Abstract
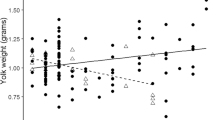
We incubated eggs of the Japanese gecko Gekko japonicus at three temperatures, and measured yolk testosterone (T) and 17β-estradiol (E2) levels at three time points in embryonic development (oviposition, 1/3 of incubation, and 2/3 of incubation), to examine whether maternal influence on offspring sex via yolk steroid hormone deposition is significant in the species. Eggs incubated at 24 °C and 32 °C produced mostly females, and eggs incubated at 28 °C almost a 50:50 sex ratio of hatchlings. Female-producing eggs were larger than male-producing eggs. Clutches in which eggs were incubated at the same temperature produced mostly same-sex siblings. Yolk T level at laying was negatively related to eggs mass, and yolk E2/T ratio was positively related to egg mass. Results of two-way ANOVA with incubation temperature and stage as the factors show that: yolk E2 level was higher at 32 °C than at 24 °C; yolk T level was higher, whereas yolk E2/T ratio was smaller, at 28 °C than at 24 °C; yolk E2 and T levels were higher at 2/3 than at 1/3 of incubation. Our data in G. japonucus show that: (1) maternal influence on offspring sex via yolk steroid hormone deposition is significant; (2) incubation temperature affects the dynamics of developmental changes in yolk steroid hormones; (3) influences of yolk steroid hormones on offspring sex are secondary relative to incubation temperature effects; and (4) offspring sex correlates with an interaction between incubation temperature and yolk steroid hormones.



Similar content being viewed by others
References
Bowden RM, Ewert MA, Freedberg S, Nelson CE (2002a) Maternally derived yolk hormones vary in follicles of the painted turtle, Chrysemys picta. J Exp Zool 293:67–72
Bowden RM, Ewert MA, Nelson CE (2000) Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc R Soc Lond B 267:1745–1749
Bowden RM, Ewert MA, Nelson CE (2002b) Hormone levels in yolk decline throughout development in the red-eared slider turtle (Trachemys scripta elegans). Gen Comp Endocrinol 129:171–177
Bull JJ (1987) Temperature-sensitive periods of sex determination in a lizard: similarities with turtles and crocodilians. J Exp Zool 241:143–148
Chen JC, Peng XB, Yu DW (1986) Studies on the karyotypes of three species of the genus Gekko. Acta Herpetol Sin 5:24–29
Conley AJ, Elf PK, Corbin CJ, Dubowsky S, Fivizzani AJ, Lang JW (1997) Yolk steroids decline during sexual differentiation in the alligator. Gen Comp Endocrinol 107:191–200
Crews D (1994) Temperature, steroids and sex determination. J Endocrinol 142:1–8
Dufaure JP, Hubert J (1961) Table de développement du lézard vivipare: Lacerta (Zootoca) vivipara Jacquin. Arch Anat Microsc Morph Exp 50:309–328
Elf PK (2003) Yolk steroid hormones and sex determination in reptiles with TSD. Gen Comp Endocrinol 132:349–355
Elf PK (2004) Yolk steroid hormones and their possible role in TSD species. In: Valenzuela N, Lance VA (eds) Temperature-dependent sex determination in vertebrates. Smithsonian Institution Press, Washington DC, pp 111–118
Elf PK, Lang JW, Fivizzani AJ (2002a) Yolk hormone levels in the eggs of snapping turtles and painted turtles. Gen Comp Endocrinol 127:26–33
Elf PK, Lang JW, Fivizzani AJ (2002b) Dynamics of yolk steroid hormones during development in a reptile with temperature-dependent sex determination. Gen Comp Endocrinol 127:34–39
Gamble T (2010) A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex Dev 4:88–103
Gutzke WHN, Chymiy DB (1988) Sensitive periods during embryogeny for hormonally induced sex determination in turtles. Gen Comp Endocrinol 71:265–267
Hamlin HJ, Lowers RH, Albergotti LC, McCoy MW, Mutz J, Guillette LJ (2010) Environmental influence on yolk steroids in American alligators (Alligator mississippiensis). Biol Reprod 83:736–741
Huang V, Sakata JT, Rhen T, Coomber P, Simmonds S, Crews D (2008) Constraints on temperature-dependent sex determination in the leopard gecko (Eublepharis macularius): response to Kratochvíl et al. Naturwissenschaften 95:1137–1142
Janes DE, Bermudez D, Guillette LJ Jr, Wayne ML (2007) Estrogens induced male production at a female-producing temperature in a reptile (leopard gecko, Eublepharis macularius) with temperature-dependent sex determination. J Herpetol 41:9–15
Janzen FJ, Wilson ME, Tucker JK, Ford SP (1998) Endogenous yolk steroid hormones in turtles with different sex-determining mechanisms. Gen Comp Endocrinol 111:306–317
Janzen FJ, Wilson ME, Tucker JK, Ford SP (2002) Experimental manipulation of steroid concentrations in circulation and in egg yolks of turtles. J Exp Zool 293:58–66
Ji X, Braña F (1999) The influence of thermal and hydric environments on embryonic use of energy and nutrients, and hatchling traits, in the wall lizards (Podarcis muralis). Comp Biochem Physiol A 124:205–213
Ji X, Wang PC, Hong WX (1991) The reproductive ecology of the gecko Gekko japonicus. Acta Zool Sin 37:185–192
Kratochvíl L, Kubička L, Landová E (2008) Does the mechanism of sex determination constrain the potential for sex manipulation? A test in geckos with contrasting sex-determining systems. Naturwissenschaften 95:209–215
Lovern MB, Wade J (2001) Maternal plasma and egg yolk testosterone concentrations during embryonic development in green anoles (Anolis carolinensis). Gen Comp Endocrinol 124:226–235
Lovern MB, Wade J (2003a) Yolk testosterone varies with sex in eggs of the lizard, Anolis carolinensis. J Exp Zool A 295:206–210
Lovern MB, Wade J (2003b) Sex steroids in green anoles (Anolis carolinensis): uncoupled maternal plasma and yolking follicle concentrations, potential embryonic steroidogenesis, and evolutionary implications. Gen Comp Endocrinol 134:109–115
Matsumoto Y, Crews D (2012) Molecular mechanisms of temperature-dependent sex determination in the context of ecological developmental biology. Mol Cell Endocrinol 354:103–110
Paitz RT, Bowden RM (2009) Rapid decline in the concentrations of three yolk steroids during development: is it embryonic regulation? Gen Comp Endocrinol 161:246–251
Radder RS (2007) Maternally derived egg yolk steroid hormones and sex determination: review of a paradox in reptiles. J Biosci 32:1213–1220
Radder RS, Ali S, Shine R (2007) Offspring sex is not related to maternal allocation of yolk steroids in the lizard Bassiana duperreyi (Scincidae). Physiol Biochem Zool 80:220–227
Radder RS, Pike DA, Quinn AE, Shine R (2009) Offspring sex in a lizard depends on egg size. Curr Biol 19:1102–1105
Radder RS, Shine R (2007) Sex-based hatchling asynchrony in an oviparous lizard (Bassiana duperreyi, Scincidae). Austral Ecol 32:502–508
Rhen T, Crews D, Fivizzani A, Elf P (2006) Reproductive tradeoffs and yolk steroids in female leopard geckos, Eublepharis macularius. J Evol Biol 19:1819–1829
Sarre SD, Georges A, Quinn A (2004) The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. Bioessays 26:639–645
Schwabl H (1993) Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA 90:11446–11450
Shine R, Elphick MJ, Donnellan S (2002) Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecol Lett 5:486–489
Tokunaga S (1985) Temperature-dependent sex determination in Gekko japonicus (Gekkonidae, Reptilia). Dev Growth Differ 27:117–120
Valenzuela N, Lance VA (2004) Temperature-dependent sex determination in vertebrates. Smithsonian Institution Press, Washington DC
Wapstra E, Warner DA (2010) Sex allocation and sex determination in squamate reptiles. Sex Dev 4:110–118
Warner DA, Lovern MB, Shine R (2008) Maternal influences on offspring phenotypes and sex ratios in a multi-clutching lizard with environmental sex determination. Biol J Linn Soc 95:256–266
Warner DA, Radder RS, Shine R (2009) Corticosterone exposure during embryonic development affects offspring growth and sex ratios in opposing directions in two lizard species with environmental sex determination. Physiol Biochem Zool 82:363–371
Xu XF, Ji X (2001) Female reproduction and influence of incubation temperature on duration of incubation and hatchling traits in the gecko, Gekko japonicus. Chin J Ecol 20(6):8–11
Yoshida M, Itoh M (1974) Karyotype of the gecko, Gekko japonicus. Chrom Inf Serv 17:29–31
Acknowledgements
Our experimental procedures complied with the current laws on animal welfare and research in China, and were specifically approved by the Animal Research Ethics Committee of Nanjing Normal University (Permit No. AREC 2009-11-015). Funding for this work was supported by grants from Natural Science Foundation of China (30670281 and 31060064), Innovative Team Project of Nanjing Normal University (0319 PM0902) and Priority Academic Program Development of Jiangsu Higher Education Institutions (CXLX11_0885). We thank Tian-Bao Fu, Lai-Gao Luo, Yan-Yan Sun, Yan-Qing Wu and Zong-Shi Zhou for their help during the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Ding, GH., Yang, J., Wang, J. et al. Offspring sex in a TSD gecko correlates with an interaction between incubation temperature and yolk steroid hormones. Naturwissenschaften 99, 999–1006 (2012). https://doi.org/10.1007/s00114-012-0981-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-012-0981-6