Abstract
A well-developed suction pump in the head represents an important adaptation for nectar-feeding insects, such as Hymenoptera, Lepidoptera and Diptera. This pumping organ creates a pressure gradient along the proboscis, which is responsible for nectar uptake. The extremely elongated proboscis of the genus Prosoeca (Nemestrinidae) evolved as an adaptation to feeding from long, tubular flowers. According to the functional constraint hypothesis, nectar uptake through a disproportionately elongated, straw-like proboscis increases flower handling time and consequently lowers the energy intake rate. Due to the conspicuous length variation of the proboscis of Prosoeca, individuals with longer proboscides are hypothesised to have longer handling times. To test this hypothesis, we used field video analyses of flower-visiting behaviour, detailed examinations of the suction pump morphology and correlations of proboscis length with body length and suction pump dimensions. Using a biomechanical framework described for nectar-feeding Lepidoptera in relation to proboscis length and suction pump musculature, we describe and contrast the system in long-proboscid flies. Flies with longer proboscides spent significantly more time drinking from flowers. In addition, proboscis length and body length showed a positive allometric relationship. Furthermore, adaptations of the suction pump included an allometric relationship between proboscis length and suction pump muscle volume and a combination of two pumping organs. Overall, the study gives detailed insight into the adaptations required for long-proboscid nectar feeding, and comparisons with other nectar-sucking insects allow further considerations of the evolution of the suction pump in insects with sucking mouthparts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flower-visiting animals, such as insects, birds and bats, show convergence in specialised and elongated mouthpart adaptations for nectar feeding (Johnson and Anderson 2010; Muchhala and Thomson 2009; Pellmyr 2002). Such specialisations particularly often occur among nectar-feeding, holometabolous insects (Krenn et al. 2005). Nectar offers insects a readily available and often abundant, carbohydrate-rich resource (Nicolson 2007). Several morphological adaptations are, however, needed for efficient nectar uptake and include elaborated sensory organs (Krenn et al. 2005), advanced flight capabilities (Dudley 2000) and a modified feeding apparatus that consists of both sucking mouthparts and pumping organs in the head (Borrell and Krenn 2006). A suction pump situated in the head of nectar-feeding insects creates a pressure gradient along the tubular proboscis that enables them to rapidly suck up nectar from flowers (Eberhard and Krenn 2005; Davis and Hildebrand 2006; Borrell and Krenn 2006; Kim et al. 2011a). Whilst the nectar with the highest sugar concentration would clearly offer the greatest energy reward, biophysical reasoning indicates that the exponential increase of viscosity with higher sugar concentrations would make it more difficult to transport sugar-rich liquids through a narrow food canal (Kim et al. 2011b; Kingsolver and Daniel 1979). However, the optimal sugar concentration appears to be independent of size and shape of the proboscis, with insects selecting nectar that optimises their energy gain. Various studies have shown that the optimal concentration of sucrose for butterflies is approximately 35–40 % (Daniel et al. 1989; Pivnick and McNeil 1985; May 1985; Borrell 2004) or, alternatively, that a 20–25 % sucrose concentration maximises the rate of energy intake, as suggested by other authors (Heyneman 1983; Kingsolver and Daniel 1979; Kim et al. 2011b).
The biophysical background of nectar feeding in butterflies (Kim et al. 2011b; Daniel et al. 1989; Kingsolver and Daniel 1979; Pivnick and McNeil 1985; Heyneman 1983), together with studies about the energy flux in suction feeding in orchid bees (Borrell 2004, 2006), has contributed to a biomechanical perspective of the evolutionary history of insect–flower interactions. In addition, a recent study described the proboscis of nectar-feeding butterflies as a combination of a nanosponge with a drinking straw, underlining the importance of both capillary force and the suction pump in Lepidoptera (Monaenkova et al. 2012).
Convergent evolution has shaped greatly elongated mouthparts in a number of Hymenoptera, Lepidoptera and Diptera that allow exclusive access to nectar in long-tubed flowers (Borrell and Krenn 2006). Several species of South African flower-visiting Nemestrinidae and Tabanidae have evolved disproportionately long proboscides that are up to fourfold longer than their body (Borrell and Krenn 2006). These relatively large-bodied flies are highly accomplished fliers, hovering over flowers and rapidly moving between flower patches during feeding. Therefore, larger energy requirements should be expected for these flies (Heinrich 1975) and associated morphological adaptations towards efficient nectar feeding, i.e. overcoming the biophysical limitations of taking up sugar-rich nectar through a long and narrow proboscis.
South African long-proboscid flies are linked into a broad network of coevolution with more than 170 species of host plants across various families (including Iridaceae, Geraniaceae, Orchidaceae and Proteaceae) and represent important pollinators across floristically rich regions of South Africa (Goldblatt et al. 1995; Johnson and Steiner 1997; Potgieter and Edwards 2005; Goldblatt and Manning 1999, 2000). These flower guilds share a similar morphology including an elongated and narrow, straight or slightly curved floral tube containing nectar volumes up to 12.8 μl with a sugar concentration between 20 and 32 % (Goldblatt and Manning 2000). Studies have indicated that flies with longer proboscides were able to take up more nectar in a single visit to deep-tubed flowers and therefore most likely received an energy benefit (Pauw et al. 2009; Anderson and Johnson 2008). However, according to the functional constraints hypothesis (Kunte 2007), individuals with a disproportionally long proboscis should have an increased flower handling time and therefore increased energy cost compared with shorter-proboscid individuals. Furthermore, flying with, aiming and inserting a longer proboscis into a long, narrow flower is expected to be more difficult and energy expensive. In contrast, flies with relatively longer proboscides can exclusively access nectar from deep, narrow flowers that are otherwise inaccessible to species with shorter proboscis lengths (Kunte 2007).
All Diptera are characterised by fluid-feeding mouthparts, and elongated proboscides evolved independently among many taxa with nectar or blood feeding (Krenn and Aspöck 2010; Krenn et al. 2005). Earlier studies have investigated the feeding apparatus of short-proboscid Tabanidae, revealing a two-pump system (Bonhag 1951), and of Bombyliidae, which are equipped with an improved sclerotized cibarial pump (Szucsich and Krenn 2000, 2002). However, the only morphologically well-studied Diptera possessing a proboscis longer than the body are nemestrinid flies from the genus Prosoeca. A recent morphological study of these flies confirmed a simple but unique composition of the extremely elongated proboscides: proximally, the proboscis consists of the labrum–epipharynx unit and the labium enclosing the laciniae and the hypopharynx, and the distal half is composed of the prementum which solely forms the food tube (Karolyi et al. 2012). Nemestrinidae, like other nectar-feeding insects, must optimise their foraging behaviour. Longer handling times, due to their extremely elongated proboscis, would clearly lower the rate of energy intake. In addition, they are most likely required to feed quickly to minimise the threat of predation (Kim et al. 2011b; Schowalter 2006).
The aim of this study was to survey flower handling time differences between longer- and shorter-proboscid individuals and to test the functional constraint hypothesis that flies with longer proboscides are expected to have a longer handling time. Additionally, the anatomy of the suction pump associated with an elongated proboscis and its allometric relationship with proboscis length were investigated. The results give additional insights into the foraging behaviour of nemestrinid flies and the morphological adaptations of the suction pump for feeding from long-spurred flowers.
Materials and methods
Study organism and study sites
Throughout this study, the parasitoid nemestrinid fly Prosoeca sp. nov 1 (Manning and Goldblatt 1996) (hereafter referred to as Prosoeca sp.) from winter-rainfall Namaqualand (Le Roux and Whal 2005), South Africa, was used. Specimens for anatomical studies were collected between August and September (2010–2012) from five different locations in the surroundings of Nieuwoudtville, Northern Cape Province: Hantam National Botanical Garden (31°24′43″S, 19°09′43″E), Grasberg Road (31°20′54″S, 19°05′30″E), Wild Flower Reserve (31°22′00″S, 19°08′51″E), Glacial Pavement (31°26′21″S, 19°08′42″E) and Melkbosfontein (31°21′12″S, 19°10′22″E). Videos of fly feeding activities on flowers were filmed at the botanical garden and the flower reserve sites in 2011 and 2012. Both observation sites provided a large population of the host plant Lapeirousia oreogena (Iridaceae). For all captured individuals, proboscis and body length were measured with a digital caliper (Helios Digi-Met 1220; 0.01 mm).
Video analyses
To estimate the effective flower visiting time and mean drinking time of Prosoeca sp., individuals were filmed during foraging on flowers of L. oreogena. The violet-coloured flowers have a long, narrow perianth tube containing between 2.5 and 7.3 μl sucrose-rich nectar with an average concentration of 25.8 % (Goldblatt et al. 1995). Both flies and L. oreogena are endemic to the region, and Prosoeca sp. is the flowers’ sole pollinator (Manning and Goldblatt 1997). Observations were conducted on warm and sunny days between 10:00 am and 3 pm (16–30 °C), which represented the peak activity of flies. In addition to recording total flower handling time (Fig. 1), flower handling behaviour was separated into three phases: (1) hovering phase (Fig. 2a) where the proboscis was moved into feeding position while the fly was hovering above the flower; (2) drinking phase (Fig. 2b), the head is in contact with the anthers, and the fly remains more or less stationary; and (3) removing phase (Fig. 2c), the proboscis is pulled out, and the fly moves away from the flower. Filmed flies were captured, measured and marked with a black dot on the wing with a permanent pen to prevent measurements of recaptured individuals. Videos were taken with a Sony HDR-XR550V video camera and analysed using Observer XT 11.0.
MicroCT and serial semithin sections
Collected flies were fixed in FAA solution (35 % formalin, 100 % glacial acetic acid, 90 % alcohol) and later stored in 70 % ethanol. All specimens were dehydrated to absolute ethanol and stained with 1 % iodine in 100 % ethanol overnight. Prior to scanning, samples were washed in 100 % ethanol (Metscher 2009). Specimens were scanned with a MicroXCT-200 system (optical lens 2×; tungsten source at 40 kV and 200 μA; reconstructed isotropic voxel size 9.9 μm). For 3D reconstructions of the suction pump, AMIRA 5.3.3 software was used. The food canal and suction pump muscles were manually segmented in the Amira segmentation editor. After segmentation, structures were visualised in the Amira Viewer using both volume and surface rendering.
For investigations of the food canal, serial semithin sections of the proboscis of additional (N = 5) flies were conducted. Preserved specimens were dehydrated and embedded in Agar Low Viscosity Resin (Pernstich et al. 2003). Sections of 1 μm at the proximal labium and the distal end of the prementum were cut with a Leica EM UC6 microtome using a diamond knife. Serial semithin sections were stained in a mixture of azure II (1 %) and methylene blue (1 %) in hydrous borax solution (1 %), diluted 1:20, at 90 °C for 20 s. Micrographs were taken with a Nikon Eclipse E800 microscope equipped with a Nikon Digital Sight DS-Fi2 camera. Food canal dimensions were measured with the NIS-Elements Imaging Software version 4.00.
Statistics
Data analyses were conducted using the statistical computing software R 2.15.2 (R Core Team 2012). Proboscis length and body length were correlated using model II regression (major axis regression) within the ‘smatr package’ (Warton et al. 2012). Relations between suction pump dimensions and proboscis lengths were calculated using linear regression models for cibarial and pharyngeal dilator muscles, for compressor muscles, and for cibarial retractor and protractor muscles.
Data for each feeding phase were transformed to attain normal distribution (square root transformation for hovering and drinking phase, inverse transformation for removing phase), and these were modelled in relation to proboscis length using linear regression. All graphs were designed with SigmaPlot 11.0.
Results
Flower handling time and nectar uptake
The mean flower handling time on flowers of L. oreogena was 2.59 s ± 0.7 SD with a minimum of 0.72 s and maximum of 6.76 s. Linear regressions revealed a significant relationship between total flower handling time and increasing proboscis length (R 2 = 0.10; p = 0.007) (Fig. 1). The flower-visiting behaviour of Prosoeca sp. on L. oreogena was divided into three proceeding functional phases. After approaching the flower in horizontal flight, the proboscis was swung forward from lying under the abdomen pointing posteriorly, into the feeding position while hovering above a flower for a few seconds (Fig. 2a). After contact with the flower, the fly alights down, inserting the proboscis into the spur as deep as possible, pushing the head and labral base against the anthers (Fig. 2b). Finally, after drinking, the proboscis was removed with a rapid upward movement (Fig. 2c). In total, 75 individuals were filmed over a period of 2 years.
Linear regression between flower handling time and proboscis length of Prosoeca sp. on flowers of Lapeirousia oreogena divided into three successive phases. In addition, for each phase, illustrating pictures are given above: a, d hovering fly with proboscis in feeding position; b, e drinking with proboscis fully inserted into the flower spur with the head in contact with the anthers; c, f proboscis is removed in a rapid movement. Of these, only drinking time shows a positive relationship to proboscis length
When handling time was split into its three distinct phases, linear regressions showed that the time required for inserting and removing the proboscis was independent of proboscis length, whereas there was a significant increase in drinking time associated with the increase in proboscis length (Fig. 2d–f).
Suction pump anatomy
Suction pump muscles and their hypothesised functions are summarised in Table 1. The suction pump was composed of two functionally distinct parts with corresponding inlet valves. The cibarial pump was located in a slightly oblique position behind the clypeus and the frons. It was separated from the cone-shaped pharyngeal pump by the cibarial–pharyngeal valve (Fig. 3a, b). Both pumping organs consisted of a set of paired dilator muscles, and the pharynx was additionally equipped with two visceral compressor muscles. The lumina of these consecutive pumps formed a right angle with the oesophagus, which proceeded from the posterior pharynx through the brain to the occipital foramen at the back of the head (Fig. 3a).
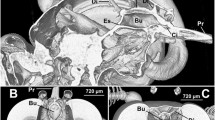
MicroCT head scans of Prosoeca sp. displaying the cibarial pump (blue) and the pharyngeal pump with oesopharynx (both green). a, b Lateral and frontal view with cibarial valve, cibarial dilator and cibarial–pharyngeal valve. c, d Frontal and lateral view with pharyngeal dilator and compressor muscles together with oesopharyngeal dilators. e, f Cibarial protractor and retractor attached to a dorsolateral cuticular ledge on the cibarium. Cb cibarium, cpv cibarial–pharyngeal valve, mcc musculus clypeo-cibarialis, mcpd m. clypeo-pharyngealis dorsalis, mcpv m. clypeo-pharyngealis ventralis, mfc musculus fronto-cibarialis, mfp m. fronto-pharyngealis, mgc m. geno-cibarialis, mlc m. labro-cibarialis, mtod m. tentorio-oesopharyngealis dorsalis, mtol m. tentorio-oesopharyngealis lateralis, oe oesopharynx, ph pharynx
Cibarial pump
A paired dilator muscle was responsible for operating the cibarial pump. Musculus clypeo-cibarialis (mcc) originated on the clypeus and inserted on the anterior cibarial wall (Fig. 3a, b). In addition, an unpaired muscle controlled the valve of the cibarial pump; at the base of the proboscis, m. labro-epipharyngealis (mle) extended between the labral base and the epipharynx (Fig. 3a, b).
Lateral to the suction pump, two paired muscles were attached to a cuticular ledge protracting dorsolaterally on the cibarium (Fig. 3e, f). Originating on the frons, next to the m. fronto-pharyngealis (Fig. 3f), m. fronto-cibarialis (mfc) attached to a dorsal tendon protracting from the cibarium ledge. Musculus geno-cibarialis (mgc) originated on the gena and extended obliquely to its insertion on the cibarium.
Pharyngeal pump
The pharyngeal pump was operated by three paired dilator and two unpaired compressor muscles. Musculus clypeo-pharyngealis ventralis (mcpv) originated on the clypeus and inserted on the cibarial–pharyngeal valve (Fig. 3c, d). The main pharyngeal dilator, m. fronto-pharyngealis (mfp), originated on the frons and was attached to the concave dorsal wall of the pharynx (Fig. 3c, d). The secondary pharynx dilator, m. clypeo-pharyngealis dorsalis (mcpd), extended between the clypeus and the pharynx (Fig. 3c, d). Two unpaired muscles enclosed the anterior and posterior pharynx surface (Fig. 3c). Musculus pharyngealis anterior (mpa) extended between the anterior dorsal pharynx ridge and the cibarial–pharyngeal valve and was breached by the fibres of m. clypeo-pharyngealis dorsalis. Lying against the posterior pharynx surface, m. pharyngealis-posterior (mpp) extended between the oesophagus outlet and the cibarial–pharyngeal valve. Finally, two small muscles, m. tentorio-oesophagialis dorsalis and lateralis (mtod, mtol, respectively), resided between the posterior tentorial arms and the oesophagus near the occipital foramen (Fig. 3c).
Allometric relationship of body and proboscis length
The proboscis measurements of Prosoeca sp. are summarised in Table 2. Proboscis lengths were twofold longer than the body, with length variations from 28 to 43 mm and with a proximal diameter of 170 to 209 μm and a distal diameter of 110 to 144 μm. The major axis regression revealed a positive allometric relationship between body and proboscis length (Fig. 4a). For every 1 mm body length gain, the proboscis length increased by 3.32 mm (95 % CI 2.64–4.41 mm; Fig. 4a). In addition, all dilator muscles of the cibarium and pharynx displayed a significant positive relationship with proboscis length (Fig. 4b, c; for further details, see Supplemental Material 1). Furthermore, volumes of the pharyngeal compressor muscles significantly increased with proboscis length (Fig. 4c). Regarding the cibarial protractor and retractor muscles, both showed a significant positive relationship with increasing proboscis length (cibarial retractor: R 2 = 0.68, P < 0.001; cibarial protractor: R 2 = 0.60, P < 0.001).
Linear regression of suction pump muscles, proboscis and body lengths. a Positive correlation between proboscis and body length. b Cibarial pump. c Pharyngeal dilator (triangles) and compressor muscles (circles). Both cibarial and pharyngeal pump muscles are positively correlated to proboscis length
Discussion
Foraging efficiency with an elongated proboscis
Long-proboscid nemestrinids co-occur with several, mostly nectar rewarding flowers that have elongated, cylindrical flower tubes (Goldblatt and Manning 2000). Proboscis and floral tube length vary between different populations within the same species and have been regarded as a reciprocal adaptation between the flower and the fly (Anderson and Johnson 2008; Pauw et al. 2009). Although allometric relationships between body size and proboscis length have already been shown in a previous study (Anderson and Johnson 2008), corolla tube lengths have been regarded as the significant predictor variable for the proboscis length. Nevertheless, given the positive scaling relationship between body and proboscis length in Prosoeca sp., allometry could be regarded as an important factor in generating the proboscis length variations in long-proboscid flies.
Given the high variation in proboscis lengths in Prosoeca sp., a difference in flower handling time was expected between individuals with different proboscis lengths as shown in Fig. 1. However, in detail, this relationship was verified only for the time spent drinking from a flower. Kunte (2007) explained the strong positive relationship between proboscis length and increased handling time as a function of a disproportionate relationship between the cibarial muscles and an elongated proboscis. With a given body size and cibarial muscle mass, longer proboscides should produce more friction to nectar travelling up the proboscis due to their greater food canal surface area. However, in Prosoeca sp., cibarial and pharyngeal muscle masses showed a positive allometric relationship with body size and proboscis length; thus, an efficient nectar uptake rate would be expected due to the increased muscle mass compensating for an increased proboscis length. Nonetheless, drinking time of flies was seen to increase with an elongated proboscis, supporting the contention that longer proboscides do offer some limitations, as suggested by the functional constraint hypothesis. Additionally, longer drinking times could also be influenced by long-proboscid individuals being able to take up the full complement of nectar out of long, narrow flowers, e.g. up to 7 μl per flower (Goldblatt et al. 1995; Manning and Goldblatt 1996).
Surprisingly, the time required to insert a long proboscis into a flower of L. oreogena appeared to be independent of proboscis length. Previous studies of the flower-visiting behaviour of Prosoeca sp. on L. oreogena revealed the importance of the white arrow markings as functional nectar guides to minimise flower handling times. Flies were no longer able to insert their proboscis into flowers with artificially manipulated nectar markings (Hansen et al. 2011). These nectar guides may help to reduce the negative effects of flower handling with an elongated proboscis. Inserting a proboscis of this length into a long-spurred flower could, however, be negatively influenced by wind and vegetation growing between and over host flowers, as was seen for some foraging flies during field experiments. This might explain the large variation observed between minimum (0.16 s) and maximum inserting times (6.6 s). Removing time was consistently rapid across all individuals observed. Flies leave flowers with a fast, forceful movement, possibly to overcome the friction between proboscis and flower, and therefore, no overall differences between individuals could be recorded.
Similar to apid bees, nemestrinid flies can be regarded as high-speed, continuous foragers (Pivnick and McNeil 1985), which should prefer nectar with sugar concentrations between 30 and 50 %. Concentrations in long-spurred flowers have been recorded to be lower than in flowers with short perianth tubes (Plowright 1987). Similar results have been shown for Lapeirousia species in Namaqualand (Goldblatt et al. 1995), and typical flowers visited by Prosoeca sp. only produce nectar with sugar concentrations of 20–30 % (Goldblatt et al. 1995). Both flowers and flies occur in a semi-arid habitat (Manning and Goldblatt 1997), where preferred nectar concentrations are expected to shift to more dilute nectar to counter water loss as predicted by mathematical models (Pivnick and McNeil 1985). Kim et al. (2011b) discovered that optimal nectar concentration is strongly related to the drinking style of foraging insects, being higher for viscous dippers, e.g. bees, than suction feeders, such as long-proboscid Nemestrinids, thus possibly offering an explanation for the relatively dilute concentrations of nectar in Prosoeca sp.’s host plants.
Suction pump design
Like in Lepidoptera (Monaenkova et al. 2012), the proboscis of Prosoeca represents a two-level fluidic system, combining a nanosponge with a straw. The mouthpart morphology suggests that Nemestrinidae are suction feeders that rely on pressure gradients within the proboscis (Karolyi et al. 2012). While feeding, on the first microlevel, paired labella at the tip of the proboscis function as a nanosponge. Rutted with a pseudothracheal system, they take in liquid via capillary action, filling the distal part of the food canal (Kingsolver and Daniel 1979). On the second microlevel, the food canal works as a pump-operated drinking straw, transporting fluids to the mouth. However, sucking up nectar from long, narrow floral tubes through a long, straw-like proboscis requires an enhanced pumping organ. Nearly all insects feature a pharyngeal pump, while a cibarial pump is developed only in insects with true sucking mouthparts (Peters 2003).
The suction pump of Prosoeca sp. is here hypothesised to combine the systaltic motion of two pumping organs regulated by two valves, each controlled by dilator muscles, dividing the feeding process into three functional phases as shown in Fig. 5. During the first phase, the functional mouth valve opens, and the cibarial pump is extended by the cibarial dilator. Due to the emerging partial vacuum in the cibarial chamber, nectar is sucked from the food canal into the cibarium (Fig. 5a). In the second phase, relaxation of the cibarial pump and combined contraction of the pharyngeal dilators draw nectar through the now opened cibarial–pharyngeal valve into the pharyngeal chamber (Fig. 5b). During the last phase, the pharyngeal dilator muscles relieve tension, and the compressor muscles push the nectar into the oesopharynx. Finally, dorsal and lateral dilators open the distal oesophagus valve to the midgut (Fig. 5c). According to this model, Prosoeca is able to efficiently suck up viscous nectar using two well-coordinated suction pumps.
Three-phase suction pump of Prosoeca sp. based on microCT scans. Contracting muscles are drawn in red with orange arrows; relaxing muscles are hollow with blue arrows. a Cibarial dilator muscles open the cibarial valve and suck nectar into the cibarium. b Pharyngeal dilators pump nectar through the cibarial–pharyngeal valve into the pharynx. In phase A and B, the cibarial protractor and retractor (light red) work as antagonists to the main dilator muscles to hold the pump stationary. c Pharyngeal compressor muscles push the nutrition into the oesopharynx, and the posterior dilator muscles open the oesopharyngeal valve. Cuticle elasticity of both pump chambers provides restoration force to reset the suction pump for the next food intake phase. Cb cibarium, cbd cibarial dilator, cbp cibarial protractor, cbr cibarial retractor, cpv cibarial–pharyngeal valve (true mouth), cv cibarial valve (functional mouth), oe oesophagus, ph pharynx, phc pharyngeal compressor, phd pharyngeal dilator
Additionally, two paired lateral muscles in the head (Fig. 3e, f) most likely provide a supporting function. Contraction of the cibarial and pharyngeal dilators exerts a force on the suction pump. Simultaneous contraction of the cibarial retractor and protractor holds the pump stationary by working antagonistically against the massive dilator muscles.
Although this feeding model has been hypothesised based on 3D reconstructions acquired from microCT scans, similar systems have been described in detail for short-proboscid Tabanidae (Bonhag 1951), Bombyliidae (Szucsich and Krenn 2000) and female mosquitoes (Kim et al. 2011a; Kim et al. 2012; Snodgrass 1959). Compared to Tabanidae, a massive posterior compressor and an additional pharyngeal dilator exist in Prosoeca sp., enhancing the pharyngeal pump.
The morphological adaptation of a two-pump system has been explained by Kim et al. (2011a). Although a rectangular path is inevitable to connect the food canal within the orthognathe proboscis with the midgut, it also leads to energy loss along the curved path. Therefore, an additional pump is necessary to regulate the flow effectively. Similarly to mosquitoes (Snodgrass 1959) and based on the morphological studies presented here, nemestrinid flies appear to be able to suck liquid efficiently using the phase-shifted motion of cibarial and pharyngeal pump in conjunction with two valves.
Compared to nemestrinid flies, the suction pump of Lepidoptera consists of only a main buccal lumen, formed by the epi- and hypopharynx, operated by several dilator and compressor muscles (Davis and Hildebrand 2006; Eberhard and Krenn 2005). In addition, a cibarial upstream valve controls the entrance to the actual pump, and a posterior valve regulates the influx to the oesophagus. In Nymphalidae, the cibarial oral valve also regulates the outflow of saliva into the proboscis (Eberhard and Krenn 2005). In contrast, the hypopharynx of Prosoeca sp. is part of the proximal proboscis, housing the salivary duct, while the epipharynx provides the lining for the food canal (Karolyi et al. 2012).
Relationship between muscle volume and proboscis length
Mechanical properties of the feeding apparatus, like the musculature needed to maintain a constant pressure gradient, are potentially limiting factors in nectar-sucking insects. Detailed considerations about biophysical and biomechanical properties of the feeding mechanism are given by Kingsolver and Daniel (1979). However, May (1985) indicated that nectar flow is not steady in butterflies and that the pressure drop (i.e. the pressure difference between the proximal and distal end of the feeding channel) is variable during nectar feeding. He further noted that butterflies with a longer proboscis have a higher energy intake rate due to greater pressure drops and greater uptake rates at any given nectar concentration.
Kunte (2007) hypothesised that insects that depend exclusively on nectar as food source should have a greater cibarial muscle mass to increase the rate of nectar uptake. Our results support this hypothesis, as seen in the positive linear relationship between proboscis length and sucking pump dimensions in Prosoeca sp., indicating that a longer proboscis demands larger pumping organs. These correlations underline the importance of both pumps in nemestrinid flies.
Evolutionary origin of the sucking pump
In various insects with sucking mouthparts, the suction pump has evolved from the cibarium, the pharynx or both (Davis and Hildebrand 2006). Snodgrass (1944, 1935) determined the evolutionary origin of the insect sucking pump as primarily cibarial with a minor pharyngeal component. He further described the frontal ganglion as a stomodaeal–cibarial border, suggesting that all muscles proceeding between clypeus and buccal cavity are cibarial. In contrast, Davis and Hildebrand (2006) postulated a stomodaeal origin of the buccal cavity, since the visceral muscles enveloping the sucking pump are characteristic of the stomodaeum. While the cibarium only forms the pre-oral valve, the main sucking pump is recognized as pharyngeal. In Prosoeca sp., the cibarial and pharyngeal pumps can be clearly assigned by their attached dilator and visceral muscles. Snodgrass (1959) described the two-part suction pump of the female mosquito which included a connectional alimentary canal with two antagonistic muscles, which represented the true mouth, and a pharyngeal pump positioned after the brain. However, in Prosoeca sp., both pumps are located anterior to the brain, and the alimentary canal is modified to the cibarial–pharyngeal valve. The oesophagus traverses the brain, with an additional posterior valve. This is quite similar to the model described for Tabanidae (Bonhag 1951), suggesting that the ancestors of Nemestrinidae might have been blood-sucking insects (Grimaldi and Engel 2005).
Dimensions of the food canal
The feeding mechanism associated with a long proboscis can be regarded as a simple pipe flow system with a laminar nectar flow. The food canal represents a long, thin cone, changing its diameter at a constant rate (Kingsolver and Daniel 1979). Intake of fluids depends on viscosity, and an increasing sugar concentration results in a curvilinear increase in the weight of sucrose per unit time which leads to an increase of viscosity (Heyneman 1983). Therefore, nectar containing 40 % sucrose is six times more viscous than water at the same temperature (Chapman 1998).
The pressure drop created by the suction pump depends also on the morphological characteristics of the proboscis. Furthermore, according to the Hagen–Poiseuille equation, a longer proboscis results in a reduced nectar flow (Kingsolver and Daniel 1979) as long as all other parameters remain constant. However, nectar flow also depends on the fourth power of the radius of the food canal. In order to maintain a constant flow rate within individuals with different proboscis lengths, flies with longer proboscides would require correspondingly increased suction pump dimensions but a relatively small increase of the food tube diameter. Reinforced suction pump musculature has also been recorded for extremely long-proboscid riodinid butterflies (Bauder et al. 2013).
Compared to Lepidoptera with similar proboscis length variations (May 1985; Bauder et al. 2011), the food canal dimensions of Prosoeca sp. are relatively large, indicating that they possibly compensate the excess length of the proboscis with extended food canal dimensions. Flower-visiting insects, like nemestrinid flies, that use energy-expensive feeding techniques benefit from minimised feeding times (Heyneman 1983), which can be achieved with efficient suction pumps and an increased nectar flux through extended food canal dimensions.
Conclusion
The present study has described the flower-visiting behaviour of long-proboscid Prosoeca sp. on flowers of L. oreogena and has verified that individuals with a longer proboscis spent more time drinking from long-spurred flowers. These results suggest that longer proboscid individuals are able to take up more nectar in a single visit and therefore gain a possible advantage over individuals with an average proboscis length. In addition, this study has shown that feeding from elongated floral tubes not only requires an efficient two-part suction pump in the head, but also a positive allometric relationship between proboscis length and suction pump muscle mass. These results indicate that Prosoeca sp. represents a highly adapted and efficient nectar feeder.
References
Anderson B, Johnson SD (2008) The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution 62(1):220–225
Bauder JA-S, Handschuh S, Metscher BD, Krenn HW (2013) Functional morphology of the feeding apparatus and evolution of proboscis length in metalmark butterflies (Lepidoptera: Riodinidae). Biol J Linn Soc 110(2):291–304. doi:10.1111/bij.12134
Bauder JAS, Lieskonig NR, Krenn HW (2011) The extremely long-tongued Neotropical butterfly Eurybia lycisca (Riodinidae): proboscis morphology and flower handling. Arthropod Struct Dev 40(2):122–127. doi:10.1016/j.asd.2010.11.002
Bonhag PF (1951) The skeleto-muscular mechanism of the head and abdomen of the adult horsefly (Diptera: Tabanidae). Trans Am Entomol Soc (1890-) 77(2):131–202
Borrell BJ (2004) Suction feeding in orchid bees (Apidae: Euglossini). Proc R Soc Lond Ser B Biol Sci 271(Suppl 4):S164–S166. doi:10.1098/rsbl.2003.0128
Borrell BJ (2006) Mechanics of nectar feeding in the orchid bee Euglossa imperialis: pressure, viscosity and flow. J Exp Biol 209(24):4901–4907. doi:10.1242/jeb.02593
Borrell BJ, Krenn HW (2006) Nectar feeding in long-proboscid insects. In: Herrel A, Speck T, Rowe N (eds) Ecology and biomechanics: a mechanical approach to the ecology of animals and plants. CRC Press, Boca Raton, pp 185–211
Chapman RF (1998) The insects: structure and function, 4th Edition. Cambridge University Press, Cambridge, pp 21–24:50
Daniel TL, Kingsolver JG, Meyhöfer E (1989) Mechanical determinants of nectar-feeding energetics in butterflies: muscle mechanics, feeding geometry, and functional equivalence. Oecologia 79(1):66–75. doi:10.1007/bf00378241
Davis NT, Hildebrand JG (2006) Neuroanatomy of the sucking pump of the moth, Manduca sexta (Sphingidae, Lepidoptera). Arthropod Struct Dev 35(1):15–33. doi:10.1016/j.asd.2005.07.001
Dudley R (2000) Flight and insect diversification. In: The biomechanics of insect flight: form, function, evolution, vol 7. First edn. Princeton University Press, New Jersey, pp. XII+476
Eberhard SH, Krenn HW (2005) Anatomy of the oral valve in nymphalid butterflies and a functional model for fluid uptake in Lepidoptera. Zoologischer Anzeiger - A J Comp Zool 243(4):305–312. doi:10.1016/j.jcz.2005.02.001
Goldblatt P, Manning JC (1999) The long-proboscid fly pollination system in Gladiolus (Iridaceae). Ann Mo Bot Gard 86(1):758–774
Goldblatt P, Manning JC (2000) The long proboscid fly pollination system in southern Africa. Ann Mo Bot Gard 87(1):146–170
Goldblatt P, Manning JC, Bernhardt P (1995) Pollination biology of Lapeirousia subgenus Lapeirousia (Iridaceae) in southern Africa; floral divergence and adaptation for long-tongued fly-pollination. Ann Mo Bot Gard 82(4):517–534
Grimaldi DA, Engel MS (2005) Evolution of the insects, 1st edn. Cambridge University Press, Cambridge, www.cambridge.org/9780521821490
Hansen DM, Van der Niet T, Johnson SD (2011) Floral signposts: testing the significance of visual ‘nectar guides’ for pollinator behaviour and plant fitness. Proc R Soc B Biol Sci. doi:10.1098/rspb.2011.1349
Heinrich B (1975) Energetics of pollination. Annu Rev Ecol Syst 6(1):139–170. doi:10.1146/annurev.es.06.110175.001035
Heyneman A (1983) Optimal sugar concentrations of floral nectars—dependence on sugar intake efficiency and foraging costs. Oecologia 60(2):198–213. doi:10.1007/bf00379522
Johnson S, Anderson B (2010) Coevolution between food-rewarding flowers and their pollinators. Evol: Educ and Outreach 3(1):32–39. doi:10.1007/s12052-009-0192-6
Johnson SD, Steiner KE (1997) Long-tongued. Fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution 51(1):45–53
Karolyi F, Szucsich NU, Colville JF, Krenn HW (2012) Adaptations for nectar-feeding in the mouthparts of long-proboscid flies (Nemestrinidae: Prosoeca). Biol J Linn Soc 107:414–424. doi:10.1111/j.1095-8312.2012.01945.x
Kim BH, Kim HK, Lee SJ (2011a) Experimental analysis of the blood-sucking mechanism of female mosquitoes. J Exp Biol 214(7):1163–1169. doi:10.1242/jeb.048793
Kim BH, Seo ES, Lim JH, Lee SJ (2012) Synchrotron X-ray microscopic computed tomography of the pump system of a female mosquito. Microsc Res Tech 75(8):1051–1058. doi:10.1002/jemt.22030
Kim W, Gilet T, Bush JWM (2011b) Optimal concentrations in nectar feeding. Proc Natl Acad Sci 108(40):16618–16621. doi:10.1073/pnas.1108642108
Kingsolver JG, Daniel TL (1979) On the mechanics and energetics of nectar feeding in butterflies. J Theor Biol 76(2):167–179. doi:10.1016/0022-5193(79)90368-0
Krenn HW, Aspöck H (2010) Bau, Funktion und Evolution der Mundwerkzeuge blutsaugender Arthropoden. In: Aspöck H (ed) Krank durch Arthropoden, vol 30, Denisia. Biologiezentrum der Oberösterreichischen Landesmuseen, Linz, pp 81–108
Krenn HW, Plant JD, Szucsich NU (2005) Mouthparts of flower-visiting insects. Arthropod Struct Dev 34(1):1–40
Kunte K (2007) Allometry and functional constraints on proboscis lengths in butterflies. Funct Ecol 21(5):982–987. doi:10.1111/j.1365-2435.2007.01299.x
Le Roux A, Whal Z (2005) Namaqualand. South African wild flower guide 1, 3rd edn. Botanical Society of South Africa, Cape Town
Manning JC, Goldblatt P (1996) The Prosoeca peringueyi (Diptera: Nemestrinidae) pollination guild in southern Africa: long-tongued flies and their tubular flowers. Ann Mo Bot Gard 83(1):67–86
Manning JC, Goldblatt P (1997) Nieuwoudtville, Bokkeveld Plateau & Hantam. South African Wild Flower Guide 9. vol 9, first edn. Botanical Society of South Africa, Kirstenbosch, Claremont 7735 RSA
May PG (1985) Nectar uptake rates and optimal nectar concentrations of two butterfly species. Oecologia 66(3):381–386. doi:10.1007/bf00378303
Metscher B (2009) MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol 9(1):11
Monaenkova D, Lehnert MS, Andrukh T, Beard CE, Rubin B, Tokarev A, Lee W-K, Adler PH, Kornev KG (2012) Butterfly proboscis: combining a drinking straw with a nanosponge facilitated diversification of feeding habits. J Royal Soc Interface 9(69):720–726. doi:10.1098/rsif.2011.0392
Muchhala N, Thomson JD (2009) Going to great lengths: selection for long corolla tubes in an extremely specialized bat–flower mutualism. Proc R Soc B Biol Sci 276(1665):2147–2152. doi:10.1098/rspb.2009.0102
Nicolson SW (2007) Nectaries and nectar. In: Nepi M, Pacini E (eds) Nectaries and nectar, 1st edn. Springer, Dordrecht, pp XVIII+–396, http://www.springer.com/life+sciences/plant+sciences/book/978-1-4020-5936-0
Pauw A, Stofberg J, Waterman RJ (2009) Flies and flowers in Darwin’s race. Evolution 63(1):268–279
Pellmyr O (2002) Pollination by animals. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions: an evolutionary approach. Blackwell Science Ltd, Oxford, pp 157–184
Pernstich A, Krenn HW, Pass G (2003) Preparation of serial sections of arthropods using 2, 2-dimethoxypropane dehydration and epoxy resin embedding under vacuum. Biotech Histochem 78(1):1–5
Peters W (2003) Ernährung und Verdauung. In: Dettner K, Peters W (eds) Lehrbuch der Entomologie, 2nd edn. Spektrum, München, pp 93–94
Pivnick K, McNeil J (1985) Effects of nectar concentration on butterfly feeding: measured feeding rates for Thymelicus lineola (Lepidoptera: Hesperiidae) and a general feeding model for adult Lepidoptera. Oecologia 66(2):226–237. doi:10.1007/bf00379859
Plowright RC (1987) Corolla depth and nectar concentration: an experimental study. Can J Bot 65(5):1011–1013. doi:10.1139/b87-139
Potgieter CJ, Edwards TJ (2005) The Stenobasipteron wiedmanni (Diptera, Nemestrinidae) pollination guild in Eastern Southern Africa. Ann Mo Bot Gard 92(1):254–267
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Schowalter TD (2006) Insect ecology, second edition: an ecosystem approach. Academic Press, Elsevier, London
Snodgrass RE (1935) Principles of insect morphology, vol First edn., pp McGraw Hill–New York
Snodgrass RE (1944) The feeding apparatus of biting and sucking insects affecting man and animals. Smithson Misc Collect 104(7):1–113
Snodgrass RE (1959) The anatomical life of the mosquito. Smithson Misc Collect 139(8):1–87
Szucsich NU, Krenn HW (2000) Morphology and function of the proboscis in Bombyliidae (Diptera, Brachycera) and implications for proboscis evolution in Brachycera. Zoomorphology 120(2):79–90
Szucsich NU, Krenn HW (2002) Flies and concealed nectar sources: morphological innovations in the proboscis of Bombyliidae (Diptera). Acta Zool 83(3):183–192
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) smatr 3—an R package for estimation and inference about allometric lines. Methods Ecol Evol 3(2):257–259. doi:10.1111/j.2041-210X.2011.00153.x
Acknowledgments
The authors would like to thank Eugene Marinus at the Hantam National Botanical Garden who provided information on locating fly populations. Furthermore, thanks to Ross Cowlin and Rogan Fourie for field work assistance. The authors are additionally grateful to Julia Bauder for assisting in the serial semithin sections. Finally, the authors would especially like to thank the two anonymous reviewers for improving the manuscript. We thank Northern Cape Nature Conservation for collecting and export permits (permit no. 471/2010, 472/2010 and 804/2012, 805/2012). This study was supported by the Austrian Foundation for Scientific Research FWF (P 222 48-B17) and the research grant 2012 of the University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Beverley Glover
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 13 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Karolyi, F., Morawetz, L., Colville, J.F. et al. Time management and nectar flow: flower handling and suction feeding in long-proboscid flies (Nemestrinidae: Prosoeca). Naturwissenschaften 100, 1083–1093 (2013). https://doi.org/10.1007/s00114-013-1114-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-013-1114-6