, transition zone, central zone and anterior zone. Prostate cancer is most likely to occur in the peripheral zone [9]. In T2-weighted imaging, prostate cancer can be recognized by a low signal intensity lesion on a high signal intensity background (Fig. 1). The high signal of the peripheral zone is generated by the high fluid content of the prostatic glands and creates an important contrast to the thin hypointense rim, which separates the peripheral zone from the transition zone. The transition zone often shows a heterogeneous signal due to different stages of benign hyperplasia. Furthermore, T1-weighted and T2-weighted imaging can also be used for local staging in terms of evaluation of extraprostatic extension, regional lymph nodes and bone structures [10]. Numerous conditions can mimic prostate cancer in these sequences. Acute or chronic inflammation, benign hyperplasia, scars and post-biopsy hemorrhage especially in the transition zone can make interpretation challenging.
Diffusion-weighted imaging
Diffusion-weighted imaging (DWI) quantifies the motion of water molecules through prostatic tissue [11]. As malignant tissue contains a higher cellular density than non-malignant tissue, it resembles an inhibitor for this shifting of water. The apparent diffusion coefficient (ADC) thus calculated quantifies the movement of water molecules on the ADC map. The ADC map is created by performing DWI at multiple b values (factor of strength and timing of the magnetic field). Guidelines recommend applying DWI sequences with b values starting from 50 to at least b1400 s/mm2 [12]. Prostate cancer represents a high intensity on each DWI sequence and low intensity on the associated ADC map. Studies showed that the DWI sequence and ADC map correlate with the Gleason score of prostate cancer [13]. The addition of DWI to T2-weighted imaging was shown to improve the detection rate of prostate cancer compared to T2-weighted imaging alone and can help to further differentiate between suspected cancer and benign hyperplasia [14]. However, DWI is also affected by artifacts caused more by disturbances of the magnetic field, e.g. artificial prosthesis or gas in the rectum, than by tissue alteration such as inflammation.
Dynamic contrast-enhanced imaging
DCE imaging is a series of rapidly acquired T1-weighted images after the intravenous injection of a gadolinium-containing contrast agent. Its diagnostic value lies in the possibility of further evaluation of suspicious lesions in T2-weighted and/or DWI sequences. Intensity and kinetics of the contrast agent such as early enhancement or a wash-out phenomenon can indicate increased angiogenesis of malignant tissue. However, suspicious contrast enhancement by itself is not indicative of prostate cancer and lesions can be classified as suspicious for prostate cancer without altered contrast enhancement. In terms of cost-saving, acquisition time and public accessibility, the use of DCE imaging is currently debated. A recent meta-analysis and multiple studies showed a high diagnostic accuracy for biparametric MRI (without DCE imaging) in the detection of prostate cancer [15,16,17], while other studies revealed a benefit from adding DCE imaging to the protocol especially for the evaluation of unclear lesions in the peripheral zone and after surgical interventions [18,19,20]. Particularly in the case of initially negative transrectal ultrasonography-guided biopsy, biparametric MRI appears to be non-inferior to mpMRI, but more cost-effective and time-efficient [21].
Prostate Imaging Reporting and Data System (PI-RADS)
In order to standardize acquisition and interpretation of mpMRI, the European Society of Urogenital Radiology (ESUR) published a guideline in 2012 [22]. Here, Barentsz et al. were the first to set up a risk assessment tool to express likelihood of clinically significant prostate cancer: the Prostate Imaging Reporting and Data System (PI-RADS) v1. Interpretation of mpMRI of the prostate by PI-RADS guidelines is a zonal based system in which every zone is interpreted in the above-mentioned sequences and given a score; these scores are then added up to yield the final PI-RADS score. On a Likert-scale of 1–5, the PI-RADS score expresses a probability of very low to very high for the presence of clinically significant prostate cancer. This guideline was only recently updated to PI-RADS v2.1 [12].
The accuracy of mpMRI in the diagnosis of prostate cancer before the release of the PI-RADS guidelines was first described in a meta-analysis by De Rooij et al. They evaluated seven studies and showed a pooled sensitivity and specificity of 0.78 (95% confidence interval [CI] 0.65–0.87) and 0.88 (95% CI 0.8–0.94), respectively. The negative predictive value ranged from 0.65 to 0.94. The reference standard in these studies was transrectal or transperineal prostate biopsy or radical prostatectomy specimen [23]. Following the introduction of the PI-RADS v1 and v2 guidelines, a meta-analysis showed an increased sensitivity of 0.89 (95% CI 0.86–0.92) and decreased specificity of 0.73 (95% CI 0.60–0.83) for prostate cancer detection [24]. A head-to-head comparison of PI-RADS v1 and v2 in six studies showed a higher pooled sensitivity for PI-RADS v2 of 0.95 (95% CI 0.85–0.98) compared to 0.88 (95% CI 0.8–0.93) for PI-RADS v1 [24].
In a multicenter randomized controlled trial, the PROMIS study group led by Ahmed compared the diagnostic performance of mpMRI to ultrasound-guided systematic biopsy of the prostate as standard of care. A total of 576 patients underwent a 1.5‑T mpMRI followed by both transrectal ultrasound-guided biopsy and transperineal template prostate mapping biopsy. The template prostate mapping biopsy served as a reference since it took a biopsy at a 5-mm sampling frame. The sensitivity for clinically significant cancer for the template biopsy was therefore estimated at 95%. For clinically significant cancer, mpMRI was more sensitive (0.93, 95% CI 0.88–0.96) than transrectal ultrasound-guided biopsy (0.48, 95% CI 0.42–0.55; p < 0.0001) and less specific (0.41, 95% CI 0.36–0.46 for mpMRI vs. 0.96, 95% CI 0.94–0.98% for transrectal ultrasound-guided biopsy; p < 0.0001). On further interpretation, Ahmed et al. found that by directing transrectal ultrasound-guided biopsy by mpMRI findings, 18% more cases of clinically significant prostate cancer would have been detected [25].
Multiparametric magnetic resonance imaging-guided biopsy
The German S3 guidelines recommend a prostate biopsy if either the PSA level is ≥4 ng/ml or shows an abnormal course over time or if the patient shows an abnormal status on digital rectal exam (DRE). The biopsy should be performed systematically and 10–12 biopsy cores should be taken [26]. This biopsy is performed transrectally using ultrasound guidance in most cases. With the implementation of mpMRI in prostate cancer diagnostics, many groups examined the feasibility and detection rate of mpMRI–ultrasound fusion-guided biopsies. A systematic review by Valerio et al. including 15 studies and 2293 patients showed that mpMRI-targeted biopsy detects more clinically significant cancers (median: 33.3% vs. 23.6%; range: 13.2–50% vs. 4.8–52%) using fewer biopsy cores (median: 9.2 vs. 37.1) compared to standard biopsy techniques [27]. This success of mpMRI and mpMRI-guided biopsy opens up the question of its position in the diagnostic pathway of prostate cancer: as a triage tool to establish whether or not to biopsy, as a replacement for systematic biopsy or as an addition to it?
In 2018, Kasivisvanathan et al. published the results of the PRECISION study to address this issue. In this randomized controlled trial, 500 biopsy-naive patients with the clinical suspicion of prostate cancer (elevated PSA value and/or abnormal DRE status) were assigned to either standard systematic biopsy or a pre-biopsy mpMRI. In the case of a PI-RADS score of ≥3, an mpMRI-targeted biopsy was performed without a concurrent systematic biopsy. In the case of a PI-RADS score of ≤2, no biopsy was performed. In 71 of the 252 patients receiving pre-biopsy mpMRI there was no suspicion of clinically significant cancer, thus biopsy was omitted. Clinically significant cancer was detected in 38% (95/252 patients) of the mpMRI group compared to 26% (64/248 patients) of the systematic-biopsy group. Fewer biopsy cores were taken in the mpMRI group and the percentage of positive biopsies out of all biopsies was higher compared to the systematic-biopsy group (mpMRI group: 44%, 422/967 biopsies vs. systematic-biopsy group: 18%, 515/2788 biopsies). The number of adverse events was higher in the systematic-biopsy group compared to the mpMRI group [28]. This non-inferiority trial conducted in 25 prostate cancer centers and 11 countries showed that mpMRI as a triage tool results in fewer men undergoing prostate biopsy, more clinically significant cancers being identified and fewer biopsy cores being obtained than standard randomized biopsy. In 2019, Venderink et al. published a retrospective analysis of 4259 patients assigned between 2012 and 2017 that only received a prostate biopsy in the case of a positive pre-biopsy mpMRI (PI-RADS ≥3). Overall, 54% (2281/4259) of patients with a negative mpMRI avoided biopsy. After 3‑year and 6‑year follow-up, clinically significant prostate cancer-free survival was 99.6% and 94.1% in patients with initial PI-RADS 1 and 2, respectively [29].
The European guidelines integrated these results and recommend performing a pre-biopsy mpMRI in all patients. In the case of a negative mpMRI (PI-RADS ≤2) in biopsy-naive patients and in the case of a PSA ≤10 ng/ml and a negative DRE, the biopsy should be omitted on the basis of shared decision-making with the patient. In the case of a positive mpMRI (PI-RADS ≥3), an mpMRI-targeted biopsy and a concurrent systematic biopsy should be performed. In the case of a prior negative standardized biopsy and a positive mpMRI, the patient should only receive an mpMRI-targeted biopsy [30]. The German S3 guidelines do not endorse general pre-biopsy mpMRI as a triage tool. They state that, if a pre-biopsy mpMRI is available, suspicious lesions should be targeted additionally to the systematic biopsy. If a pre-biopsy mpMRI shows no suspicious lesions (PI-RADS ≤2), the patient should be offered a systematic biopsy or PSA-based follow-up. Furthermore, patients with a prior negative biopsy and persistent clinical suspicion of prostate cancer and patients eligible for active surveillance therapy of prostate cancer should receive an mpMRI and, if positive, an mpMRI-targeted biopsy with a concurrent systematic biopsy [26].
With regard to the question of whether or not to perform a systematic biopsy additionally to mpMRI-targeted biopsy, the majority of studies show the highest detection rates of clinically significant prostate cancer in the combination of both modalities [6, 31,32,33,34,35]. In a prospective multicenter study, Rouvière et al. compared the oncological outcome of systematic ultrasound-guided biopsy versus mpMRI-targeted biopsy performed by two separate operators on the same patient (n = 275). While there was no difference between systematic and mpMRI-targeted biopsy in the detection of clinically significant prostate cancer (29.9%, 95% CI 24.3–36.0 vs. 32.3%, 95% CI 26.5–38.4; p = 0.38), combining both techniques substantially improved the detection rate (66%) [36]. Furthermore, Ploussard et al. showed that concordance rates between biopsy pathology and subsequent radical prostatectomy pathology significantly differed between mpMRI-targeted biopsy and mpMRI-targeted biopsy plus systematic biopsy (45.2% and 51.7%, respectively). In 478 patients included in the study, the upgrading rate in radical prostatectomy specimens compared to biopsy pathology decreased by 22% when systematic biopsy was added to mpMRI-targeted biopsy [37].
Fusion strategies
According to current guidelines, a biopsy of the prostate should be performed under transrectal ultrasound imaging [26]. Fusion of the mpMRI and the live ultrasound imaging can be done either technically by software-assisted registration or visually, also referred to as cognitive fusion. Cognitive fusion is performed by the operator, who places the biopsy core according to the information of the pre-biopsy mpMRI. The advantage of this approach is its simplicity and the fact that additional equipment is not required. However, it requires interdisciplinary skills to locate the mpMRI and ultrasound lesion and involves a learning curve [38]. Software-assisted fusion usually works by outlining the prostate gland and suspicious lesion on mpMRI and then overlaying it with live ultrasound imaging (Fig. 2). Another approach is the in-bore biopsy technique. Here, the biopsy is performed inside the MR scanner under live mpMRI imaging. This allows immediate registration of the needle and mpMRI target lesion and is supposed to rule out the risk of failure of mpMRI–ultrasound overlay. However, this approach is associated with increased costs and acquisition time and an additional systematic biopsy of the rest of the prostate can usually not be performed. Biopsy of additional targets increases the time of the procedure by a further 15 min [39]. Most studies comparing these three techniques showed no significant advantage in the detection rate of clinically significant prostate cancer and no official recommendation for either one approach has been made as yet [40]. A meta-analysis evaluating 43 studies showed no significant difference in the detection of clinically significant prostate cancer [41]. However, by assigning patients to either software-assisted or visual fusion, Stabile et al. showed a superior detection rate of clinically significant prostate cancer with mpMRI-targeted biopsy by software-assisted fusion compared to visual fusion (57% vs. 36%; p = 0.002) [38]. According to the results of the SmartTarget Biopsy trial, Hamid et al. showed no difference in detection rate between visual and technical fusion. In this study, the highest detection rate was achieved by combining both strategies [42].
Biopsy approaches
The biopsy can be performed using a transrectal or transperineal approach. Today, most biopsies are performed transrectally under local anesthesia and antibiotic prophylaxis [27]. According to the annual report of the National Prostate Cancer Audit 2018, 12% of all patients in England underwent transperineal biopsy [43]. This approach usually requires more equipment and general sedation of the patient. However, it is possible to perform transperineal biopsy under local anesthesia. In a retrospective analysis of 1287 patients, Stefanova et al. reported good tolerance and feasibility of transperineal biopsy under local anesthesia. Infiltration of the anesthesia was reported to be more painful than the biopsy itself [44]. A major advantage of the transperineal approach compared to the transrectal approach is the lower risk of systemic inflammation and sepsis [45]. However, higher rates of urinary retention have been reported for the transperineal approach compared to the transrectal approach [45]. In terms of the detection rate of prostate cancer, comparative studies of both procedures are scant. A systematic review and meta-analysis indicates a similar detection rate [46].
Pitfalls
Given the recent success of mpMRI in the diagnostic pathway of prostate cancer, it should be applied with reasonable caution. By comparing 3‑T mpMRI to whole-mount pathology of radical prostatectomy specimens from 588 patients, Johnson et al. showed that mpMRI detected only 45% of all prostate cancer lesions (95% CI 42–47%), including 65% of clinically significant lesions (95% CI 61–69%) and 80% of high-grade tumors. Of the missed solitary tumors, 74% were clinically significant [47]. In the PRECISION Trial, mpMRI was performed in high-volume centers by experienced radiologists at an average of 350–1000 mpMRI per year. With the 2019 EAU recommendation of a mandatory pre-biopsy mpMRI, a major increase in the use of mpMRI will be expected. Although regular updates of PI-RADS guidelines are intended to reduce inter-observer variability, a number of studies showed insufficient performance in the general community [48, 49]. Greer et al. showed that less experienced radiologists are more likely to assign PI-RADS 3 (equivocal risk of clinically significant prostate cancer) compared to experienced radiologists [50]. Radiology training programs were shown to be able to improve concordance of non-university and university inter-reader agreement and biopsy decision [51].
Conclusion
MpMRI of the prostate and mpMRI guided-biopsy have been shown to significantly improve the detection rate of prostate cancer and have become a valuable part of clinical risk stratification. The value of MpMRI as a triage test for prostate biopsy is as yet uncertain and guideline recommendations are inconsistent. The expected rise in referrals for mpMRI of the prostate will present a challenge in terms of public accessibility, health-related costs and validity.
References
Robert-Koch-Institut (2017) Krebs in Deutschland, Prostata. Zentrum für Krebsregisterdaten
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
Bill-Axelson A, Holmberg L, Garmo H, Taari K, Busch C, Nordling S, Haggman M, Andersson SO, Andren O, Steineck G et al (2018) Radical prostatectomy or watchful waiting in prostate cancer—29-year follow-up. N Engl J Med 379:2319–2329
Neal DE, Metcalfe C, Donovan JL, Lane JA, Davis M, Young GJ, Dutton SJ, Walsh EI, Martin RM, Peters TJ et al (2019) Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the ProtecT randomised controlled trial according to treatment received. Eur Urol. https://doi.org/10.1016/j.eururo.2019.10.030
Futterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, Taneja SS, Thoeny H, Villeirs G, Villers A (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 68:1045–1053
de Gorski A, Roupret M, Peyronnet B, Le Cossec C, Granger B, Comperat E, Cussenot O, Renard-Penna R, Mozer P (2015) Accuracy of magnetic resonance imaging/ultrasound fusion targeted biopsies to diagnose clinically significant prostate cancer in enlarged compared to smaller prostates. J Urol 194:669–673
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S et al (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71:618–629
Giganti F, Rosenkrantz AB, Villeirs G, Panebianco V, Stabile A, Emberton M, Moore CM (2019) The evolution of MRI of the prostate: the past, the present, and the future. Ajr Am J Roentgenol 213:384–396
Sinnott JA, Rider JR, Carlsson J, Gerke T, Tyekucheva S, Penney KL, Sesso HD, Loda M, Fall K, Stampfer MJ et al (2015) Molecular differences in transition zone and peripheral zone prostate tumors. Carcinogenesis 36:632–638
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM et al (2016) PI-RADS prostate imaging—reporting and data system: 2015, version 2. Eur Urol 69:16–40
Somford DM, Futterer JJ, Hambrock T, Barentsz JO (2008) Diffusion and perfusion MR imaging of the prostate. Magn Reson Imaging Clin N Am 16:685–695 (ix)
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, Tempany CM, Choyke PL, Cornud F, Margolis DJ et al (2019) Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 76:340–351
Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, Scheenen T, Barentsz JO (2011) Relationship between apparent diffusion coefficients at 3.0‑T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259:453–461
Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN (2012) The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. Ajr Am J Roentgenol 199:103–110
Niu XK, Chen XH, Chen ZF, Chen L, Li J, Peng T (2018) Diagnostic performance of biparametric MRI for detection of prostate cancer: a systematic review and meta-analysis. Ajr Am J Roentgenol 211:369–378
Scialpi M, Prosperi E, D’Andrea A, Martorana E, Malaspina C, Palumbo B, Orlandi A, Falcone G, Milizia M, Mearini L et al (2017) Biparametric versus multiparametric MRI with non-endorectal coil at 3T in the detection and localization of prostate cancer. Anticancer Res 37:1263–1271
van der Leest M, Israel B, Cornel EB, Zamecnik P, Schoots IG, van der Lelij H, Padhani AR, Rovers M, van Oort I, Sedelaar M et al (2019) High diagnostic performance of short magnetic resonance imaging protocols for prostate cancer detection in biopsy-naive men: the next step in magnetic resonance imaging accessibility. Eur Urol 76:574–581
Ullrich T, Quentin M, Arsov C, Laqua N, Abrar D, Hiester A, Albers P, Antoch G, Schimmoller L (2019) Value of dynamic contrast-enhanced (DCE) MR imaging in peripheral lesions in PI-RADS‑4 patients. Rofo. https://doi.org/10.1055/a-1020-4026
Greer MD, Shih JH, Lay N, Barrett T, Kayat Bittencourt L, Borofsky S, Kabakus IM, Law YM, Marko J, Shebel H et al (2017) Validation of the dominant sequence paradigm and role of dynamic contrast-enhanced imaging in PI-RADS version 2. Radiology 285:859–869
Del Vescovo R, Pisanti F, Russo V, Battisti S, Cazzato RL, D’Agostino F, Giurazza F, Quattrocchi CC, Faiella E, Setola R et al (2013) Dynamic contrast-enhanced MR evaluation of prostate cancer before and after endorectal high-intensity focused ultrasound. Radiol Med 118:851–862
Kuhl CK, Bruhn R, Kramer N, Nebelung S, Heidenreich A, Schrading S (2017) Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology 285:493–505
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Futterer JJ, European Society of Urogenital R (2012) ESUR prostate MR guidelines. Eur Radiol 22(2012):746–757
de Rooij M, Hamoen EH, Futterer JJ, Barentsz JO, Rovers MM (2014) Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. Ajr Am J Roentgenol 202:343–351
Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2017) Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol 72:177–188
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822
Deutsche Krebsgesellschaft (DK), AWMF (2019) Leitlinienprogramm Onkologie: Interdisziplininäre Leitlinie der Qualität S3 zur Führerkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinom
Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, Mozer P, Rastinehad AR, Ahmed HU (2015) Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol 68:8–19
Kasivisvanathan V, Emberton M, Moore CM (2018) MRI-targeted biopsy for prostate-cancer diagnosis. N Engl J Med 379:589–590
Venderink W, van Luijtelaar A, van der Leest M, Barentsz JO, Jenniskens SFM, Sedelaar MJP, Hulsbergen-van de Kaa C, Overduin CG, Futterer JJ (2019) Multiparametric magnetic resonance imaging and follow-up to avoid prostate biopsy in 4259 men. BJU Int 124:775–784
Mottet N (Chair) RCNvdB, Briers E (Patient Representative), Cornford P (Vice-chair), De Santis M, Fanti S, Gillessen S, Grummet J, Henry AM, Lam TB, Mason MD, van der Kwast TH, van der Poel HG, Rouvière O, Tilki D, Wiegel T, & Guidelines Associates: Van den Broeck TMC, Fossati N, Gross T, Lardas M, Liew M, Moris L, Schoots IG, Willemse P‑PM (2019) EAU Guidelines. Edn. presented at the EAU Annual Congress Barcelona 2019
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM et al (2015) Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 313:390–397
Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, Reiter RE, Marks LS (2016) Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 122:884–892
Delongchamps NB, Portalez D, Bruguiere E, Rouviere O, Malavaud B, Mozer P, Fiard G, Cornud F and Group MS (2016) Are magnetic resonance imaging-transrectal ultrasound guided targeted biopsies noninferior to transrectal ultrasound guided systematic biopsies for the detection of prostate cancer? J Urol 196:1069–1075
Peltier A, Aoun F, Lemort M, Kwizera F, Paesmans M, Van Velthoven R (2015) MRI-targeted biopsies versus systematic transrectal ultrasound guided biopsies for the diagnosis of localized prostate cancer in biopsy naive men. Biomed Res Int 2015:571708
Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, Svindland A, Eggesbo HB, Ukimura O (2016) A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol 69:149–156
Rouviere O, Puech P, Renard-Penna R, Claudon M, Roy C, Mege-Lechevallier F, Decaussin-Petrucci M, Dubreuil-Chambardel M, Magaud L, Remontet L et al (2019) Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 20:100–109
Ploussard G, Beauval JB, Lesourd M, Almeras C, Assoun J, Aziza R, Gautier JR, Loison G, Portalez D, Salin A et al (2019) Added value of concomitant systematic and fusion targeted biopsies for grade group prediction based on radical prostatectomy final pathology on positive magnetic resonance imaging. J Urol 202:1182–1187
Stabile A, Dell’Oglio P, Gandaglia G, Fossati N, Brembilla G, Cristel G, Deho F, Scattoni V, Maga T, Losa A et al (2018) Not all multiparametric magnetic resonance imaging-targeted biopsies are equal: the impact of the type of approach and operator expertise on the detection of clinically significant prostate cancer. Eur Urol Oncol 1:120–128
Pokorny M, Kua B, Esler R, Yaxley J, Samaratunga H, Dunglison N, Gianduzzo T, Coughlin G, Holt R, Laing B et al (2019) MRI-guided in-bore biopsy for prostate cancer: what does the evidence say? A case series of 554 patients and a review of the current literature. World J Urol 37:1263–1279
Wegelin O, Exterkate L, van der Leest M, Kummer JA, Vreuls W, de Bruin PC, Bosch J, Barentsz JO, Somford DM, van Melick HHE (2019) The FUTURE Trial: a multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur Urol 75:582–590
Wegelin O, van Melick HHE, Hooft L, Bosch J, Reitsma HB, Barentsz JO, Somford DM (2017) Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol 71:517–531
Hamid S, Donaldson IA, Hu Y, Rodell R, Villarini B, Bonmati E, Tranter P, Punwani S, Sidhu HS, Willis S et al (2019) The SmartTarget Biopsy Trial: a prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for prostate cancer risk stratification. Eur Urol 75:733–740
The Royal College of Surgeons of England BAoUSB, British Uro-Oncology (BUG) (2019) National Prostate Cancer Audit - Fifth Year Annua Report-Results of the NPCA Prospective Audit in England and Wales for men diagnosed 1 April 2016-Mach 2017
Stefanova V, Buckley R, Flax S, Spevack L, Hajek D, Tunis A, Lai E, Loblaw A, Collaborators (2019) Transperineal prostate biopsies using local anesthesia: experience with 1,287 patients. Prostate cancer detection rate, complications and patient tolerability. J Urol 201:1121–1126
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, Weidner W, Loeb S (2017) Complications after systematic, random, and image-guided prostate biopsy. Eur Urol 71:353–365
Xiang J, Yan H, Li J, Wang X, Chen H, Zheng X (2019) Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol 17:31
Johnson DC, Raman SS, Mirak SA, Kwan L, Bajgiran AM, Hsu W, Maehara CK, Ahuja P, Faiena I, Pooli A et al (2019) Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol 75:712–720
Branger N, Maubon T, Traumann M, Thomassin-Piana J, Brandone N, Taix S, Touzlian J, Brunelle S, Pignot G, Salem N et al (2017) Is negative multiparametric magnetic resonance imaging really able to exclude significant prostate cancer? The real-life experience. BJU Int 119:449–455
Muller S, Lilleaasen G, Sand TE, Lofsgaard L, Estop-Garanto M, Helgo D, Sund P, Mygland V (2018) Poor reproducibility of PIRADS score in two multiparametric MRIs before biopsy in men with elevated PSA. World J Urol 36:687–691
Greer MD, Brown AM, Shih JH, Summers RM, Marko J, Law YM, Sankineni S, George AK, Merino MJ, Pinto PA et al (2017) Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: a multireader study. J Magn Reson Imaging 45:579–585
van der Leest M, Cornel E, Israel B, Hendriks R, Padhani AR, Hoogenboom M, Zamecnik P, Bakker D, Setiasti AY, Veltman J et al (2019) Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naive Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur Urol 75:570–578
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Chaloupka, M. Apfelbeck, P. Pfitzinger, R. Bischoff, E. Lellig, L. Rath, B. Schlenker, C.G. Stief and D.-A. Clevert declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
The supplement containing this article is not sponsored by industry.
Rights and permissions
About this article
Cite this article
Chaloupka, M., Apfelbeck, M., Pfitzinger, P. et al. Multiparametric magnetic resonance imaging and multiparametric magnetic resonance imaging-guided biopsy in the diagnostic pathway of prostate cancer. Radiologe 60 (Suppl 1), 63–69 (2020). https://doi.org/10.1007/s00117-020-00716-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00117-020-00716-z