Abstract
Migration is used by many species as a strategy to deal with a seasonally changing environment. For some species, migration patterns can vary across different or even within the same breeding area. The Common Woodpigeon Columba palumbus, an abundant and widespread Palearctic species, exhibits three migratory strategies (strictly migratory, partially migratory and resident) across its European breeding grounds. Based on ring recoveries and satellite tracking data, we investigated the migration and foraging behaviour of Woodpigeons breeding in Southwestern Europe (Portugal) and Central Europe (Germany). We found that individuals could be classified as residents (Portugal) or partial migrants (Germany), with migrating individuals following the European sector of the East Atlantic flyway, and mainly wintering in France. In addition to general data on migration phenology, we provide evidence for different migration strategies (migration of varying distances or resident behaviour), low wintering site fidelity and the use of multiple wintering sites. Furthermore, tracking data provided information on migratory behaviour in consecutive years, clearly showing that individuals may switch migratory strategies (resident vs. migrant) between years, i.e. are facultative partial migrants. While individuals from Portugal mainly stayed within a large park (‘green urban area’) year-round, Woodpigeons from the city of Giessen (Germany) regularly left the urban area to forage on surrounding farmland (with an average distance covered of 5.7 km), particularly from July to September. Overall, our results highlight the behavioural plasticity in Woodpigeons in terms of foraging and migration strategies within and amongst individuals as well as populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal movements are performed in a variety of ways, ranging from daily foraging movements, one-way dispersal movement, nomadism, to seasonally predictable round-trip migratory movements (Shaw 2020). In some bird species, strictly migrant to strictly resident wintering behaviours can occur (Chambon et al. 2018). However, in others, within-population migratory dimorphism exists, with some individuals migrating between habitats whilst others remain resident in a single habitat, so-called partial migration (Chapman et al. 2011a). Whether individuals in a population migrate can be determined genetically, i.e. fixed across lifetime, or depend on condition (e.g. age, sex, and personality) and the environment (e.g. resources, temperature and predation) or on a combination of these factors. Moreover, migratory behaviour may change within an individual’s life (Lundberg 1987; Ogonowski and Conway 2009; Nilsson et al. 2016). For instance, individual Western Burrowing Owls Athene cunicularia hypugaea change their migratory tendency from one year to the next (Ogonowski and Conway 2009) and White-Winged Snowfinches Montifringilla nivalis nivalis adopt a partial migratory strategy, likely correlated to winter temperatures (Resano-Mayor et al. 2020).
The Common Woodpigeon Columba palumbus (henceforth Woodpigeon) is an abundant and widespread Palearctic bird species, which breeds throughout Europe (von Blotzheim and Bauer 1994; Saari 1997), where all of the aforementioned wintering behaviours appear. Woodpigeons breeding in Western Europe are mainly residents. Most Central European individuals are expected to be partial migrants, whereas populations from Eastern Europe and Fennoscandia are strictly migratory (Rouxel and Czajkowski 2004). For migrating Woodpigeons, three flyways in Europe were reported. Woodpigeons from Northern and Eastern Europe use the East Atlantic flyway, stretching along the coasts of the Baltic and North Seas to Atlantic coastal regions of South-West France and further to Spain and Portugal. Birds breeding in Hungary, the Czech Republic and Southern Germany use the Mediterranean flyway and winter in the West Mediterranean region, including Italy and Southern France, and particularly in Corsica and Sardinia. Individuals from South-Eastern Russia, Eastern Ukraine and birds breeding further east are likely to follow the Black Sea flyway (von Blotzheim and Bauer 1994; Bankovics 2001; Sruoga et al. 2005; Boere and Stroud 2006; Hobson et al. 2009; BirdLife International 2010; Butkauskas et al. 2013, 2019; Cavina et al. 2018).
Until recently, these migratory routes were mainly determined on the basis of available ring recovery data (Saari 1979; Bankovics 2001; Švažas 2001; Fiedler et al. 2004). However, ring recovery data are limited by poor recovery rates, particularly in Eastern and Northern Europe, and are biased due to different degrees of harvesting activity across Europe (Fiedler et al. 2004; Butkauskas et al. 2019). Furthermore, intensive ringing of Woodpigeons started only recently, in the last few decades (Negrier et al. 2020). Alternative approaches, such as hydrogen isotope analysis of feathers (Hobson et al. 2009) and genetic methods (Sruoga et al. 2005; Grosso et al. 2006; Butkauskas et al. 2013, 2019), were used to check for geographically-based divergence amongst Woodpigeon populations and to designate their flyways. Studies on genetics indicate a high genetic variability in Woodpigeons across Europe (Sruoga et al. 2005; Butkauskas et al. 2013, 2019), detecting the largest genetic distances amongst breeding Woodpigeons sampled in Central Europe and Portugal. Genetically similar were breeding individuals sampled in Germany and migratory Woodpigeons harvested in Northwest France (Sruoga et al. 2005). However, data from cytochrome b sequences did not support the existence of a geographically based divergence between populations (Grosso et al. 2006). Results from stable isotope analysis suggest that individuals harvested in Spain were primarily migrants from more northerly areas in Europe, and Woodpigeons taken in Corsica were from Eastern Europe (Hobson et al. 2009). While the aforementioned approaches provide important information on a population level, some questions, particularly on individual level, such as migration timing and routes, fidelity to wintering sites and wintering behaviour, remain outstanding.
Once a typical woodland species, inhabiting deciduous, mixed and coniferous forests, an increase in population size had resulted in an expansion to urban areas across the European breeding grounds (Tomiałojć 1976; Slater 2001; Bea et al. 2011; Schuster 2017). Currently, the Woodpigeon is one of the most common bird species in many European cities and towns (Bea et al. 2011; König et al. 2015; Sakhvon and Kövér 2020). Nevertheless, individuals from urban areas usually fly to agricultural areas to feed upon farmland, i.e. perform foraging movements outside the actual city area (Slater 2001). In temperate, seasonal environments, such as Germany, the non-breeding season, i.e. winter, is often characterized by a deterioration of abiotic factors, e.g. shorter day length, lower temperatures and lack of some food sources, which might promote migration to more benign areas (Nilsson et al. 2011). However, in some regions of Germany, winter records of Woodpigeons increased since the year 2000. Yet, it is not clear whether rising numbers of resident birds or an influx of migrating individuals from more northerly areas cause this observed increase, in particular as it is challenging to observe individual Woodpigeons due to their possible extensive activity range (Schuster 2017).
The present study aims to update and improve our knowledge on Woodpigeon migration strategy as well as foraging behaviour throughout the annual cycle by analysing and comparing (a) ringing recoveries, (b) Argos satellite tracks from individuals tagged during the winter in France and Portugal, as well as (c) GPS tracks from individuals tagged at their breeding sites in Germany and Portugal. Based on these three datasets, the following hypotheses were investigated:
-
1.
Woodpigeons breeding in Germany are partial migrants
-
2.
Migrating individuals from Central Europe follow the East Atlantic flyway
-
3.
Foraging movements and habitat use of Woodpigeons vary throughout the annual cycle and differ depending on the breeding region (Portugal vs. Germany)
Material and methods
Analysis of ring recoveries
The EURING Data Bank provided long-term ringing recoveries of Woodpigeons from Europe (EURING Data Bank extract 8th May 2020; du Feu et al. 2009). Recoveries of birds ringed from July 1929 until October 2019 were analysed (n = 11,842 recoveries). For the final analysis, only records of Woodpigeons ringed during the breeding season in Germany (defined as 01 April – 30 September; von Blotzheim and Bauer 1994) and recovered during the wintering season within Germany (01 November – 30 February; Fiedler et al. 2004) or in any other European country (01 October – 30 March; von Blotzheim and Bauer 1994) and individuals ringed during the wintering season at any location and recovered during the breeding season in Germany were selected. Records with a time span of more than 5 years between ringing and recapture and an accuracy of date worse than 6 weeks were discarded. This resulted in a final data set of 315 ring recoveries belonging to Woodpigeons with breeding sites in Germany. The individual records were visualized as straight lines via mapping in QGIS 2.18 (QGIS Development Team 2016).
To indicate the main migration corridor, an analysis of line density kernels of mark-recovery lines for individuals wintering outside of Germany (n = 216) was performed using the line density tool under Spatial Analyst in ArcGIS 10.7.1 (ESRI, Redlands California). Furthermore, kernel densities of ring recovery positions outside of Germany during the wintering time were calculated in R (R Core Team 2018) with the package ‘adehabitatHR’ (Calenge 2015) in order to illustrate main wintering sites. We used a generic grid of 100 cells and the bandwidth href (ad hoc method) as the smoothing parameter. No further analyses were conducted on the data set of Woodpigeons recovered within Germany during wintering time due to the low sample size of recovery records (n = 99).
Analysis of Argos data
Data from 12 Woodpigeons, equipped with Argos transmitters (PTT non-solar or solar tags, Microwave telemetry, Inc., USA) in France (n = 11) and Portugal (n = 1) during the wintering season, were analysed (Table 1). These 12 Woodpigeons, caught from 2003 to 2014, comprised a sub dataset of a larger project by GIFS France (Groupe d’Investigations sur la Faune Sauvage).
Argos transmitters deployed as backpacks were programmed with a duty cycle of 10 h ON/48 h OFF for 12 g transmitters and 10 h ON/24 h OFF for 18 g transmitters. Only location data as received from Argos of location classes (LC) 3, 2, and 1, which were afterwards checked for possible outliers manually (n = 29 locations removed), were used for analysis. The filtered locations were plotted in QGIS in their original projection (WGS84) and likely migration tracks were displayed by using the ‘Points2One’ plugin (Kapusta 2015). To determine the different phases in the annual cycle, i.e. breeding, migration, stopover and wintering, we used a similar approach as described in Lormée et al. (2016). Clear switches in the pattern of the location data together with movements of at least 100 km from the wintering or breeding site defined the onset of spring or autumn migration, respectively. A stopover site was defined as consecutive set of locations overlapping spatially for a minimum of 3 days during the migration period.
To estimate breeding and wintering site fidelity for individuals providing data for consecutive years (n = 5), repeatability of site utilization (based on longitude) was calculated as described in Lessells and Boag (1987). To avoid overrepresentation of individuals with multiple wintering sites, only the site occupied first was chosen for these birds. Migration phenology was specified by calculating the mean between all tracked individuals. If there was a time gap in the transmission of consecutive locations, the mean was selected and if the time gap was more than 14 days, the data were excluded from phenology analysis.
Analysis of GPS data
Between June 2018 and March 2021, we captured 21 Woodpigeons in Hesse, Germany (20 in the city of Giessen: 50°35′ N, 8°40′ E and one in the forest of Caldern: 50°50′ N, 8°39′ E). Most (n = 19) of these were captured during the breeding season (mid-March – August), and only two during winter (Table 2). Furthermore, 10 Woodpigeons were captured in Lisbon, Portugal (38°43′ N, 9°10′ W) prior to the migratory season (June – August, Table 2). We determined the age of each bird by plumage examination (Demongin 2016) and the sex by molecular analysis (Griffith et al. 1998, Table 2). Individuals were fitted with an OrniTrack-15 solar powered GPS-GSM/GPRS transmitter (Ornitela, Lithuania), fixed as a backpack using a 4-mm-width Teflon ribbon harness. OrniTrack-15 transmitters were programmed to take a GPS-position every 5 min if the battery was more than 75%, every 30 min (battery > 50%) and every 4 h (battery > 25%). No GPS-fixes were received during the night (GPS set to sleep from a sun angle of − 6° from dusk to dawn). GPS data were checked for erroneous locations by applying a speed filter to omit all data points exceeding 30 m/s (Bruderer and Boldt 2001). Migration tracks were displayed in QGIS in WGS 84 projection as described for Argos data. Breakdown in the different phases of the annual cycle, site fidelity and migration phenology were evaluated as delineated for the Argos tracking data.
Movements and habitat use
The habitat use and foraging movements between individuals of the cities Giessen, Germany (n = 19; #190758 individual from forest and #190759 individual from Herborn were excluded as they were not comparable due to the different types of occupied habitat) and Lisbon, Portugal (n = 10) were compared. Data from Argos transmitters were not considered for kernel utilization distributions (KUD) analysis due to partly large time gaps between consecutive localizations. To estimate the area used by the individual Woodpigeons, Epanechnikov kernels (95% and 50% KUD; Epanechnikov 1969) were calculated in R with the function kernelUD in the package ‘adehabitatHR’ (Calenge 2015) and the R package ‘sp Classes and Methods for Spatial Data’ (Pebesma 2020) with a generic grid of 100 cells (n = 370) or 500 cells (n = 49) and the smoothing parameter was estimated with a href parameter (ad hoc method). KUDs were calculated of GPS positions from wintering and breeding sites per month. Months for which localizations were only partially available (e.g. in month of capturing) were excluded. To characterize the land cover in the occupied home ranges (95% KUD), the KUDs were superimposed and subsequently clipped in QGIS with Corine Land Cover CLC 2018 v.2020_20u1 raster land cover data (Copernicus Land Monitoring Service 2021).
For Woodpigeons with breeding sites in the city of Giessen (n = 19), the distance travelled between city habitats and agricultural (foraging) sites outside the city was estimated by calculating the distance between the mean coordinates of the monthly 95% KUD polygon parts in the city and the agricultural area in R with the function distm in the ‘geosphere’ package (Hijmans et al. 2019). The mean coordinates were used also for circular statistics of foraging flight direction in Oriana 4 (Kovach Computing Services, Anglesey, Wales, https://www.kovcomp.co.uk/oriana/).
Results
Migratory behaviour and movements
Ring recoveries
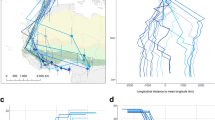
A 28% of the ring recoveries from Woodpigeons ringed in Germany during the breeding season and recovered during wintering period were found within Germany, while 72% outside Germany. Most recoveries out of Germany were from France (85%). Ring recoveries within Germany indicate resident or short-distance migrating Woodpigeons (Fig. S1). Migrating Woodpigeons leaving Germany wintered near the German border (Netherlands, Belgium, Denmark, East and South-East France) or flew greater distances to South-Western France, Spain and Portugal (Fig. 1).
Ring recoveries of Common Woodpigeons Columba palumbus with breeding sites in Germany spending the wintering period outside of Germany. a Validated ring recoveries are represented as a line between ringing and recovery site. Different colours correspond to the respective country in which the wintertime was spent. b Line density kernels for Woodpigeon ring recoveries. Kernel densities of ring recovery positions outside of Germany during the non-breeding period are displayed as dashed line (50% kernel) and dotted line (95% kernel). Background colours indicate the terrain (Background map: Stamen terrain (map tiles by Stamen Design: maps.stamen.com; data by OpenStreetMap: www.openstreetmap.org))
According to the ring recoveries, all individuals followed the western European part of the East Atlantic flyway. Line density kernels showed a south-westerly migration direction with the majority of Woodpigeons ending their migration in France (Fig. 1b). Density kernels of positions of ring recoveries during wintering time indicate South-Western France (regions: Occitanie and Nouvelle-Aquitaine) as the wintering region for the majority of migrating Woodpigeons with breeding sites in Germany. The majority of ring recoveries (90%) in all countries were due to hunting activities (Table S1).
Argos tracking data
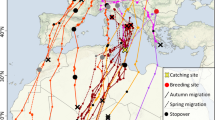
Woodpigeons equipped during the non-breeding season in France and Portugal (n = 12) departed for spring migration on average on 13 March (21 February − 21 March) and arrived at the breeding sites on 07 April (20 March – 14 May; Fig. 2, S2 and S3). On average, one stopover (range 0 – 4) lasting 8.9 ± 4.5 days (n = 20 stopovers) was made during spring migration. Breeding sites were located in the German federal states Bavaria (n = 4), Baden-Württemberg (n = 1), Rhineland-Palatinate (n = 1), Thuringia (n = 1), Lower Saxony (n = 1), North Rhine-Westphalia (n = 1) as well as in Switzerland (n = 1; Fig. S2).
Migratory movements of Common Woodpigeons Columba palumbus equipped with Argos-transmitters (n = 12) during the winter season in Portugal and France (dark blue lines and symbols) or during the breeding season with GPS-GSM transmitters (n = 4) in Hesse, Germany (pink lines and symbols). The map gives the spatial organization with spring migration (solid line) and autumn migration (dashed line) between the breeding sites (circles) and the winter sites (squares). The star symbol indicates that breeding and winter time in at least 1 year were spent at the same location. Crosses indicate that the last position was transmitted outside the breeding or winter site. The insets show the temporal organization with percentages of time for period spent at the breeding site (dark grey), the non-breeding site (light grey) and on migration (striped = spring migration; dotted = autumn migration) and average arrival and departure for each respective period. Background colours indicate the terrain (Background map: Stamen terrain (map tiles by Stamen Design: maps.stamen.com; data by OpenStreetMap: www.openstreetmap.org))
For five Woodpigeons, data transmission lasted beyond the first breeding season after transmitter deployment. Migrating individuals (n = 4) departed from the breeding sites on 11 October (26 September–18 October) and arrived at the wintering sites on 29 October (22 October–10 November) with 0.5 stopovers on average (range 0–2) which lasted 7.0 ± 0.0 days (n = 2).
In the tagging year, only individual #141869 (Table 1) used several distinct wintering sites (n = 3; Fig. S2). However, most individuals (83%) were tagged in February (Table 1), which is rather at the end of the wintering period; therefore, the use of multiple wintering sites in the year of tagging might be underestimated in the Argos data set. For migrating birds of which we have data for a second wintering period (n = 4), 75.0% used two distinct wintering sites (Fig. S2). We observed high repeatability of breeding site utilization (Longitude: r = 1.0, Anova: F3,4 = 162,009.0, p < 0.01), whereas a low repeatability of location for the wintering sites (Longitude: r = 0.008, Anova: F8,9 = 1.0, p = 0.484) between two consecutive years.
GPS tracking data
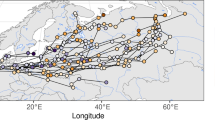
GPS tracking data revealed three migratory strategies of Woodpigeons (Table 2; Fig. S4). 4 out of 21 individuals tagged in Hesse performed migratory movements, spending the wintering time in France (regions: Grand-Est and Bourgogne-Franche Comté, Figs. 2 and S5). These 4 individuals started autumn migration on average on 28 October (20 October – 16 November) and reached the wintering sites on 24 November (15 November – 2 December) with an average migration time of 27.5 ± 8.9 days, including an average number of 3.0 stopovers (range 1 - 6) and total stopover duration of 20.5 ± 9.0 days. After wintering for 123.7 ± 6.8 days at 1 to 3 wintering sites, Woodpigeons started spring migration on 29 March (24 March – 9 April) and arrived after 11.0 ± 8.9 days of spring migration, including 0 to 1 stopover up to 15 days, on 9 April (1–24 April) at their breeding sites in Hesse.
Three further individuals (#190763, #190764 and #191391; Table 2) spent the wintertime in different sites than during the breeding season, though without leaving Germany. These wintering sites were approx. 10 km, 20 km and 40 km away from the breeding area. Woodpigeons reached them in less than one day (Fig. S6). One individual (#190759; Table 2) changed several times (n = 11) between two sites, however, not only during the winter period (Fig. S6), and this was not considered as migration. Migratory movements of Woodpigeons tagged in Hesse could be observed in the wintering seasons 2019/20 (5 of 12 individuals, 41.7%) and 2020/2021 (2 of 15 individuals, 13.3%), whereas no migratory movements were observed during the winter of 2018/19 (2 individuals). For migrating individuals from Hesse of which data were available for two consecutive years (n = 5, Table 3), repeated site utilization suggested a high breeding site fidelity (Longitude: r = 0.909, Anova: F4,5 = 42.9, p < 0.001), whereas wintering site fidelity was weak (Longitude: r = − 0.381, Anova: F3,4 = 0.4, p = 0.732).
None of the Woodpigeons tagged in Portugal (n = 10) exhibited migratory movements. However, two individuals (#191389 and #180783; Table 2) showed movements between sites approx. 20 km apart from each other throughout the annual cycle similar to one of the German birds (#190769; Table 2).
Habitat use and foraging movements of Woodpigeons from two European cities
According to GPS positions, the average size of Woodpigeons core area of use (50% KUD) was 0.7 ± 0.3 km2 or 1.0 ± 0.3 km2 and home range (95% KUD) was 4.2 ± 1.5 km2 or 7.5 ± 1.3 km2, for individuals from Lisbon and Giessen, respectively. Whereby monthly core areas and home ranges were mostly < 1.0 km2 (87% and 62%, respectively; Table S2, Figs. S7 and S8). In general, core areas as well as home ranges were larger for Woodpigeons in Giessen compared to individuals in Lisbon (Mann–Whitney: 50% KUD: W = 22072, p < 0.001; 95% KUD: W = 21645, p < 0.001).
Whereas the sizes of core area and home range varied across the annual cycle, i.e. was significantly different for the different months, for individuals in Giessen, it was not the case for Woodpigeons in Lisbon (Kruskal–Wallis: Giessen: 50% KUD: χ2 = 56.45, d.f. = 11, p < 0.001; 95% KUD: χ2 = 67.68, d.f. = 11, p < 0.001; Lisbon: 50% KUD: χ2 = 6.10, d.f. = 11, p = 0.866; 95% KUD: χ2 = 7.36, d.f. = 11, p = 0.770).
Woodpigeons in Lisbon mainly stayed within the ‘Parque Florestal de Monsanto’, an approximately 800 ha wooded park categorized as ‘green urban area’ by the CLC land cover data (Fig. 3), in which they were caught and tagged, leaving the park area only occasionally (Table S3). When they left the park area, their monthly home range was significantly larger (Mean 95% KUD: visits outside park: 26.3 km2, only inside park: 0.4 km2; Wilcoxon rank sum test: W = 16, p < 0.001).
Average proportions of land cover categories in monthly home ranges used by tagged Common Woodpigeons Columba palumbus. Shown are Woodpigeons from two regions (Lisbon, Portugal, n = 10 and Giessen, Germany, n = 19). The different wintering strategies of individuals from Giessen are shown separately for the wintering period: residents (no symbol), individuals using another distinct site during the winter than during the breeding season, but migratory movements occurred within Germany (black circle symbol), and Woodpigeons migrating to France (black triangle symbol). Categories occurring with < 1% were combined into ‘Others’. Land cover categories and associated colours were chosen according CORINE land cover (CLC) nomenclature. Black lines represent the average proportion of the land cover main categories ‘Artificial surfaces’ (continuous line) and ‘Agricultural areas’ (dashed line). Sample sizes and detailed proportions can be found in Table S3
Woodpigeons tagged in Giessen regularly left the city area to fly to agricultural areas/farmland located mainly south-westerly of the city, particularly between July and September (Figs. 3 and 4; Table S3), resulting in an enlarged home range size (mean 95% KUD: flights to farmland: 13.0 km2, only within city area: 0.7 km2; Wilcoxon rank sum test: W = 518, p < 0.001). The average distance travelled to the agricultural sites was 5.7 ± 0.2 km (maximum: 19.7 km).
Foraging behaviour of Common Woodpigeons Columba palumbus (n = 19) with breeding sites in the city of Giessen, Hesse (n = 19). Left: Circular diagram showing the proportion of individuals (0–100%) leaving the city area, i.e. artificial surfaces, to forage outside the city on agricultural used areas. Foraging outside was assumed if parts of the home range (95% Kernel Utilization Distributions KUD) were located on agricultural areas outside the city area. Right: The circular diagram represents the direction (geographic North N corresponds to 0°) and distance (0–20 km) Woodpigeons flew from the city of Giessen to foraging sites outside the city area. Each data point indicates the orientation of one individual bird for its monthly 95% KUD. Arrow indicates the mean direction (α) and vector length (r; * p < 0.001, Rayleigh test). Symbols used as in Fig. 3
Discussion
Foraging movements and habitat use
While forests were the original breeding habitat, breeding Woodpigeons are increasingly recorded in many European towns since the 1970s (Tomiałojć 1976; Sruoga et al. 2005; Bea et al. 2011). Typically, urban areas contain novel food items, such as non-native species and intentionally provisioned food. This can cause a diet shift (Murray et al. 2018), which in turn may also alter foraging behaviour. GPS data revealed differences in foraging movements and habitat use between individuals from Lisbon and Giessen and seasons. Birds in Lisbon rarely and seasonally independently left the ‘green urban area’, whilst individuals from Giessen regularly visited surrounding farmland (Figs. 3 and 4). This difference is also reflected in the habitat use: Whilst for individuals in Lisbon the proportion of ‘artificial surfaces’ barely varies throughout the year, it clearly decreases and is replaced by ‘agricultural areas’ in summer and early autumn for Woodpigeons in Giessen, resulting in an enlarged home range size.
Woodpigeons are granivorous-frugivorous with an opportunistic nature adapting their dietary choices according to (seasonal) food availability, resulting in significant variations of consumed items between seasons (Ó hUallachain and Dunne 2013; Gutiérrez-Galán et al. 2017; Negrier et al. 2020). Particularly during summer and beginning of autumn, previous studies pointed out grains of cereal crops as major part of the diet (Murton et al. 1964; Gutiérrez-Galán et al. 2017; Negrier et al. 2020). This is in line with our result showing that Woodpigeons from an urban population of Giessen undertook foraging trips to surrounding agricultural areas mainly from July to September (Fig. 4). Anthropogenic plant species provided at bird feeders were found in faecal samples of columbiform birds in the UK (Dunn et al. 2018) and Woodpigeons are nowadays regularly recorded at bird feeders (Reynolds et al. 2017; Darryl 2018). It is thus evident that Woodpigeons breeding in urban areas find part of their food, and in the case of individuals wintering in Giessen, the majority of their food in their urban areas (but see Tomiałojć 1999). However, comparing the two study sites, it is obvious that different foraging strategies exist: Woodpigeons in Lisbon appear to find their food almost exclusively within the urban park area throughout the year, whereas individuals in Giessen left the urban area to forage on farmland. The covered distance to reach the farmland feeding sites observed in this study (5.7 km averagely) is similar to previously observed distances in other locations (5–15 km: Wrocław, Tomiałojć 1999; min. 6 km: Liverpool, Slater 2001; > 10 km: Bejaia, Moali et al. 2003). The observed main foraging flight direction (south-westerly, Fig. 4) might be influenced by the regional distribution of farmland. However, farmland is surrounding the city of Giessen in various cardinal directions. The spatial directed foraging behaviour might be also caused by the gregarious feeding behaviour of Woodpigeons (Murton et al. 1966, 1971), as previous tracking data support a memory-based model with a flocking behaviour rather than an optimal foraging model as their foraging strategy (Kułakowska et al. 2014). Our results point to a distinct plasticity in foraging habits for ‘urban’ Woodpigeons, most likely adapted to different uses of foraging habitats as the productivity, i.e. available food, of these habitats changes over time (Bendjoudi et al. 2015), e.g. cereal ripening in July, and variable food supply in different cities (Rose et al. 2006), such as the proportion of green urban areas or distance to closest surrounding fields.
Resident or migrant species?
While the Woodpigeons tracked in Lisbon were definitely residents (as expected, see Sruoga et al. 2005), all three methods demonstrated that some Woodpigeons with breeding ground in Germany winter in Germany, whilst other individuals migrate along the East Atlantic flyway to mainly France and less frequently to Spain, Portugal, Belgium, Netherlands and Denmark (Figs. 1 and 2, Table 3). Tracking-based methods provide information on migratory behaviour of individuals for consecutive years, clearly showing that the individual based migratory decision can vary from year to year. Therefore, Woodpigeons with breeding sites in Germany can be classified as facultative partial migrants (Nilsson et al. 2016; Chambon et al. 2018), performing a non-breeding partial migration, i.e. sympatric breeding and allopatric wintering (Chapman et al. 2011a), with individuals switching migratory strategies (resident vs migrant) between years. This results in annually fluctuating numbers of migrating and resident individuals (e.g. our study: winter 2019/20: 42% vs. winter 2020/21: 13% migrating Woodpigeons).
Fluctuating numbers of Woodpigeons were also recorded at French, Spanish and Portuguese wintering sites (Beitia et al. 2001; Bea et al. 2003; Cohou et al. 2006, 2007; Lanusse et al. 2006; Lormée and Aubry 2018). It was hypothesized that the inter-annual fluctuations of migrants might occur due to a shift of their migratory route and/or wintering sites (Bea et al. 2003; Cohou et al. 2007). Such a shift to wintering sites to South-Western France was associated with the intensification of maize monoculture there (Lanusse et al. 2006). So far, there was no unambiguous data on the Woodpigeon site fidelity to certain wintering sites (Sruoga et al. 2005). Based on Argos and GPS tracking data, we provided evidence for a low wintering site fidelity, in contrast to being faithful to their breeding sites. Furthermore, the tracking data revealed the use of multiple wintering sites (Figs. S2 and S5), presumably following the availability and accessibility of food resources (Díaz and Martín 1998; Lanusse et al. 2006; Cohou 2013). Annually varying winter sites due to a low wintering site fidelity and exploitation of multiple wintering sites might explain fluctuating counts of Woodpigeons at wintering sites partly. Alternative hypotheses for the observed fluctuations in wintering Woodpigeons might be that the numbers are influenced by fluctuations in breeding success (cf. Robillard et al. 2016) or that due to warmer winters previous migrants may now winter at their breeding sites or only perform shorter-distance migration movements (Hobson et al. 2009; Butkauskas et al. 2019). Migratory movements of only around 10 km to 40 km, leaving the city area to winter in close wood- and farmland, were observed for three individuals in our study (Fig. S6). Interestingly, independent of migration distance (> 100 km outside of Germany vs. < 50 km within Germany), the onset of autumn migration was quite similar (average 11 November and 12 November, respectively), whereas the individuals wintering outside Germany arrived almost 1 month later (08 April) at their breeding sites compared to the migrants wintering closer to their breeding site (09 March; Fig. S4). Wintering closer to the breeding sites might constitute an intermediate tactic between more distant migration and residence, minimizing the disadvantage of migrants in competition over high-quality territories due to later arrival at the breeding sites (see ‘arrival time’ hypothesis in Chapman et al. 2011a).
Most individuals with breeding sites in the city of Giessen spent the winter season mainly within the urban area without exhibiting any migratory movements (Table 2, Figs. 3 and S4). Generally, urbanization may affect individual migration strategies, favouring resident behaviour, because urban areas are characterized by large and predictable anthropogenic food resources and due to the urban heat island effect are warmer than rural areas (Evans et al. 2012; Jokimäki and Kaisanlahti-Jokimäki 2012; Jokimäki et al. 2016; Bonnet-Lebrun et al. 2020).
Observed plasticity in individual migratory decisions of Woodpigeons suggests that migratory strategy is unlikely to be strictly and solely genetically fixed (see also Ogonowski and Conway 2009; Lundblad and Conway 2020). However, for the data examined here, the question why some individuals migrate and others do not within the same population and even same city still remains. A multi-taxa meta-analysis found consistently higher fitness of residents over migrants in birds (Buchan et al. 2020, but see Zúñiga et al. 2017). However, further exploration of the effects on fitness in terms of survival and reproductive outcome dependent on the chosen wintering tactic and vice versa would be helpful to evaluate the differences between the migratory strategies (Chambon et al. 2018; Buchan et al. 2020).
Conclusion and outlook
Our study provides the first tracking data of Woodpigeons in Europe for consecutive years, revealing pronounced plasticity in intra-species and intra-individual migration and foraging behaviour. In this way, our results add to the body of evidence that migratory movements in partial migratory birds are not solely a genetically fixed behaviour as they can change from year to year. The observed individual and within-species variation in migratory decision might be influenced by numerous factors and their interactions (reviewed in Chapman et al. 2011a and Hegemann et al. 2019) such as varying (local) food supply, e.g. mast seeding of oaks or beeches (Nilsson et al. 2006; Selås 2017), climatic conditions like winter temperature or snow cover (Mulsow 1979; Resano-Mayor et al. 2020) or individual traits (Chapman et al. 2011b; Fudickar et al. 2013). However, the small sample size available for migrating individuals precluded rigorous statistical comparison and modelling. In general, studying species with a plastic migratory behaviour can lend insight into the intrinsic and extrinsic factors that mediate this decision and how animals respond to environmental dynamics in terms of migration and, hence, gain insight into the evolution of which mechanisms underlie migratory behaviour more generally (Berthold 1999; Bowlin et al. 2010; Bonnet-Lebrun et al. 2020; Lundblad and Conway 2020).
Future studies tracking individuals year-round and over several years, tying migratory decisions to measures of individual fitness and environmental parameters but also to sex and age class and examining effects that carry-over across different stages of the annual cycle will help to understand the proximate and ultimate drivers and consequences of migratory decisions (cf. Lundblad and Conway 2020). In particular, in the framework of ongoing climate change, predicted to have profound effects on migrants (Berthold 1999; Bonnet-Lebrun et al. 2020), and increasing urbanization, which both may interact in their effects on the birds (Greig et al. 2017), studying species with pronounced variation in migratory behaviour, such as Woodpigeons, might be particularly valuable.
Data availability
Tracking data of GPS-GSM transmitters is archived on movebank.org (Movebank ID: 746410443 and 897868497) and available upon request. Tracking data of Argos transmitters need to be requested from VC. The EURING Data Bank provided the dataset of ringing recoveries of Woodpigeons (European Union for Bird Ringing: https://euring.org/).
Change history
07 October 2022
Missing Open Access funding information has been added in the Funding Note.
References
Bankovics A (2001) The migration of Wood Pigeon (Columba palumbus) and Turtle Dove (Streptopelia turtur) in Hungary. Naturzale 16:83–93
Bea A, Beitia R, Fernández JM (2003) The census and distribution of wintering woodpigeons Columba palumbus in the Iberian peninsula. Ornis Hungarica 12-13:157-167
Bea A, Svazas S, Grishanov G, Kozulin A, Stanevicius V, Astafieva T, Olano I, Raudonikis L, Butkauskas D, Sruoga A (2011) Woodland and urban populations of the woodpigeon Columba palumbus in the Eastern Baltic region. Ardeola 58:315-321. https://doi.org/10.13157/arla.58.2.2011.315
Beitia R, Daguerre L, Cloute M-L (2001) Observation of the trans-pyrenean migration of Wood Pigeons in 1999/2000. Naturzale 16:13–30
Bendjoudi D, Voison J-F, Doumandji S, Merabet A, Benyounes N, Chenchouni H (2015) Rapid increase in numbers and change of land-use in two expanding Columbidae species (Columba palumbus and Streptopelia decaocto) in Algeria. Avian Res 6:18. https://doi.org/10.1186/s40657-015-0027-9
Berthold P (1999) A comprehensive theory for the evolution, control and adaptability of avian migration. Ostrich 70:1–11. https://doi.org/10.1080/00306525.1999.9639744
BirdLife International. 2010. The flyways concept can help coordinate global efforts to conserve migratory birds. Downloaded from http://datazone.birdlife.org/sowb/casestudy/the-flyways-concept-can-help-coordinate-global-efforts-to-conserve-migratory-birds. Accessed 04 Nov 2021
Boere GC, Stroud DA (2006) The flyway concept: what it is and what it isn’t. In: Boere GC, Galbraith CA, Stroud DA (eds) Conference: Waterbirds around the world. Edinburgh, UK, The Stationery Office, pp 40–47
Bonnet-Lebrun A-S, Manica A, Rodrigues ASL (2020) Effects of urbanization on bird migration. Biol Conserv 244:108423. https://doi.org/10.1016/j.biocon.2020.108423
Bowlin MS, Bisson I-A, Shamoun-Baranes J, Reichard JD, Sapir N, Marra PP, Kunz TH, Wilcove DS, Hedenström A, Guglielmo CG, Åkesson S, Ramenofsky M, Wikelski M (2010) Grand challenges in migration biology. Integr Comp Biol 50:261–279. https://doi.org/10.1093/icb/icq013
Bruderer B, Boldt A (2001) Flight characteristics of birds: I. radar measurements of speeds. Ibis 143:178–204. https://doi.org/10.1111/j.1474-919X.2001.tb04475.x
Buchan C, Gilroy JJ, Catry I, Franco AMA (2020) Fitness consequences of different migratory strategies in partially migratory populations: a multi-taxa meta-analysis. J Anim Ecol 89:678–690. https://doi.org/10.1111/1365-2656.13155
Butkauskas D, Švažas S, Sruoga A, Bea A, Grishanov G, Kozulin A, Olano I, Stanevičius V, Tubelytė V, Ragauskas A (2013) Genetic techniques for designation of main flyways of the woodpigeon (Columba palumbus) in Europe as a tool for control and prevention of pathogenic diseases. Vet Med Zoot 63:12–16
Butkauskas D, Švažas S, Bea A, Prakas P, Olano I, Grishanov G, Mischenko A, Kozulin A, Stanevičius V, Báldi A, Huysentruyt F, Vaitkuvienė D, Red’kin Y (2019) Designation of flyways and genetic structure of Woodpigeon Columba palumbus in Europe and Morocco. Eur J Wildl Res 65:91. https://doi.org/10.1007/s10344-019-1336-9
Calenge C (2015) Home range estimation in R: the adehabitatHR Package. https://cran.r-project.org/web/packages/adehabitatHR
Cavina E, Bucchi R, Busse P (2018) The general pattern of seasonal dynamics of the autumn migration of the Wood Pigeon Columba palumbus in Italy. Ring 40:3–18. https://doi.org/10.1515/ring-2018-0001
Chambon R, Dugravot S, Paillisson J-M, Lemesle J-C, Ysnel F, Gélinaud G (2018) Partial migration in inexperienced pied avocets Recurvirostra avosetta: distribution pattern and correlates. J Avian Biol 49:e01549. https://doi.org/10.1111/jav.01549
Chapman BB, Brönmark C, Nilsson J-Å, Hansson L-A (2011) The ecology and evolution of partial migration. Oikos 120:1764–1775. https://doi.org/10.1111/j.1600-0706.2011.20131.x
Chapman BB, Hulthén K, Blomqvist DR, Hansson LA, Nilsson J-Å, Brodersen J, Nilsson PA, Skov C, Brönmark C (2011) To boldly go: individual differences in boldness influence migratory tendency. Ecol Lett 14:871–876. https://doi.org/10.1111/j.1461-0248.2011.01648.x
Cohou V, Beitia R, Mourguiart P, Veiga J (2006) Nouvelles données sur la migration post-nuptiale transpyrénéenne du Pigeon ramier Période 1999–2004. Faune Sauvage 273:14–18
Cohou V, Lanusse D, Mourguiart P, Recarte J, Veiga J, Wemo J (2007) Le pigeon ramier et son état de conservation dans le Sud-Ouest de la France: bilan de six années de suivi. Faune Sauvage 276:10–14
Cohou V (2013) Spatio-temporal tracking of common wood pigeons. Argos Forum 77:12–13
Copernicus Land Monitoring Service (2021) Corine Land Cover (CLC) 2018, Version 2020_20u1. https://land.copernicus.eu/pan-european/corine-land-cover/clc2018. Accessed 29 Jan 2021
Darryl J (2018) The birds at my table: why we feed wild birds and why it matters. Cornell University Press, New York
Demongin L (2016) Identification guide to birds in the hand. Privately published, Beauregard-Vendon, France
Díaz M, Martín P (1998) Habitat selectivity in wintering wood pigeons (Columba palumbus) in holm oak dehesas of Central Spain. Faune Sauvage 15:167–181
du Feu CR, Joys AC, Clark JA, Fiedler W, Downie IS, van Noordwijk AJ, Spina F, Wassenaar R, Baillie SR (2009) EURING Data Bank geographical index 2009. http://www.euring.org/edb. Accessed 08.05.2020
Dunn JC, Stockdale JE, Moorhouse-Gann RJ, McCubbin A, Hipperson H, Morris AJ, Grice PV, Symondson WOC (2018) The decline of the Turtle Dove: dietary associations with body condition and competition with other columbids analysed using high-throughput sequencing. Mol Ecol 27:3386–3407. https://doi.org/10.1111/mec.14766
Epanechnikov VA (1969) Non-parametric estimation of a multivariate probability density. Theory Probab Appl 14:153–158. https://doi.org/10.1137/1114019
Evans KL, Newton J, Gaston KJ, Sharp SP, McGowan A, Hatchwell BJ (2012) Colonisation of urban environments is associated with reduced migratory behaviour, facilitating divergence from ancestral populations. Oikos 121:634–640. https://doi.org/10.1111/j.1600-0706.2011.19722.x
Fiedler W, Bairlein F, Köppen U (2004) Using large-scale data from ringed birds for the investigation of effects of climate change on migrating birds: pitfalls and prospects. Adv Ecol Res 35:49–67. https://doi.org/10.1016/S0065-2504(04)35003-8
Fudickar AM, Schmidt A, Hau M, Quetting M, Partecke J (2013) Female-biased obligate strategies in a partially migratory population. J Anim Ecol 82:863–871. https://doi.org/10.1111/1365-2656.12052
Greig EI, Wood EM, Bonter DN (2017) Winter range expansion of a hummingbird is associated with urbanization and supplementary feeding. Proc R Soc B 284:20170256. https://doi.org/10.1098/rspb.2017.0256
Griffith R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x
Grosso AR, Bastos-Silveira C, Coelho MM, Dias D (2006) Columba palumbus Cyt b-like Numt sequence: comparison with functional homologue and the use of universal primers. Folia Zool 55:131–144
Gutiérrez-Galán A, Alonso González C, Maroto de Mercado J (2017) Woodpigeon Columba palumbus diet compositions in mediterranean Southern Spain. Ardeola 64:17–30. https://doi.org/10.13157/arla.64.1.2017.ra2
Hegemann A, Fudickar AM, Nilsson J-Å (2019) A physiological perspective on the ecology and evolution of partial migration. J Ornithol 160:893–905. https://doi.org/10.1007/s10336-019-01648-9
Hijmans RJ, Williams E, Vennes C (2019) Package ‘geosphere’. https://CRAN.R-project.org/package=geosphere
Hobson K, Lormée H, van Wilgenburg S, Wassenaar L, Boutin J (2009) Stable isotopes (D) delineate the origins and migratory connectivity of harvested animals: the case of European Woodpigeons. J Appl Ecol 46:572–581. https://doi.org/10.1111/j.1365-2664.2009.01651.x
Jokimäki J, Kaisanlahti-Jokimäki ML (2012) Residential areas support overwintering possibilities of most species. Ann Zool Fennici 49:240–256. https://doi.org/10.5735/086.049.0404
Jokimäki J, Suhonen J, Jokimäki-Kaisanlathi M-L, Carbó-Ramírez P (2016) Effects of urbanization on breeding birds in European towns: Impacts of species traits. Urban Ecosyst 19:1565–1577. https://doi.org/10.1007/s11252-014-0423-7
Kapusta P (2015) Points2One. QGIS Plugin. https://plugins.qgis.org/plugins/points2one/
König C, Stübing S, Wahl J (2015) Vögel in Deutschland aktuell: Herbst 2014: Rotmilane, Ringeltauben und Rotfußfalken. Der Falke 62:30–35
Kułakowska KA, Kułakowski TM, Inglis IR, Smith GC, Haynes PJ, Prosser P, Thorbek P, Sibly RM (2014) Using an individual-based model to select among alternative foraging strategies of woodpigeons: Data support a memory-based model with a flocking mechanism. Ecol Model 280:89–101. https://doi.org/10.1016/j.ecolmodel.2013.09.019
Lanusse D, Allou J, Bellot F, Sabathé F, Cohou V, Mourguiart P, Robin E, Werno J (2006) L’hivernage du Pigeon ramier dans le Sud-Ouest de la France. Faune Sauvage 273:19–23
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121. https://doi.org/10.2307/4087240
Lormée H, Boutin JM, Pinaud D, Bidault H, Eraud C (2016) Turtle Dove Streptopelia turtur migration routes and wintering areas revealed using satellite telemetry. Bird Study 63:425–429. https://doi.org/10.1080/00063657.2016.1185086
Lormée H, Aubry P (2018) Estimation des tableaux de chasse de colombidés en France pour la saison 2013–2014. Faune Sauvage 318:15–22
Lundblad CG, Conway CJ (2020) Testing four hypotheses to explain partial migration: balancing reproductive benefits with limits to fasting endurance. Behav Ecol Sociobiol 74:26. https://doi.org/10.1007/s00265-019-2796-3
Lundberg P (1987) Partial bird migration and evolutionary stable strategies. J Theor Biol 125:351–360. https://doi.org/10.1016/S0022-5193(87)80067-X
Moali A, Moali-Grine N, Fellous A, Isenmann P (2003) Expansion spatiale de la Tourterelle turque Streptopelia decaocto et présence dans les parcs urbains du Pigeon ramier Columba palumbus en Algérie. Alauda 71:371-374
Mulsow R (1979) Ringeltaube (Columba palumbus L.) – Populationsökologische Untersuchungen im Raum Hamburg. Hamb Avifaun Breitr 16:25–42
Murray MH, Kidd AD, Curry SE, Hepinstall-Cymerman J, Yabsley MJ, Adams HC, Ellison T, Welch CN, Hernandez SM (2018) From wetland specialist to hand-fed generalist: shifts in diet and condition with provisioning for a recently urbanized wading bird. Phil Trans R Soc B 373:20170100. https://doi.org/10.1098/rstb.2017.0100
Murton RK, Westwood NJ, Isaacson A (1964) The feeding habits of the woodpigeon Columba palumbus, Stock Dove C. oenas and Turtle Dove Streptopelia turtur. Ibis 106:174–188. https://doi.org/10.1111/j.1474-919X.1964.tb03694.x
Murton R, Isaacson A, Westwood N (1966) The relationships between wood-pigeons and their clover food supply and the mechanism of population control. J Appl Ecol 3:55–96. https://doi.org/10.2307/2401666
Murton R, Isaacson A, Westwood N (1971) The significance of gregarious feeding behaviour and adrenal stress in a population of wood-pigeons Columba palumbus. J Zool 165:53–84. https://doi.org/10.1111/j.1469-7998.1971.tb02176.x
Negrier C, Fantinati M, Jouglar J-Y, Lyazrhi F, Cohou V, Priymenko N (2020) Dietary regimen of the woodpigeon (Columba palumbus). J Anim Physiol Anim Nutr 00:1–9. https://doi.org/10.1111/jpn.13409
Nilsson ALK, Lindström Å, Jonzén N, Nilsson SG, Karlsson L (2006) The effect of climate change on partial migration—the blue tit paradox. Glob Change Biol 12:2014–2022. https://doi.org/10.1111/j.1365-2486.2006.01237.x
Nilsson ALK, Nilsson JÅ, Alerstam T (2011) Basal metabolic rate and energetic cost of thermoregulation among migratory and resident blue tits. Oikos 120:1784–1789. https://doi.org/10.1111/j.1600-0706.2011.19440.x
Nilsson ALK, Nilsson JÅ, Mettke-Hofmann C (2016) Energy reserves, information need and a pinch of personality determine decision-making on route in partially migratory blue tits. PLoS ONE 11:e0163213. https://doi.org/10.1371/journal.pone.0163213
Ogonowski MS, Conway CJ (2009) Migratory decisions in birds: extent of genetic versus environmental control. Oecologia 161:199–207. https://doi.org/10.1007/s00442-009-1356-3
Ó hUallachain D, Dunne J (2013) Seasonal variation in the diet and food preference of the Woodpigeon Columba palumbus in Ireland. Bird Study 60:417–422. https://doi.org/10.1080/00063657.2013.798259
Pebesma E (2020) sp Classes and Methods for Spatial Data v1.4–1. https://www.rdocumentation.org/packages/sp
QGIS Development Team (2016) QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.org
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Resano-Mayor J, Bettega C, Delgado MdM, Fernández-Martín Á, Hernández-Gómez S, Toranzo I, España A, Gabriel Md, Roa-Alvarez I, Gil JA, Strinella E, Hobson KA, Arlettaz R (2020) Partial migration of White-winged snowfinches is correlated with winter weather conditions. Glob Ecol Conserv 24:e011346. https://doi.org/10.1016/j.gecco.2020.e01346
Reynolds SJ, Galbraith JA, Smith JA, Jones DN (2017) Garden bird feeding: insights and prospects from a North-South comparison of this global urban phenomenon. Front Ecol Evol 5:24. https://doi.org/10.3389/fevo.2017.00024
Robillard A, Therrien JF, Gauthier G, Clark KM, Bêty J (2016) Pulsed resources at tundra breeding sites affect winter irruptions at temperate latitudes of a top predator, the snowy owl. Oecologia 181:423–433. https://doi.org/10.1007/s00442-016-3588-3
Rose E, Nagel P, Haag-Wackernagel D (2006) Spatio-temporal use of the urban habitat by feral pigeons (Columba livia). Behav Ecol Sociobiol 60:242–254. https://doi.org/10.1007/s00265-006-0162-8
Rouxel R, Czajkowski A (2004) Le Pigeon Ramier (Columba palumbus). Societe de Presse Adour-Pyrenees, Lourdes, France
Saari L (1979) Ring recoveries of Finnish Woodpigeons (Columba palumbus) and Stock Doves (C. oenas). Finn Game Res 38:17–30
Saari L (1997) Woodpigeon. In: Hagemeijer W, Blair M (Eds.) The EBCC Atlas of European Breeding Birds: Their Distribution and Abundance. Poyser, London, UK, pp 384–385
Sakhvon V, Kövér L (2020) Distribution and habitat preferences of the urban Woodpigeon (Columba palumbus) in the north-eastern breeding range in Belarus. J Urban Landsc Plan 201:103846. https://doi.org/10.1016/j.landurbplan.2020.103846
Schuster S (2017) Verhaltensänderungen bei Ringeltauben Columba palumbus im Voralpenraum. Ornithol Jh Bad-Württ 33:71–80
Selås V (2017) Autumn irruptions of Eurasian Jay (Garrulus glandarius) in Norway in relation to acorn production and weather. Ornis Fennica 94:92–100
Shaw AK (2020) Causes and consequences of individual variation in animal movement. Mov Ecol 8:12. https://doi.org/10.1186/s40462-020-0197-x
Slater P (2001) Breeding ecology of a suburban population of Woodpigeons Columba palumbus in northwest England. Bird Study 48:361–366. https://doi.org/10.1080/00063650109461235
Sruoga A, Butkauskas D, Švažas S, Bea A, Mozalienė E (2005) Identification of flyways of Woodpigeon (Columba palumbus) in Europe by using genetic methods. Acta Zool Litu 15:248–253. https://doi.org/10.1080/13921657.2005.10512618
Švažas S (2001) Population status of pigeons and doves in the Eastern Baltic Region. Naturzale 16:71–81
Tomiałojć L (1976) The urban population of the Woodpigeon Columba palumbus Linnaeus, 1758, in Europe - Its origin, increase and distribution. Acta Zool Crac 18:585–632
Tomiałojć L (1999) A long-term study on changing predation impact on breeding woodpigeons. In: Cowand DP, Feare CJ (eds) Advances in vertebrate pest management. Filander Verlag, Fürth, Germany, pp 205–218
von Blotzheim UG, Bauer KM (1994) Handbuch Der Vögel Mitteleuropas, vol 9. Columbiformes-Piciformes. Aula-Verlag, Wiesbaden, Germany, pp 64–97
Zúñiga D, Gager Y, Kokko H, Fudickar AM, Schmidt A, Naef-Daenzer B, Wikelski M, Partecke J (2017) Migration confers winter survival benefits in a partially migratory songbird. eLife 6:e28123. https://doi.org/10.7554/eLife.28123
Acknowledgements
We are grateful to the European Union for Bird Ringing (EURING) that made the recovery data available through the EURING Data Bank and to the ringers and ringing scheme staff who have gathered and prepared the data. We appreciate amongst others Marie Claire Gatt, Lennart Wegner, Jennifer Greiner, Maya Ehmig and Franziska Schmidt for providing support during the fieldwork. We acknowledge funding from the German Ornithologists’ Society (DO-G) and the Hessen State Ministry for Higher Education, Research and the Arts, Germany, as part of the LOEWE priority project Nature 4.0—Sensing Biodiversity.
Funding
Open Access funding enabled and organized by Projekt DEAL. GPS transmitters were funded by a research grant to YRS by the German Ornithologists’ Society (DO-G) and the LOEWE priority project Nature 4.0. JFM and SR were funded by the Hessen State Ministry for Higher Education, Research and the Arts, Germany, as part of the LOEWE priority project Nature 4.0 – Sensing Biodiversity.
Author information
Authors and Affiliations
Contributions
Yvonne R. Schumm, Petra Quillfeldt and Juan F. Masello contributed to the study conception and Yvonne R. Schumm analysed the data. All authors performed fieldwork, material preparation and data collection. The first draft of the manuscript was written by Yvonne R. Schumm and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. Animal handling, including an ethical approval according to the national animal protection laws, was carried out under permits of the Regierungspräsidium Gießen (permit numbers G 51/2017 and G 10/2019), the Instituto da Conservação da Natureza e das Florestas (permit numbers 933/2018/CAPT, 15/2019/CAPT and 23/2020/CAPT) and the Centre de Recherches sur la Biologie des Populations d'Oiseaux (CRBPO; program number 392).
Conflict of interests
The authors declare no competing interests.
Additional information
Communicated by: Matthias Waltert
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schumm, Y.R., Masello, J.F., Cohou, V. et al. Should I stay or should I fly? Migration phenology, individual-based migration decision and seasonal changes in foraging behaviour of Common Woodpigeons. Sci Nat 109, 44 (2022). https://doi.org/10.1007/s00114-022-01812-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-022-01812-x