Abstract
Documentation of cryptic trilobite behavior has presented important insights into the paleoecology of this fully extinct arthropod group. One such example is the preservation of trilobites inside the remains of larger animals. To date, evidence for trilobites within cephalopods, gastropods, hyoliths, and other trilobites has been presented. Importantly, most of these interactions show trilobite molts, suggesting that trilobites used larger animals for protection during molting. To expand the record of molted trilobites within cephalopods, we present a unique case of a Toxochasmops vormsiensis trilobite within the body chamber of a Gorbyoceras textumaraneum nautiloid from the Upper Ordovician Kõrgessaare Formation of Estonia. By examining this material, we present new insights into the ecology of pterygometopid trilobites, highlighting how these forms used large cephalopods as areas to successfully molt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Examination of trilobite molting patterns and processes has presented insight into the paleoecology of these extinct arthropods (Henningsmoen 1975; McNamara and Rudkin 1984; Speyer 1985; Daley and Drage 2016; Drage and Daley 2016; Drage et al. 2018; Drage 2019, 2024). The exceptional preservational potential of trilobite exoskeletons permitted this line of inquiry (Whittington 1990; Daley and Drage 2016), resulting in a comprehensive understanding of molting configurations and long-term evolutionary patterns (Daley and Drage 2016; Drage and Daley 2016; Drage et al. 2018, 2023; Drage 2019, 2024). As such, trilobite molting processes are known across most of the Paleozoic and among higher order groupings.
Various forms of cryptic trilobite behavior have been documented, including clustering, hiding, and gregariousness (Brett 1977; Davis et al. 2001; Paterson et al. 2008; Popp and Pärnaste 2011; Fatka and Budil 2014; Bicknell et al. 2019; Fatka et al. 2021; Bicknell and Kimmig 2023). Additionally, there are rare records of smaller trilobites preserved within the remains of larger animals, such as cephalopods, other trilobites, and brachiopods (Table 1; Brett 1977; Valent et al. 2008; Fatka et al. 2009, 2021; Fatka and Szabad 2011; Fatka and Kozak 2014). This so-called conchicolous habit (= “the use by other animals of shells as residences after the original builders have died” Vermeij 1987, p. 240), or inquilinism (sensu Fraaye and Jäger 1995b; Landman et al. 2014; Fraaije et al. 2020; Bicknell et al. 2021), has been documented in 15 trilobite genera spanning the Cambrian through the Carboniferous (Table 1; Fatka and Kozak 2014; Fatka et al. 2021). These associations have been attributed to feeding on carcasses (Fatka et al. 2021), habitation and/or shelter (Rakociński 2009; Vokáč et al. 2015; Fatka et al. 2021), or using larger animals for protection during molting (Ladd 1929; Chatterton et al. 2003; Fatka et al. 2008, 2021; Zong et al. 2016). To expand the record of molted trilobites preserved within cephalopods, we present a molted Toxochasmops vormsiensis Rõõmusoks 1998 within the body chamber of a nautiloid cephalopod (Gorbyoceras textumaraneum (Roemer 1861), identified by Björn Kröger pers. comms.) from the Upper Ordovician Kõrgessaare Formation, Vormsi, Estonia. This record presents the first example of cryptic molting for pterygometopid trilobites.
Geological context
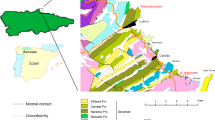
Vormsi is a low and flat island west of mainland Estonia that emerged from the Baltic Sea ~ 3000 years ago and rises to a maximum height of 11 m above sea level. Deposits below the thin Quaternary material are historically called the Lyckholm Layer and consist of the Upper Ordovician (middle to upper Katian shallow sea sediments mainly of bioclastic limestones that correlate upwards with the Nabala, Vormsi, and Pirgu regional stages; Fig. 1a; Schmidt 1858; Jaanusson 1944, 1956). The first detailed fossil collection on Saxby seashore, on the northwestern section of Vormsi, was by Sauramo (1929) who recorded a rich fauna over a 2-km-long section, proximal to the beach. A diverse assemblage of trilobites, brachiopods, cephalopods, gastropods, and corals totaling 46 taxa has been recorded. Furthermore, the fauna from the northern coastal region differs from the southern section. This was confirmed in Jaanusson (1944; 1956) who elected the substages based on this lateral faunal change—the older Kõrgessaare (in the northern region) and the younger Nõmmküla (in the southern region). This is due to a slight, 4–7° dip in the beds, which parallels the Paleozoic sedimentological belts observed in Estonia (Fig. 1). Despite this, both zones are considered part of the Kõrgessaare Formation, with the younger coral and calcitic vermiporellid algae-rich beds gradually transitioning into the overlying Moe Formation (see Kröger et al. 2017).
Geological, stratigraphic, and geographical information for specimen location. a Map showing local geology and specimen location (red star) in Estonia. b Paleogeography showing Baltica, 444 mya. Red star indicates the specimen location. Reconstruction constructed using BugPlates (Torsvik and Cocks 2009). c Stratigraphic section of local geology, showing position of the Kõrgessaare Formation
The Upper Ordovician Kõrgessaare Formation is a 10–20-m-thick limestone intercalated with marls and clay interlayers, becoming more argillaceous up-section. There is a ~ 1.7 m exposure along Saxby Beach at a cliff, but this is commonly covered by rubble and erosion. In the northern beach region, a discontinuity surface marks the upper Vormsi Stage (Einasto 2012). This is the most accessible area, as there is a road to the beach. This situation may have resulted in selective sampling bias in more recent collections, including our specimen. The fauna in the formation consists of brachiopods, gastropods, cephalopods, bryozoans, tabulate and rugose corals, and trilobites (Schmidt 1858, 1881; Sauramo 1929; Jaanusson 1944, 1956; Rõõmusoks 1998, 2000), mostly from the Vormsi Regional Stage. Documented trilobites include illaenids (Parillaenus roemeri (Volborth 1864) and Parillaenus angustifrons (Holm 1886)), proetids (e.g., Ascetopeltis kertelensis (Schmidt 1894), and Cyphaspis sp.), rare calymenids, enrinurids (Erratencrinurus moe (Männil 1958), Erratencrinurus nebeni Krueger 1971), pterygometopids (Toxochasmops vormsiensis Rõõmusoks 1998; Valdariops angustus Rõõmusoks 2000; Valdariops eichwaldi (Schmidt 1881)), lichids (Amphilichas lineatus (Angelin 1854), Conolichas angustatus (Beyrich 1846)), scutelluids (Eobronteus laticauda Wahlenberg 1818), harpetids (Hibbertia aff. costatus (Angelin 1854)), and asaphids (Brachyaspis robustus (Roemer 1861)). Except for the pterygometopids, all trilobite genera inhabited the deeper depositional environments of the Boda mud-mound (Suzuki et al. 2009) and during the Hirnantian trilobites with schizochroal eyes and better vision expanded into the pterygometopid niche (Hints et al. 2012; Ebbestad et al. 2015). The examined T. vormsiensis therefore likely lived relatively nearshore, in a warm, low-latitude epicontinental sea environment, rich in corals and shelly fauna.
Methods and materials
The examined specimen is housed in the Geological collections of the University of Tartu (TUG) and assigned the specimen number TUG 1355–193. The specimen was photographed under LED lighting using an Olympus E-M1 MarkIII camera with a 12–45-mm lens. Images were stacked using OM Capture. Measurements of specimens were gathered using ImageJ (Schneider et al. 2012) and 3D Slicer 4.11 (Fedorov et al. 2012).
The specimen was micro-CT scanned on a GE PHOENIX v|tome|x scanner with a 240 kV X-ray tube at the Microscopy and Imaging Facility at the American Museum of Natural History (AMNH). The scan was run at 200 kV and 230 μA. Scan data were reconstructed using the GE software datos|x 2.1 and segmented in the software 3D Slicer 4.11 using the SlicerMorph toolkit (Rolfe et al. 2021). The reconstruction of the scan was exported as a.PLY file (Supplemental Document 1).
Results
The specimen shows a molted exoskeleton of an individual of the trilobite Toxochasmops vormsiensis within the steinkern of a Gorbyoceras textumaraneum nautiloid (Fig. 2). The G. textumaraneum specimen measures 95.1 mm in length and 36.6 mm in width (across the aperture) tapering to 22.4 mm posteriorly. The trilobite is situated within the body chamber of the nautiloid. The chamber is entirely filled with sediment.
Examined Toxochasmops vormsiensis trilobite molt in Gorbyoceras textumaraneum nautiloid shell. TUG 1355–193. a View showing cephalon and trunk in oblique orientation. b View showing trunk with cephalon in oblique orientation. c View showing nautiloid siphuncle. d View showing external morphology and ornament (white arrows)
The Toxochasmops vormsiensis is partially preserved. The cephalon is disarticulated, missing the left eye (possibly due to erosion), and is partially covered by matrix on the right side; the cephalon sagittal length is 19.8 mm. The pygidium is disarticulated and partially covered by matrix on the left side; the pygidium sagittal length is 21.7 mm. The pygidial sagittal axis lies at an angle from the cephalic sagittal axis: ~ 10° to the right on a horizontal plane and ~ 60° declining posteriorly on a vertical plane. The cephalon and pygidium are separated by 11.6 mm, with the pygidium resting behind the cephalon in a telescoped configuration. The counterpart of a thoracic segment is located between the cephalon and pygidium. Upon examining the micro-CT scans, five to six articulated thoracic segments are identified in the matrix, under the cephalon.
Discussion
Explanations for trilobites (Brett 1977; Davis et al. 2001; Chatterton et al. 2003; Fatka et al. 2009, 2021; Fatka and Budil 2014; Zong et al. 2016) and agnostids (Brongniart 1822; Suzuki and Bergström 1999; Chatterton et al. 2003; Fatka et al. 2009; Fatka and Kozak 2014) within larger animals have been summarized into four main behaviors: (1) molting in shelter, (2) hiding from predation, (3) shelter from sea floor disturbance, and (4) scavenging on food (Fatka and Kozak 2014). Although the exact reasons cannot be unambiguously presented here, these primary explanations can be explored to understand our specimen.
Molting in shelter
Trilobites required quiet environments to complete ecdysis (Henningsmoen 1975; Brett 1977; Brandt 1993; Zong et al. 2016) and inhabiting the remains of other animals, such as empty cephalopod shells, would have been ideal for this purpose (Chatterton et al. 2003). The trilobite considered here is preserved within a molting configuration, demonstrating that the individual had entered the dead cephalopod conch to molt and excludes the possibility of the carcass having been washed into the nautiloid by passive fluid flow. While cephalopod shells may have been an ideal location for successful molting in paleoenvironments that lacked other safer areas to molt (Ladd 1929; Chatterton et al. 2003), the association of a trilobite within a nautiloid from the Kõrgessaare Formation is unique. This indicates that the environment likely had locations for successful molting, and, in this case, the trilobite was simply fortunate enough to locate a shell to molt within.
Hiding from predation
Large, empty shells would have provided ideal shelter from predation, as well as molting (Brett 2003; Chatterton et al. 2003; Fatka and Kozak 2014). While there is no evidence for predation on the observed specimen, records of failed predation are known from Ordovician trilobites (see Owen 1985; Rudkin 1985; Zong 2021; Bicknell et al. 2022a, b; Fatka et al. 2022; Bicknell and Kimmig 2023). Although this specimen may not have been subject to predation, this interaction may indicate a preference for molting within cavities to avoid possible attacks (Brett 1990, 2003; Brett and Walker 2002; Fatka and Budil 2014; Fatka et al. 2021).
Sea floor disturbance
Large shells represent ideal localities to survive periods of disturbances from storm events or rapid sediment inundation (Fatka and Kozak 2014). We are unable to determine if this situation can be excluded. As the paleoenvironment associated with this fossil is considered relatively nearshore, the trilobite may have entered the cephalopod to avoid disturbances and then may subsequently have molted.
Scavenging on food
The cephalopod may have had decaying soft material ideal for benthic scavengers (Fatka and Kozak 2014) and would have attracted trilobites and other smaller animals to the shell (Fatka et al. 2009). There is no evidence for any other feeding activity preserved in the specimen. As such, we can exclude this option here as a primary justification for the trilobite entering the cephalopod.
Taken together, the evidence suggests that this interaction represents molting within a sheltered condition. However, this condition would also have allowed the trilobite to be protected from predators and from poor environmental conditions.
The molt configuration observed in this specimen—the pygidium wedged directly behind the cephalon in a telescoped manner—is common in Toxochasmops (McNamara and Rudkin 1984, figs. 1, 3; Rõõmusoks 1998, pl 1, fig. 8; pl 2, fig. 13). During exuviation, the cephalothoracic joint disarticulated, similar to Salter’s molting mode (Henningsmoen 1975). The cephalon was therefore molted in a way that allowed the trilobite to move anteriorly on an angle and wedge the pygidium against the cephalon, facilitating its removal (McNamara and Rudkin 1984; Budil and Bruthansová, 2005). Previously records of this molt configuration are of specimens in non-sheltered conditions (McNamara and Rudkin 1984). In these examples, few to no thoracic segments are observed, indicating the thorax is shed elsewhere (McNamara and Rudkin 1984). In our specimen, most of the thorax is preserved under the cephalon, as would be expected from molting in shelter. Further, TUG 1355–193 demonstrates that this complex exuviation technique was possible within enclosed spaces.
The limited space within the shell would have presented some complications, such as escaping from the shell after molting. Passive sediment in-filling of an open structure on the sea floor likely resulted in a partly filled shell when the trilobite entered to molt (Hewitt 1988). This would have further limited the space for movement. Given the molt size, the trilobite likely filled up over half of the remaining space and would have had to move farther into the conch after molting. If the individual did leave the shell after molting, it likely rotated itself and moved over its molt. Such flexibility would have been possible in its soft-shelled condition (Drage et al. 2019) as the trilobite may have been more dorso-ventrally compressible, permitting movement through the limited space (Drage and Daley 2016). This exit from the conch could have taken minutes to days (Zong et al. 2016). Alternatively, the individual may have been trapped behind its molt, unable to escape. The Kõrgessaare Formation does not preserve soft-bodied fossils and, as such, we cannot test this possibility. Due to this limitation of the fossil record, we present both possible outcomes here.
By considering the history of trilobite molting within larger organisms, we can explore when in time and where in the group this cryptic behavior arose (Table 1). Although it was more common within agnostids (see Chatterton et al. 2003; Valent et al. 2008; Fatka et al. 2009; Fatka and Kozak 2014), hiding during molting had an origin in Cambrian agraulid trilobites (Valent et al. 2008). There is an increase in the prevalence of these interactions in the Ordovician asaphids, calymenids, harpetids, odontopleurids, and pliomerids (Table 1). This increase may reflect a biological signal, or it may be sampling bias. Additional examination of trilobite interactions during the Cambrian would help resolve this question. The long record of trilobites concealing themselves within larger animals may also reflect the rise of larger, more effective durophagous predators that targeted smaller trilobites (Brett 1990; 2003; Bicknell and Paterson 2018). Additionally, the increased abundance of this behavior likely gave trilobite species a protective advantage, adding to their success.
Habitation of animals in cephalopod shells is also observed in the Mesozoic (Fraaye and Jäger 1995a, b; Davis et al. 2001; Fraaije and Pennings 2006; Vullo et al. 2009; Klompmaker and Fraaije 2012; Landman et al. 2014; Nyborg et al. 2014; Smith and Holland 2016; Fraaije et al. 2020; Bicknell et al. 2021). The apparent increase in the diversity of animals showing this association reflects the use of cavities as a means of shelter, a food source, or for reproduction (Fraaye and Jäger 1995a, b; Nyborg et al. 2014). Despite the Mesozoic record, there are large gaps in information regarding these behaviors and associations, especially in the later Paleozoic trilobites (Table 1). We therefore propose the ongoing examination of cephalopod shells with the aim of documenting the use of these remains by benthic animals across the Phanerozoic.
References
Adrain JM, Westrop SR (2005) Lower Ordovician trilobites from the Baumann Fiord Formation, Ellesmere Island, Arctic Canada. Can J Earth Sci 42(9):1523–1546
Angelin NP (1854) Palaeontologica Scandinavica. Pars 1. Crustacea Formationis Transitionis. Fasc 2. T.O. Weigel, Lund, pp I–IX, 21–92, pls 25–41
Barrande J (1846) Notice Préliminaire sur le systême Silurien et les Trilobites de Bohême. Hirschfeld, Leipzig, p 97
Barrande J (1860) Troncature normale ou périodique de la coquille dans certains céphalopodes paléozoïques. Bulletinde La Societé Géologique De France, Séries 2(17):573–601
Barrande J (1867) Systêm silurien du centre de la Bohême. Ordre des Ptéropodes 3. Chez l’auteur, Prague, p 197
Barrande J (1872) Systêm silurien du centre de la Bohême. Supplément au vol. 1 (Trilobites, Crustacés divers et Poissons). Chez l’auteur, Prague, p 647
Beyrich E (1846) Untersuchungen über Trilobiten: zweites Stück als Fortsetzung zu der Abhandlung" Ueber einige böhmische Trilobiten". G. Reimer, Berlin, p 37
Bicknell RDC, Kimmig J (2023) Clustered and injured Pseudogygites latimarginatus from the late Ordovician Lindsay Formation, Canada. N Jb Geol Paläont 309:199–208
Bicknell RDC, Paterson JR (2018) Reappraising the early evidence of durophagy and drilling predation in the fossil record: implications for escalation and the Cambrian Explosion. Biol Rev 93(2):754–784
Bicknell RDC, Paterson JR, Hopkins MJ (2019) A trilobite cluster from the Silurian Rochester Shale of New York: predation patterns and possible defensive behavior. Am Mus Novit 39(3937):1–16
Bicknell RDC, Smith PM, Holland T, Klompmaker AA (2021) Cretaceous clam chowder: the first evidence of inquilinism between extinct shrimps and bivalves. Palaeogeogr Palaeoclimatol Palaeoecol 584:110669
Bicknell RDC, Smith PM, Bruthansová J, Holland B (2022a) Malformed trilobites from the Ordovician and Devonian. PalZ 96:1–10
Bicknell RDC, Smith PM, Howells TF, Foster JR (2022b) New records of injured Cambrian and Ordovician trilobites. J Paleontol 96:921–929
Brandt DS (1993) Ecdysis in Flexicalymene meeki (Trilobita). J Paleontol 67(6):999–1005
Brett CE (1990) Predation. In: Briggs DEG, Crowther PR (eds) Palaeobiology: A Synthesis. Blackwell Press, Oxford, pp 368–372
Brett CE (2003) Durophagous predation in Paleozoic marine benthic assemblages. In: Kelley PH, Kowalewski M, Hansen HJ (eds) Predator-prey interactions in the fossil record. Springer, Boston, MA, pp 401–432
Brett CE, Walker SE (2002) Predators and predation in Paleozoic marine environments. Paleontol Soc Pap 8:93–118
Brett CE (1977) Entombment of a trilobite within a closed brachiopod shell. J Paleontol 51:1041–1045
Brongniart A (1822) Sur la classification et la distribution des végétaux fossiles. Mémoires Du Museum D’histoire Naturelle Paris 8:203–348
Budil P, Bruthansová J (2005) Moulting in Ordovician dalmanitoid and acastoid trilobites of the Prague Basin. Prelim Observ Geol Acta 3:373–384
Chatterton BDE (1971) Taxonomy and ontogeny of Siluro-Devonian trilobites from near Yass, New South Wales. Palaeontogr Abt A 137:1–108
Chatterton BDE, Collins DH, Ludvigsen R, Lane P (2003) Cryptic behaviour in trilobites: Cambrian and Silurian examples from Canada, and other related occurrences. Spec Pap Palaeontol 70:157–173
Daley AC, Drage HB (2016) The fossil record of ecdysis, and trends in the moulting behaviour of trilobites. Arthropod Struct Dev 45(2):71–96
Davis RA, Fraaye RHB, Holland CH (2001) Trilobites within nautiloid cephalopods. Lethaia 34(1):37–45
de Sowerby JC (1839). In: Murchison RI (ed) The Silurian system. John Murray, London, p 768
DeKay JE (1824) Observations on the structure of trilobites, and descriptions of an apparently new genus. With notes on the geology of Trenton Falls by J. Renwick. Ann. Lyceum Nat. Hist New York 1:174–189
Drage HB (2019) Quantifying intra-and interspecific variability in trilobite moulting behaviour across the Palaeozoic. Palaeontol Electron 22(2):1–39
Drage HB (2024) Trilobite moulting behaviour variability had little association with morphometry. Palaeontol Electron 27(1):a9
Drage HB, Daley AC (2016) Recognising moulting behaviour in trilobites by examining morphology, development and preservation: comment on Błażejowski et al. 2015. BioEssays 38(10):981–990
Drage HB, Holmes JD, García-Bellido DC, Daley AC (2018) The fossil record of ecdysis, and trends in the moulting behaviour of trilobites. Lethaia 51(4):473–492
Drage HB, Vandenbroucke TR, Van Roy P, Daley AC (2019) Sequence of post-moult exoskeleton hardening preserved in a trilobite mass moult assemblage from the Lower Ordovician Fezouata Konservat-Lagerstätte, Morocco. Acta Palaeontol Pol 64(2):261–273
Drage HB, Holmes JD, García-Bellido DC, Paterson JR (2023) Associations between trilobite intraspecific moulting variability and body proportions: Estaingia bilobata from the Cambrian Emu Bay Shale, Australia. Palaeontology 66(3):e12651
Ebbestad JOR, Högström AES, Frisk ÅM, Martma T, Kaljo D, Kröger B, Pärnaste H (2015) Terminal Ordovician stratigraphy of the Siljan district, Sweden. GFF 137(1):44–57
Einasto R (2012) Vaadates kivi sisse Saxby rannikupaljandis Vormsil. Keskkonnatehnika 6:41–43
Fatka O, Budil P (2014) Sheltered gregarious behavior of Middle Ordovician harpetid trilobites. Palaios 29(9):495–500
Fatka O, Kozák V (2014) A new type of entombment of Peronopsis (Agnostida) in a hyolithid conch. Carnets De Géologie-Notebooks on Geology 14(10):191–198
Fatka O, Szabad M (2011) Agnostids entombed under exoskeletons of paradoxidid trilobites. Neues Jahrbuch Für Geologie Und Paläontologie-Abhandlungen 259:207–215
Fatka O, Szabad M, Budil P, Micka V (2008) Position of trilobites in Cambrian ecosystem: Preliminary remarks from the Barrandian region (Czechia). Adv Trilobite Res 9:117–121
Fatka O, Vokáč V, Moravec J, Šinágl M, Valent M (2009) Agnostids entombed in hyolith conchs. Memoirs of the Association of Australasian Palaeontologists 37:481–489
Fatka O, Budil P, Kraft P (2021) Sheltered preservation in Ordovician trilobites. Fossil Record 24(1):193–205
Fatka O, Budil P, Mikuláš R (2022) Healed injury in a nektobenthic trilobite: “octopus-like” predatory style in Middle Ordovician? Geol Cro 75(2):189–198
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30(9):1323–1341
Feist R (2001) Clustered trilobite assemblages formed under shelter: case studies of paleoecological behaviour. Third International Symposium on Trilobites and Their Relatives, Oxford, pp 12
Flick U, Flick H (2009) Unterkarbon-Trilobiten in Wohnkammern von Goniatiten-Fossilfalle oder Häutungsplatz. Der Aufschluss 60:245–250
Foerste AF (1910) Preliminary notes on Cincinnatian and Lexington fossils of Ohio, Indiana, Kentucky and Tennessee. Bull Sci Lab Denison Univ 16:17–100
Fraaije RHB, Pennings HWJ (2006) Crab carapaces preserved in nautiloid shells from the Upper Paleocene of Huesca: Pyrenees, Spain. Rev Mex Cienc Geol 23(3):361–363
Fraaije RHB, Jagt JWM, Van Bakel BWM, Tshudy DM (2020) A new early Late Cretaceous nephropid lobster (Crustacea, Decapoda) from Kazakhstan, entombed within an ammonite body chamber. Cretac Res 115:104552
Fraaye RHB, Jäger M (1995a) Ammonite inquilinism by fishes: examples from the Lower Jurassic of Germany and England. Neues Jahrbuch Für Paläontologie Monatshefte 9:541–541
Fraaye RHB, Jäger M (1995b) Decapods in ammonite shells: examples of inquilinism from the Jurassic of England and Germany. Palaeontology 38(1):63–76
Green J (1832) A monograph of the trilobites of North America: with coloured models of the species. J. Brano, Philadelphia, p 93, pl 1
Gürich G (1896) Das Palaeozoicum im Polnischen Mittelgebirge. Verhandlungen Der Russisch Kaiserlichen Mineralogischen Gesellschaft, St Petersburg 32:1–539
Gutiérrez-Marco JC, Sá AA, García-Bellido DC, Rábano I, Valério M (2009) Giant trilobites and trilobite clusters from the Ordovician of Portugal. Geology 37(5):443–446
Hall J, Whitfield RP (1875) Section I. Description of invertebrate fossils, mainly from the Silurian system. Geol Surv Ohio 2:65–110, p 111–114
Henningsmoen G (1975) Moulting in trilobites. Fossils Strata 4(1):179–200
Hewitt RA (1988) Nautiloid shell taphonomy: interpretations based on water pressure. Palaeogeogr Palaeoclimatol Palaeoecol 63(1):15–25
Hicks H (1875) On the succession of the ancient rocks in the vicinity of St. David’s, Pembrokeshire, with special reference to those of the Arenig and Llandeilo groups, and their fossil contents. Quart J Geol Soc 31(1–4):167–195
Hints L, Pärnaste H, Gailite L-I (2012) Hirnantia sagittifera (Brachiopoda) and Mucronaspis mucronata s.l. (Trilobita) in the Upper Ordovician of the East Baltic: taxonomy and distribution. Est J Earth Sci 61(2):65–81
Holm G (1886) Illaeniden. Revision der ostbaltischen silurischen Trilobiten von Fr. Schmidt, Abt 3. Illaeniden. Mémoires de lAcadémie Impériale des Sciences de St Petersbourg Series 7(33):1–173, p 171–112
Jaanusson V (1944) Übersicht der Stratigraphie der Lyckholm-Komplexstufe. Bull Geol Soc Finl 132:92–100
Jaanusson V (1956) Untersuchungen über den oberordovizischen Lyckholm-Stufenkomplex in Estland. Bull Geol Inst Uppsala 36:369–401
Klompmaker AA, Fraaije RHB (2012) Animal behavior frozen in time: gregarious behavior of Early Jurassic lobsters within an ammonoid body chamber. PLoS ONE 7(3):e31893
Kordule V (2006) Ptychopariid trilobites in the Middle Cambrian of Central Bohemia (taxonomy, biostratigraphy, synecology). Bull Geosci 81(4):277–304
Kříž J (1992). Silurian field excursions: Prague Basin (Barrandian), Bohemia. National Museum of Wales, Cardiff, pp. 110.
Kröger B, Hints L, Lehnert O (2017) Ordovician reef and mound evolution: the Baltoscandian picture. Geol Mag 154(4):683–706
Krueger HH (1971) Encrinuriden aus ordovizischen Geschieben (Teil I). Geologie 20:1132–1169
Ladd HS (1929) The stratigraphy and paleontology of the Maquoketa Shale of Iowa, Pt. 1. Iowa Geol Surv Annu Rep 34(1):307–440
Landman NH, Fraaije RHB, Klofak SM, Larson NL, Bishop GA, Kruta I (2014) Inquilinism of a baculite by a dynomenid crab from the Upper Cretaceous of South Dakota. Am Mus Novit 2014(3818):1–16
Lindström G (1885) Förteckning på Gotlands Siluriska Crustacéer. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar 6:37–100, pls 12-16
Männil R (1958) Trilobites of the families Cheiruridae and Encrinuridae from Estonia. ENSV Teaduste Akadeemia Geoloogia Instituudi Uurimused 3:165–212
McNamara KJ, Rudkin DM (1984) Techniques of trilobite exuviation. Lethaia 17(2):153–173
Meek FB, Worthen AH (1875) Descriptions of invertebrates. Ill Geol Surv Geol Paleontol 6:491–532
Mikulic DG (1994) Sheltered molting by trilobites. Geological Society of America. Boulder, pp 55
Nyborg T, Ifrim C, Moreno-Bedmar JA, Muzquiz HP, Giersch S, Vega FJ (2014) Late Cretaeous fish cans: fish preserved in ammonite body chambers from the middle Santonian of Coahuila State, northeastern Mexico. Neues Jahrbuch Für Geologie Und Paläontologie-Abhandlungen 273(1):75–88
Owen AW (1985) Trilobite abnormalities. Trans R Soc Edinb: Earth Sci 76(2–3):255–272
Paterson JR, Hughes NC, Chatterton BDE (2008) Trilobite clusters: what do they tell us? A preliminary investigation. Adv Trilobite Res 9:313–318
Pedder AEH, Feist R (1998) Lower Devonian (Emsian) Rugosa of the Izarne Formation, Montagne Noire, France. J Paleontol 76:967–991
Pereira S, Pires M, Marques Guedes A, Silva CMd & Sá AA (2015). Sheltered preservation of Upper Ordovician harpetid trilobites from Portugal. Jornadas de Paleontologia Sociedad Española de Paleontología: 230–232.
Pompeckj JF (1895) Die Fauna des Cambrium von Tejřovic und Skrej in Böhmen. Jahrbuch Der Kaiserlich Königlichen Geologischen Reichsanstalt 45:495–614
Popp A, Pärnaste H (2011) Biometry and lifestyle of the Ordovician proetide trilobite Cyamella stensioei Owens, 1979. GFF 133(3–4):111–123
Přibyl A (1950) On the Carboniferous trilobites of Moravia-Silesia. Bulletin International De L’académie Tchèque Des Sciences 51(24):209–232
Přibyl A, Vaněk J (1965) Neue trilobiten des böhmischen Ordoviziums. Věstník Ústředního Ústavu Geologického 40(4):277–282
Rakociński M (2009) Zjawisko „zachowania w ukryciu” skamieniałości—przegląd i przykłady z górnego dewonu Polski. Przegląd Geologiczny 57(7):584–590
Reed FRC (1920) Description of two trilobites. In: Gardiner CI (ed) The Silurian rocks of May Hill. Proceedings of the Cotteswold Naturalists’ Field Club 20(3):219–222
Richter R, Richter E (1933) Die letzten Phacopidae. Bulletin du Musée royal d’Histoire naturelle de Belgique 9(21):1–19, pl. 12
Roemer F (1861) Die fossile fauna der silurischen diluvial-geschiebe von Sadewitz bei Oels in Nieder-Schlesien: Eine palaeontologische monographie. Robert Nischkowsky, Breslau, p 81, pls 8
Rolfe S, Pieper S, Porto A, Diamond K, Winchester J, Shan S, Kirveslahti H, Boyer D, Summers A, Maga AM (2021) SlicerMorph: an open and extensible platform to retrieve, visualize and analyse 3D morphology. Methods Ecol Evol 12(10):1816–1825
Romano M (1975) Harpid tribolites from the Ordovician of North Portugal. Comunicações Service Geológicas Portugal 59:27–36
Rõõmusoks A (1998) Trilobites of the genus Toxochasmops from the Ordovician of Estonia. Proc Est Acad Sci 47:173–194
Rõõmusoks A (2000) The new trilobite genus Valdariops from the Harju Series (Upper Ordovician) of Estonia. Proc Est Acad Sci Geol 49:28–43
Rudkin DM (1985) Exoskeletal abnormalities in four trilobites. Can J Earth Sci 22(3):479–483
Sauramo M (1929) Zur Kenntnis der Geologie von Worms und Nuckö, Estland. Bull Geol Soc Finl 87:1–20
Schmidt F (1858). Untersuchungen über die Silurische Formation von Ehstland, Nord-Livland und Oesel. Archiv für die Naturkunde Liv-, Ehst- und Kurlands, 1 Serie (Mineralogische Wissenschaften, nebst Chemie, Physik und Erdbeschreibung) 2: 1–249
Schmidt F (1881) Revision der ostbaltischen silurischen Trilobiten, nebst geognostischer Übersicht des ostbaltischen Silurgebiets Abt 1. Phacopiden, Cheiruriden und Encrinuriden. Mémoires de l´Académie Impériale des Sciences de St Petersbourg Series 7 30(1):1–238, pls 16
Schmidt F (1894) Revision der Ostbaltischen Silurischen Trilobiten , Abt 4. Calymmeniden, Proetiden, Bronteiden, Harpediden, Trinucleiden, Remopleuriden und Agnostiden. Mémoires de l´Académie Impériale des Sciences de St Petersbourg Series 7 42(5):1–93, pls 6
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Smith PM, Holland T (2016) Cretaceous time capsules: remarkable preservation of fish and crustaceans inside the bivalve Inoceramus sutherlandi McCoy, 1865 from the Allaru Mudstone (late Albian), Eromanga Basin, Queensland. In: Laurie JR, Kruse PD, García-Bellido DC, Holmes JD (eds) Palaeo Down Under 2. Geological Society of Australia, Adelaide, pp 55–56
Šnajdr M (1990). Bohemian Trilobites. Geological Survey Prague, Prague.
Speyer SE (1985) Moulting in phacopid trilobites. Earth Environ Sci Trans R Soc Edinb 76(2–3):239–253
Suzuki Y, Bergström J (1999) Trilobite taphonomy and ecology in Upper Ordovician carbonate buildups in Dalarna, Sweden. Lethaia 32(2):159–172
Suzuki Y, Shiino Y, Bergström J (2009) Stratigraphy, carbonate facies and trilobite associations in the Hirnantian part of the Boda Limestone, Sweden. GFF 131(4):299–310
Torsvik TH & Cocks LRM (2009). BugPlates: linking biogeography and palaeogeography, http://www.geodynamics.no/Web/Content/Software.
Valent M, Fatka O, Micka V, Šinágl M (2008) Hyoliths with entombed trilobites-Cryptic behaviour of trilobites? In: Rábano I, Gozalo R, García-Bellido DC (eds) Advances in Trilobite Research. Instituto Geológico y Minero de España, Madrid, pp 411–413
Vermeij GJ (1987) Evolution and escalation: an ecological history of life. Princeton, Princeton University Press, p 544
Vokáč V, Hartl F, David M, Pavlovič M, Doubrava M, Kozák V, Grigar V (2015) Notable findings of trilobites from the Ordovician (Dapingian–Sandbian) of the Prague Basin (Barrandian area, Czech Republic). Erica 22:141–157
Vokáč V, Hartl F, Pavlovič M, Beneš P, Zicha O, Grigar L, Tichávek F, Henkl L (2019) Remarkable new findings of Ordovician (Darriwillian–Sandbian) trilobites in the southwest part of the Prague Basin (Rokycany region, Czech Republic). Erica 26:67–93
Volborth A (1864) Über einige neue ehstländische Illaenen. Mémoires de l´Académie Impériale des Sciences de St Petersbourg Series 7 8(9): 1–11, pl 1
Vullo R, Cavin L, Clochard V (2009) An ammonite–fish association from the Kimmeridgian (Upper Jurassic) of La Rochelle, western France. Lethaia 42(4):462–468
Wahlenberg G (1818) Petrificata telluris Svecanae. Nova Acta Regiae Societatis Scientiarum Upsaliensis 8:1–116, 293–296, pls 1–4, 7
Webster CL (1921) Notes on the genus Atrypa, with description of new species. Am Midl Nat 7(1):13–20
Whittington HB (1990) Articulation and exuviation in Cambrian trilobites. Philos Trans R Soc Lond B Biol Sci 329(1252):27–46
Zong R-W (2021) Abnormalities in early Paleozoic trilobites from central and eastern China. Palaeoworld 30:430–439
Zong R-W, Fan R-Y, Gong Y-M (2016) Seven 365-million-year-old trilobites moulting within a nautiloid conch. Sci Rep 6(1):34914
Zwanzig M & Liebermann S (2012). A Silurian Bohemoharpes twice used an empty shell of an orthocone nautiloid as refuge for moulting. The 5th Conference on Trilobites and their relatives, 1st–4th July 2012, Prague, Czech Republic, Czech geological Survey, Prague, pp 58
Acknowledgements
This research was supported by funding from a MAT Postdoctoral fellowship (to R.C.D.B.), the Lerner Gray Fund for Marine Research (to E.E.V.P.), and the National Science Foundation (award #1848145 to E.E.V.P). We also thank Morgan Hill Chase and Andrew Smith (AMNH Microscopy and Imaging Facility) for assistance in scanning specimens, Mare Isakar (University of Tartu Museum of Natural History) for assistance in collections, and Björn Kröger (Helsinki University) who kindly identified the cephalopod. Finally, we thank two anonymous reviewers for their comments that improved the manuscript and Matthias Waltert for editorial assistance.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Julien Denayer
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bicknell, R.D.C., Vargas-Parra, E.E., Landman, N.H. et al. Evidence for cryptic molting behavior in the trilobite Toxochasmops vormsiensis from the Upper Ordovician Katian Kõrgessaare Formation, Estonia. Sci Nat 111, 22 (2024). https://doi.org/10.1007/s00114-024-01906-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-024-01906-8