Abstract
Extracellular vesicles (EVs) are important carriers of signaling molecules, such as nucleic acids, proteins, and lipids, and have become a focus of increasing interest due to their numerous physiological and pathological functions. For a long time, most studies on EV components focused on noncoding RNAs; however, in recent years, extracellular vesicle proteins (EVPs) have been found to play important roles in diagnosis, treatment, and drug resistance and thus have been considered favorable biomarkers and therapeutic targets for various tumors. In this review, we describe the general protocols of research on EVPs and summarize their multifaceted roles in precision medicine applications, including cancer diagnosis, dynamic monitoring of therapeutic efficacy, drug resistance research, tumor microenvironment interaction research, and anticancer drug delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs), also known as exosomes, microvesicles, microparticles, ectosomes, oncosomes, apoptotic bodies, and many other names [1], are cell-secreted vesicles of different sizes and intracellular origins and are of increasing interest to scientists due to their numerous functions in physiology and pathology [2, 3]. EVs contain diverse biomolecules, including proteins, lipids, metabolites, mitochondrial DNA [4], mRNA [5], other noncoding RNAs, and regulatory RNA [6], that are important for homeostasis; they act by transferring nucleic acids and specific repertoires of proteins and lipids [2, 7]. Of these, extracellular vesicle protein (EVP) components can be categorized into two major types. One type is EVPs found in various cell types. They are involved in vesicle formation and secretion and commonly used as exosome markers; these EVPs include membrane transport and fusion-related proteins (e.g., GTPase), heat shock proteins (e.g., HSP70), members of the four-transmembrane protein superfamily (e.g., CD63), ESCRT complex-related proteins (e.g., Tsg101), and integrins. The other type is EVPs closely related to cell specificity, such as tetraspanins (e.g., CD37 and CD53 in leukocytes), ERBB2 in breast cancer, CD45 in immune cells, and major histocompatibility complex class (MHC) and II [1, 8]. EVs perform many functions in fundamental physiological processes, such as neuronal communication [9], immune response [10], organ development [11], reproductive performance [12], cancer progression [13], cardiovascular disease [14], and inflammation [15]. The role of EVs in cancer research is increasingly appreciated because cancer cells secrete at least tenfold more EVs than normal cells [16, 17] and can be stably detected in various kinds of body fluids, such as blood, urine, saliva, and bronchoalveolar fluid. In addition, by transferring oncogenic proteins and nucleic acids, tumor-derived EVs interact with the microenvironment and are involved in tumorigenesis, growth, progression, and drug resistance [18,19,20].
The incidence of cancer is growing rapidly worldwide, with an estimation of more than 18 million new cases and more than 9 million deaths per year [21,22,23]. EVPs of tumor cells are increasingly drawing attention for tumor monitoring because of their diverse characteristics [24, 25] and applications, ranging from tumor diagnosis [26], prognostication [27], dynamic monitoring, drug resistance, and precise targeted drug delivery [28] (Fig. 1). In this review, we discuss the developments in the isolation and characterization strategies of EVPs and highlight their functions in the tumor microenvironment and recent applications in precision oncology, such as tumor diagnosis, treatment monitoring, and drug delivery.
Precision biomedical applications of extracellular vesicles. A Extracellular vesicles obtained from body fluids, tissues, and cells can be derived from exocytosis and gemmation. B Precision biomedical applications of EV intracellular components. Basic research: detecting biomarker abundance and further targeting anti-tumor immunity through signaling pathways. Applied research: biomarkers are used as disease diagnosis and therapeutic response, further developing vaccines and drug delivery to target tumor
Isolation and identification of EVPs
Most of the studies on EVs focus on their proteins and RNA; however, the research methods are different due to the various purposes and characteristics of RNA and protein (Fig. 2). Existing studies of EVs usually involve (1) isolation, purification, and characterization of EVs and (2) identification of the composition of EVs and analysis of their data profile. The small size, low density, and contaminants that normally coexist with EVs make their isolation and purification difficult and not reproducible. Therefore, efficient enrichment of EVs is currently a major issue and is crucial for downstream analysis. Separation methods should be carefully chosen for different purposes and applications relying on either the physical or biochemical properties. The traditional methods include differential ultracentrifugation, size-exclusion-based chromatography, density gradients, polymer precipitation, and immunoaffinity capture techniques [29]. In addition, recent studies have developed several novel techniques for EV separation and enrichment. Lactoferrin-conjugated 2,2-bis-(hydroxymethyl)-propionic acid dendrimer-modified magnetic nanoparticles (LF-bis-MPA-MNPs), chimeric nanocomposites, were used for simple and sensitive EV isolation and cancer detection from human urine samples [30]. A surface plasmon ultrasensitive method based on polymer dots and AuNPs was used for detecting EVs in pancreatic cancer with high sensitivity [31]. Microfluidic systems with multiple confinement structures can be used for capturing and quantifying circulating EVs from small sample volumes and applied to clinical studies [32]. For the characterization of isolated EVs, electron microscopy, nanoparticle tracking analysis, Western blotting, and flow cytometry are commonly used. These methods have accelerated the study of extracellular vesicles.
Proteins in EVs have a high potential for application in tumor diagnosis due to their stable nature, which can provide rich information, and the extraction of proteins from EVs has become a new hotspot. Traditionally, EVPs are generally extracted in RIPA buffer supplemented with protease inhibitors [33] or obtained by digestion in trypsin. There are differences in the EVPs content separated by different methods and buffers, and it was recommended in MISEV2018 that the properties and concentration of the reagents be clearly indicated [1]. EV lysis with RIPA buffer resulted in the highest detectable amount of proteins when compared against other buffers, such as Tris-SDS lysis, Tris-Triton lysis, and Guanidium chloride lysis, suggesting that RIPA buffer is an optimal lysis buffer for EV proteomics analysis based on mass spectrometry [34]. Trypsin has been also widely used in the literature for protein extraction, and various colorimetric assays, Western blotting, and mass spectrometry have been applied for measurement after isolation of EVs from cells or body fluids [35, 36]. Nevertheless, EV proteins that belong to intravesicular cargo are thought to be resistant to trypsin treatment because trypsin cannot penetrate the vesicular membrane [37]. A strategy based on using membrane impermeant biotin coupled to mass spectrometry analysis has been reported for the analysis of EV surface protein expression, which would avoid compromising EVP integrity by Proteinase K [38]. Several methods have been developed to identify EVPs after the extraction of EVs from body fluids or cells, conventionally including mass spectrometry and enzyme-linked immunosorbent assay (ELISA) methods combined with quantitative reverse transcription polymerase chain reaction (qPCR), Western blotting, or flow cytometry. In addition, several new techniques, such as microfluidic chips [39] and adaptive dynamic artificial poly-ligand targeting (ADAPT) [33], are now available.
With the rapid development of proteomic technologies and analytical methods for EVPs, targeted analysis of the membrane proteins on the surface is of great scientific importance. Tian and colleagues reported a rapid method based on a laboratory-built high-sensitivity flow cytometer (HSFCM) for protein profiling and size detection of EVs in colorectal cancer (CRC) patients. The researchers found that HSFCM allows immunofluorescence staining and enables protein analysis of individual EVs and quantitative counting of different EV subsets [40]. Ko et al. proposed an antibody-based immuno-sequencing method called seiSEQ, which enabled highly sensitive detection of specific proteins at the single EV level by isolating and coding individual EVs using droplet microfluidics, followed by sequencing of barcodes/antibody-DNA to determine protein composition [41]. Moreover, with the development of omics technologies, several databases for studying EV have become available. ExoCarta (http://www.exocarta.org) is a commonly used database on EVs that contains data from more than 286 EV studies, including data on 41,860 proteins, providing data support for conducting EVP studies. All separation, extraction, and analytical techniques have helped us recognize EV and EVP and have accelerated EV-related research.
Functions of EVPs in the tumor microenvironment
The malignancy of a tumor depends not only on the tumor cells themselves but also on the interaction between the tumor and its microenvironment (TME) [42]. The TME consists of various cell types, including stromal cells (e.g., fibroblasts, mesenchymal stromal cells, pericytes, and adipocytes) and immune cells (e.g., T and B lymphocytes, natural killer (NK) cells, and tumor-associated macrophages (TAMs)), all of which can be embedded in the extracellular matrix (ECM) [43, 44]. In recent years, numerous studies have proven that tumor-derived EVPs play important roles in shaping the tumor microenvironment by activating tumorigenesis, remodelling the ECM, re-educating stromal cells, or regulating immune cell interactions, thereby facilitating cancer development [45,46,47].
Tumor-derived EVs contain a range of tumor-specific antigens and ligands that interact with corresponding receptors on immune cells. Immune checkpoint molecules, including PD-1/PD-L1 and CTLA-4, have been reported to be present in EVs isolated from various cancer types, and these EVPs have been implicated in the regulation of the immune microenvironment and response to immunotherapy. Exosomal PD-L1 from tumor cells systemically suppress antitumor immunity by directly binding to PD-1-expressing CD8+ T cells, and the level of plasma exosomal PD-L1 may predict the clinical effectiveness of anti-PD-1 therapy [48]. Poggio et al. also proved that cancer cells can secrete their PD-L1 protein into exosomes and hence suppress T-cell activation [49]. In addition, other tumor-derived EVPs associated with the microenvironment have been reported. A study indicated that HSP72 expressed at the surface of tumor-derived exosomes, acting as a ligand that binds to TLR2 on myeloid-derived suppressor cells (MDSCs), is responsible for activating human MDSCs and triggering their suppressive function via the activation of Stat3 [50]. Similarly, tumor exosomal PGE2 and TGF-β can also induce the accumulation of MDSCs [51]. Fas ligand expressed on tumor-derived exosomes was proven to induce apoptosis of activated T lymphocytes [52]. Moreover, CDCP1 was found to be a novel tumor-associated antigen in EVs from irradiated tumor cells, which can trigger antitumor immunity against primary tumors and experimental lung metastases by enhancing the infiltration of CD8+ and CD4+ T cells [53].
The survival rate of cancer patients treated with immune checkpoint therapy (ICT) and vitamin E (VitE) was significantly increased, and increasing evidence has shown that EVs are involved in the regulation of the tumor immune microenvironment and influence the efficacy of antitumor therapy. VitE enters dendritic cells (DCs) through the SCARB1 receptor and restores the function of tumor-associated DCs by directly binding and inhibiting the protein tyrosine phosphatase SHP1. Enhanced cross-presentation of tumor antigens on DCs and DC-derived extracellular vesicles (DC-EVs) triggers systemic antigen-specific T-cell antitumor immunity [54]. HSPC111-rich EVs secreted by colorectal cancer cells can transform quiescent fibroblasts into tumor-associated fibroblasts, which reprogram their lipid metabolism and promote the synthesis and secretion of acetyl-CoA. Acetyl-CoA promotes the synthesis and secretion of the chemokine CXCL5 through the acetylation of histone H3K27, which binds to the chemokine receptor CXCR2 on the surface of CR cells, promoting high expression of HSPC111 in CR cells and its secretion in EVs. Moreover, acetyl-CoA promotes the proliferation and migration of colorectal cancer cells, triggers epithelial-mesenchymal transition, and induces the metastasis of colorectal cancer cells to the liver [55]. Within the tumor microenvironment, EVs can help cancer cells proliferate and spread, as well as help them evade the immune system, and they can act as carriers of molecules that can kill cancer cells and reactivate the immune system.
Application of EVPs in tumor diagnosis
Currently, mounting evidence supports a role for tumor-derived EVPs in diagnosing or predicting the state of cancer (Table 1), as EVPs have a higher concentration in cancer patients than in nontumor individuals and contain much cancer-associated biological information. In addition, EVPs are considered to have a higher sensitivity and specificity than proteins directly detected in body fluids [56,57,58]. Plasma exosomal ephrin type-A receptor 2 (EphA2) levels in pancreatic cancer (PC) patients who received neoadjuvant chemotherapy or chemoradiotherapy were significantly lower before and after treatment in patients with curable PC but not in those without PC, suggesting that EphA2 could be used for monitoring the curative effect in PC [59]. CPN1, IGHV2-26, ITIH3, CLU, and DNAJB11 derived from plasma exosomes were also found to be associated with PC progression and may represent alternative biomarkers for diagnostic [60, 61]. In another study, Glypican-1 (GPC-1)-positive EVs were proven to be a diagnostic indicator of early PC [62]. Reports have found that CD317, epidermal growth factor receptor (EGFR) in plasma EVs, and human leucine rich alpha-2-glycoprotein 1 (LRG1) in urinary EVs are reliable biomarkers for diagnosing non-small cell lung cancer (NSCLC) [63, 64]. CD91 in EVs, as a lung adenocarcinoma (ADC)-specific antigen, can help identify patients with tumors [65]. In both small cell lung cancer (SCLC) and NSCLC, plasma-derived MUC5B, SELL, and APOH have been shown to be potential biomarkers for the diagnosis of brain and liver metastasis. Latent membrane protein 1 (LMP1) could be detected in EVs from nasopharyngeal carcinoma (NPC) cell lines [66]. Additionally, LMP1 and BARF1 could be detected in EVs in the serum and saliva from teenagers and adults with nasopharyngeal carcinoma [67]. Exosomal CYPA, which associated with Epstein-Barr virus (EVB), has been verified as potential diagnostic biomarker for EVP-positive NPC [68]. Additionally, analysis of the EVPs expression level in the blood sample could distinguish hepatocellular carcinoma (HCC) patients from healthy individuals and revealed significantly elevated galectin-3-binding protein (G3BP) and polymeric immunoglobulin receptor (PIGR) in EVPs of hepatocellular carcinoma (HCC) patients [69]. Tripartite motif-containing 3 (TRIM3) and gastrokine 1 (GKN1) were found to be downregulated in plasma exosomes in gastric cancer (GC) compared with those in healthy controls, so these factors are thought to be novel biomarkers for GC diagnosis and therapeutic targets [70, 71]. Heat shock protein 60 (HSP60), serpin peptidase inhibitor clade A member 1 (SERPINA1) and fibrinogen (PLG) in the plasma EVs of patients is a promising candidate for CR diagnosis [72, 73]. Different cancer types, including BC, CRC, PC, lung cancers, and mesothelioma, could be distinguished by specific combinations of patients’ plasma-derived EVPs, and these cancer-specific EVPs can be used as liquid biopsy tools to help diagnose patients with cancers of unknown primary tumor origin and to guide the treatment of these patients [74].
Numerous studies have confirmed that proteomic analysis could distinguish cancer-derived EVs from normal cell-derived EVs, and EVPs vary with the tissue origin of cancer cells. Szajnik et al. found from 22 patients’ plasma samples that TGF-beta and MAGE3/6 proteins present on exosomes can distinguish ovarian carcinoma from benign tumors or nontumor tissues, and the levels of these tumor-associated proteins correlate with patients’ responses to therapy [75]. A study performed on the proteomic profile of EVs secreted by sixty cell lines from the National Cancer Institute (NCI-60) found that only 213 of 6071 identified EV cargo proteins were common to all cell lines. The researchers further confirmed that most EVPs clustered based on cancer type; that is, each cancer cell type secretes EVs with a unique proteomic cargo, indicating the potential value of EVPs as circulating biomarkers for identifying and diagnosing different cancer types [76]. Hoshino et al. performed a comprehensive proteomic analysis of EVPs from 426 human samples (including tissue explants, plasma, and other bodily fluids) and identified a panel of tumor-associated EVPs (e.g., VCAN, TNC, and THBS2) that could be used as biomarkers for early-stage cancer detection with more than 90% sensitivity and specificity. The specific combinations of these EV proteins could be used to diagnose tumors of unknown primary origin [74]. Hinestrosa et al. developed a blood-based EVP detection method and identified a panel of 13 EVPs that allowed the detection of early-stage pancreatic, ovarian, and bladder cancers with an average sensitivity of 71.2% and specificity of 99.5% [77]. By comparing plasma EVs from 37 CRC patients and 32 healthy individuals using the HSFCM platform, Tian et al. confirmed the high predictive value of CD147 expression in EVs for CRC diagnosis [40]. Therefore, tumor-derived EVPs could be used for early cancer detection and identifying tumors of unknown origin.
Application of EVPs in predicting therapeutic effects
Currently, biomarkers that can predict therapeutic effects are urgently needed to rapidly screen for therapeutically superior options and assist in the clinical adjustment of treatment strategies through real-time monitoring. EVPs, as liquid biopsy tools, similar to CTCs and ctDNA, have been proven to have good efficacy, predictive value, and clinical application prospects in various tumors (Table 2). The results of a clinical trial (NCT02862470) in patients with recurrent thyroid cancer showed that although serum thyroglobulin was not detected in 3 patients with thyroid cancer, the levels of urinary exosomal thyroglobulin were consistently increased, indicating that urinary exosomal thyroglobulin protein is a potential marker for predicting thyroid cancer recurrence [82]. Lin and colleagues found that serum EVP concentration and protein characteristics could be used as prognostic predictors in colorectal liver metastasis (CRLM) patients in both discovery and validation cohorts, which included 56 and 154 CRLM patients, respectively [83]. In addition, the extracellular vesicle protein chemokine ligand 7 (CXCL7) can be used as a biomarker to predict early response in patients with CRLM undergoing chemotherapy [83]. A study established an EVDX signature (defined as a weighted sum of the expression of the following 8 EV markers) by linear discriminant analysis of eight breast cancer-associated serum EVPs (CA15-3, CA125, CEA, HER2, EGFR, PSMA, EpCAM, and VEGF), which can distinguish metastatic breast cancer (MBC) patients from non-MBC patients and healthy donors with an overall accuracy of 91.1% [84]. More importantly, the EVDX signature showed high accuracy in monitoring the MBC treatment response in the training (88.9%), validation (87.9%), and prospective (85.2%) cohorts and was associated with progression-free survival (PFS). Additionally, investigations using EVPs to predict the efficacy of immunotherapy and targeted therapies have been applied in cancer. It was reported that PD-L1 could be secreted extracellularly via EV or in the soluble form, except for its expression on the cell membrane [85]. Moreover, plasma exosomal PD-L1 is closely related to the response to immunotherapy, affecting the prognosis of patients. Furthermore, it has been confirmed in melanoma, GC, and NSCLC that patients with low baseline peripheral blood exo-PD-L1 levels usually have complete or partial responses to PD-1/PD-L1 inhibitor therapy [86,87,88]. Therefore, circulating exosomal PD-L1 is expected to be a new predictive marker for the efficacy of immunotherapy with PD-1 inhibitors. In conclusion, EVPs are increasingly being used to predict and monitor the response to treatment.
Application of EVPs in monitoring drug resistance
Primary or secondary drug resistance was considered the main cause of treatment failure. The secretion of EVs can act as mediators of signal transduction, altering the sensitivity of tumors to chemotherapeutic drugs and participating in tumor progression. Investigation of the drug resistance mechanism of EVs and their associated proteins can be helpful to improve the therapeutic effect of tumors. It has been shown that ovarian cancer cells excrete drug molecules directly by secreting EVs. EVs alter the sensitivity of target cells to chemotherapeutic drugs by transporting active proteins and affect the chemoresistance of ovarian cancer cells by modulating the tumor microenvironment [92]. EVs released from drug-resistant breast cancer cells are rich in EphA2 protein, which promotes tumor invasion and metastasis through the EphA2-Ephrin A1 reverse pathway [93], and the increase in EphA2 in EVs released from drug-resistant cell-derived cells may be an important mechanism of chemoresistance-induced breast cancer progression. EVs from gemcitabine-resistant cells in triple-negative breast cancer promote gemcitabine resistance in sensitive cells through the regulation of EGFR by the extracellular vesicle annexin A6 (ANXA6) protein [94]. Cisplatin-resistant NSCLC cells can be induced to secrete EVs in a hypoxic environment and transfer drug resistance to sensitive NSCLC cells via intercellular delivery of pyruvate kinase isozyme type M2 (PKM2) [95]. Exosomal Wnts from fibroblasts can induce dedifferentiation of tumor cells to promote chemoresistance in CRC, suggesting that interference with exosomal Wnt signaling contributes to greater chemoresistance [96]. The elevated levels of four membrane protein biomarkers in melanoma plasma EVs, melanoma chondroitin sulfate proteoglycan (MCSP), melanoma cell adhesion molecule (MCAM), low-affinity nerve growth factor receptors (LNGFR), and receptor tyrosine protein kinase and ErbB3 may predict resistance to B-Raf proto-oncogene serine/threonine kinase (BRAF) inhibitor therapy in melanoma patients [97]. Research to date strongly supports the pivotal role of EVs in tumor resistance.
Application of EVPs in drug delivery
Compared with liposomes and viral vectors, EVs are characterized by high stability, good biocompatibility, low immunogenicity, and excellent biological barrier penetrability (such as the blood‒brain barrier and placental barrier). Therefore, EVs are considered natural nanomaterials that can be used as drug delivery vehicles with great prospects for targeted therapies [98, 99]. A study fused platelet EVs with photothermal-sensitive liposomes to obtain biomimetic nanocarriers, which not only retained their physiological functions, such as platelet-macrophage escape, tumor selective adhesion, and damaged blood vessel targeting, but also improved drug-carrying capacity [100]. Targeted drug delivery to tumor tissue exerted a synergistic therapeutic effect. Zhang et al. developed an engineered neutrophil-like EV-like nanovesicle that could precisely target tumor tissue for efficient antitumor effects [101]. Antibody-modified and tumor antigen peptide-stimulated EVs from dendritic cells were used as T-cell activators to build “bridges” between cancer cells and activated T cells, thereby enhancing the antitumor immune response of T cells [102]. Engineered macrophage EVs were modified with biodegradable nanodrug carriers for precise sonodynamic therapy of gliomas [103]. In summary, engineered EVs and their protein cargos are expected to be new tools for tumor treatment with minimal side effects.
Conclusion and perspective
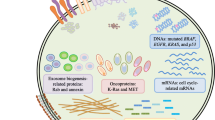
EVPs, which play crucial roles in TME (tumor microenvironment) regulation and drug resistance, represent a new field as an effective therapeutic approach in cell-free medicine and are increasingly used in tumor diagnosis, therapeutic monitoring, and drug-carrying with amazing results based on the advantages of EV stability (Fig. 3). However, for precise clinical treatment, the source of EVs must be selected cautiously. For example, tumor-derived EVs exhibit remarkable targeting ability to tumor cells but also contain bioactive substances that promote tumor progression [104]. To further improve the targeting efficiency of EVs and reduce adverse effects, the integration of multifield technologies can be used to modify EVs so they can respond to specific external triggers (e.g., light, sound, heat, and magnetic fields) to specifically target lesions. Currently, drug loading of EVs can be realized by incubation, transfection, sonication, and electroporation to achieve clinical translation of the therapeutic effects of EVs in tumors.
Notably, although the current findings suggest the great potential of EVPs in precise diagnosis and treatment, there is still much work to be done before they can be used in clinical practice. There is a lack of simple, rapid, and standardized clinical assays for EVs and a lack of experience with clinical trials of EV-related contents. However, the development of a large number of EV-based formulations bodes well for the widespread clinical use of EVPs in the future. Tumor-associated EVPs could be used as biomarkers for early cancer detection, therapeutic response, and potentially for the diagnosis of primary unknown tumors. There is potential for the implementation of routine EVP-based screening in the clinical setting. In the future, exploring the important role of EVPs in vivo and their unique properties will facilitate the beneficial applications of EVs for patients and society.
Data availability
Not applicable.
Abbreviations
- EV:
-
Extracellular vesicle
- MV:
-
Microvesicle
- EVP:
-
Extracellular vesicle protein
- TME:
-
Tumor microenvironment
- ECM:
-
Extracellular matrix
- NK:
-
Natural killer cell
- TAM:
-
Tumor-associated macrophage
- DC:
-
Dendritic cell
- MDSC:
-
Myeloid-derived suppressor cell
- NSCLC:
-
Non-small-cell lung cancer
- HCC:
-
Hepatocellular carcinoma
- BC:
-
Breast cancer
- MBC:
-
Metastatic breast cancer
- PC:
-
Pancreatic cancer
- GC:
-
Gastric cancer
- CRC:
-
Colorectal cancer
- CRLM:
-
Colorectal cancer liver metastases
References
Thery C, Witwer KW, Aikawa E et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750
He C, Zheng S, Luo Y et al (2018) Exosome theranostics: biology and translational medicine. Theranostics 8(1):237–255
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science (American Association for the Advancement of Science) 367(6478):640
Guescini M, Genedani S, Stocchi V et al (2010) Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 117(1):1–4
Valadi H, Ekstrom K, Bossios A et al (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659
Ferguson SW, Nguyen J (2016) Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release 228:179–190
Kaushik S, Cuervo AM (2015) Proteostasis and aging. Nat Med 21(12):1406–1415
Zhang Y, Bi J, Huang J et al (2020) Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed 15:6917–6934
Fruhbeis C, Frohlich D, Kuo WP et al (2013) Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11(7):e1001604
Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14(3):195–208
Korkut C, Ataman B, Ramachandran P et al (2009) Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139(2):393–404
Machtinger R, Laurent LC, Baccarelli AA (2016) Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update 22(2):182–193
Skog J, Wurdinger T, van Rijn S et al (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10(12):1470–1476
Ailawadi S, Wang X, Gu H et al (2015) Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1852(1):1–11
Kulshreshtha A, Ahmad T, Agrawal A et al (2013) Proinflammatory role of epithelial cell–derived exosomes in allergic airway inflammation. Journal of Allergy and Clinical Immunology 131(4):1194–1203
Lee H, Castro CM (2019) Thermophoretically enriched detection. Nat Biomed Eng 3(3):163–164
Balaj L, Lessard R, Dai L et al (2011) Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2:180
Zhang X, Yuan X, Shi H et al (2015) Exosomes in cancer: small particle, big player. J Hematol Oncol 8:83
Kosaka N (2016) Decoding the secret of cancer by means of extracellular vesicles. J Clin Med 5(2):22
Minciacchi VR, Freeman MR, Di Vizio D (2015) Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 40:41–51
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 71(3):209–249
Subedi P, Nembrini S, An Q et al (2019) Telomere length and cancer mortality in American Indians: the Strong Heart Study. Geroscience 41(3):351–361
Csiszar A, Balasubramanian P, Tarantini S et al (2019) Chemically induced carcinogenesis in rodent models of aging: assessing organismal resilience to genotoxic stressors in geroscience research. Geroscience 41(2):209–227
Cheng L, Hill AF (2022) Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discovery 21(5):379–399
Chen Y, McAndrews KM, Kalluri R (2021) Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 18(12):792–804
Morad G, Helmink BA, Sharma P et al (2021) Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184(21):5309–5337
Thakur A, Parra DC, Motallebnejad P et al (2022) Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioactive Materials 10:281–294
Herrmann IK, Wood M, Fuhrmann G (2021) Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol 16(7):748–759
Li P, Kaslan M, Lee SH et al (2017) Progress in exosome isolation techniques. Theranostics 7(3):789–804
Dao T, Kim MG, Koo B et al (2022) Chimeric nanocomposites for the rapid and simple isolation of urinary extracellular vesicles. J Extracell Vesicles 11(2):e12195
Xiong H, Huang Z, Lin Q et al (2022) Surface plasmon coupling electrochemiluminescence immunosensor based on polymer dots and AuNPs for ultrasensitive detection of pancreatic cancer exosomes. Anal Chem 94(2):837–846
Liu C, Guo J, Tian F et al (2017) Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11(7):6968–6976
Domenyuk V, Zhong Z, Stark A et al (2017) Plasma exosome profiling of cancer patients by a next generation systems biology approach. Sci Rep 7:42741
Subedi P, Schneider M, Philipp J et al (2019) Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal Biochem 584:113390
Kowal J, Arras G, Colombo M et al (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113(8):E968–E977
Liu P, Wang W, Wang F et al (2023) Alterations of plasma exosomal proteins and motabolies are associated with the progression of castration-resistant prostate cancer. J Transl Med 21(1):40
Choi D, Go G, Kim DK et al (2020) Quantitative proteomic analysis of trypsin-treated extracellular vesicles to identify the real-vesicular proteins. J Extracell Vesicles 9(1):1757209
Rai A, Fang H, Claridge B et al (2021) Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. Journal of extracellular vesicles 10(13):e12164
Kanwar SS, Dunlay CJ, Simeone DM et al (2014) Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 14(11):1891–1900
Tian Y, Ma L, Gong M et al (2018) Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano 12(1):671–680
Ko J, Wang Y, Sheng K et al (2021) Sequencing-based protein analysis of single extracellular vesicles. ACS Nano 15(3):5631–5638
Galon J, Bruni D (2020) Tumor immunology and tumor evolution: intertwined histories. Immunity 52(1):55–81
Roma-Rodrigues C, Mendes R, Baptista P et al (2019) Targeting tumor microenvironment for cancer therapy. Int J Mol Sci 20(4):840
Gabai Y, Assouline B, Ben-Porath I (2023) Senescent stromal cells: roles in the tumor microenvironment. Trends in Cancer 9(1):28–41
Guo X, Xue H, Shao Q et al (2016) Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget 7(49):80521–80542
Guo X, Qiu W, Wang J et al (2019) Glioma exosomes mediate the expansion and function of myeloid-derived suppressor cells through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways. Int J Cancer 144(12):3111–3126
Qian M, Wang S, Guo X et al (2020) Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene 39(2):428–442
Chen G, Huang AC, Zhang W et al (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560(7718):382–386
Poggio M, Hu T, Pai C et al (2019) Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 177(2):414–427
Chalmin F, Ladoire S, Mignot G et al (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120(2):457–471
Xiang X, Poliakov A, Liu C et al (2009) Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 124(11):2621–2633
Kim JW, Wieckowski E, Taylor DD et al (2005) Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res 11(3):1010–1020
Chen X, Lin W, Huang Z et al (2020) Radiation-induced small extracellular vesicles as “carriages” promote tumor antigens release and trigger antitumor immunity. International Journal of Radiation Oncology Biology Physics 108(3, Supplement):e561
Yuan X, Duan Y, Xiao Y et al (2022) Vitamin E enhances cancer immunotherapy by reinvigorating dendritic cells via targeting checkpoint SHP1. Cancer Discov 12(7):OF1–OF18
Zhang C, Wang XY, Zhang P et al (2022) Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis 13(1):57
He M, Zeng Y (2016) Microfluidic exosome analysis toward liquid biopsy for cancer. SLAS Technology 21(4):599–608
Chen IH, Xue L, Hsu CC et al (2017) Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci U S A 114(12):3175–3180
Li A, Zhang T, Zheng M et al (2017) Exosomal proteins as potential markers of tumor diagnosis. J Hematol Oncol 10(1):175
Liang K, Liu F, Fan J et al (2017) Nanoplasmonic quantification of tumor-derived extracellular vesicles in plasma microsamples for diagnosis and treatment monitoring. Nat Biomed Eng 1:0021
Marin AM, Batista M, Korte De Azevedo AL et al (2023) Screening of exosome-derived proteins and their potential as biomarkers in diagnostic and prognostic for pancreatic cancer. Int J Mol Sci 24(16):12604
Liu P, Zu F, Chen H et al (2022) Exosomal DNAJB11 promotes the development of pancreatic cancer by modulating the EGFR/MAPK pathway. Cell Mol Biol Lett 27(1):87
Li H, Chiang C, Kwak KJ et al (2024) Extracellular vesicular analysis of glypican 1 mRNA and protein for pancreatic cancer diagnosis and prognosis. Adv Sci 11(11):e2306373
Jakobsen KR, Paulsen BS, Baek R et al (2015) Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles 4:26659
Li Y, Zhang Y, Qiu F et al (2011) Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis 32(15):1976–1983
Ueda K, Ishikawa N, Tatsuguchi A et al (2014) Antibody-coupled monolithic silica microtips for highthroughput molecular profiling of circulating exosomes. Sci Rep 4:6232
Keryer-Bibens C, Pioche-Durieu C, Villemant C et al (2006) Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer 6:283
Houali K, Wang X, Shimizu Y et al (2007) A new diagnostic marker for secreted Epstein-Barr Virus–encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res 13(17):4993–5000
Liu L, Zuo L, Yang J et al (2019) Exosomal cyclophilin A as a novel noninvasive biomarker for Epstein-Barr virus associated nasopharyngeal carcinoma. Cancer Med 8(6):3142–3151
Arbelaiz A, Azkargorta M, Santos-Laso Á et al (2017) Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. J Hepatol 66(1, Supplement):S207–S208
Fu H, Yang H, Zhang X et al (2018) Exosomal TRIM3 is a novel marker and therapy target for gastric cancer. J Exp Clin Cancer Res 37(1):162
Yoon JH, Ham IH, Kim O et al (2018) Gastrokine 1 protein is a potential theragnostic target for gastric cancer. Gastric Cancer 21(6):956–967
Campanella C, Rappa F, Sciume C et al (2015) Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 121(18):3230–3239
Li L, Song X, Chen G et al (2023) Plasma exosomal protein PLG and SERPINA1 in colorectal cancer diagnosis and coagulation abnormalities. J Cancer Res Clin Oncol 149(11):8507–8519
Hoshino A, Kim HS, Bojmar L et al (2020) Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 182(4):1044–1061
Szajnik M, Derbis M, Lach M et al (2013) Exosomes in plasma of patients with ovarian carcinoma: potential biomarkers of tumor progression and response to therapy. Gynecol Obstet (Sunnyvale) 4:3
Hurwitz SN, Rider MA, Bundy JL et al (2016) Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 7(52):86999–87015
Hinestrosa JP, Kurzrock R, Lewis JM et al (2022) Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Communications Medicine 2(1):29
Chang W, Zhu J, Yang D et al (2023) Plasma versican and plasma exosomal versican as potential diagnostic markers for non-small cell lung cancer. Respir Res 24(1):140
Luo B, Que Z, Lu X et al (2023) Identification of exosome protein panels as predictive biomarkers for non-small cell lung cancer. Biol Proced Online 25(1):29
Li S, Qu Y, Liu L et al (2023) Comparative proteomic profiling of plasma exosomes in lung cancer cases of liver and brain metastasis. Cell Biosci 13(1):180
Lapitz A, Azkargorta M, Milkiewicz P et al (2023) Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J Hepatol 79(1):93–108
Huang TY, Wang CY, Chen KY et al (2020) Urinary exosomal thyroglobulin in thyroid cancer patients with post-ablative therapy: a new biomarker in thyroid cancer. Front Endocrinol (Lausanne) 11:382
Lin K, Baenke F, Lai X et al (2022) Comprehensive proteomic profiling of serum extracellular vesicles in patients with colorectal liver metastases identifies a signature for non-invasive risk stratification and early-response evaluation. Mol Cancer 21(1):91
Tian F, Zhang S, Liu C et al (2021) Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat Commun 12(1):2536
Fan Y, Che X, Qu J et al (2019) Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann Surg Oncol 26(11):3745–3755
Zhang C, Fan Y, Che X et al (2020) Anti-PD-1 therapy response predicted by the combination of exosomal PD-L1 and CD28. Front Oncol 10:760
Del Re M, van Schaik RHN, Fogli S et al (2021) Blood-based PD-L1 analysis in tumor-derived extracellular vesicles: applications for optimal use of anti-PD-1/PD-L1 axis inhibitors. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1875(1):188463
Serratì S, Guida M, Di Fonte R et al (2022) Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol Cancer 21(1):20
Li Q, Lv M, Lv L et al (2023) Identifying HER2 from serum-derived exosomes in advanced gastric cancer as a promising biomarker for assessing tissue HER2 status and predicting the efficacy of trastuzumab-based therapy. Cancer Med 12(4):4110–4124
Viktorsson K, Hååg P, Shah CH et al (2022) Profiling of extracellular vesicles of metastatic urothelial cancer patients to discover protein signatures related to treatment outcome. Mol Oncol 16(20):3620–3641
Shuen T, Alunni-Fabbroni M, Ocal E et al (2022) Extracellular vesicles may predict response to radioembolization and sorafenib treatment in advanced hepatocellular carcinoma: an exploratory analysis from the SORAMIC trial. Clin Cancer Res 28(17):3890–3901
Tian W, Lei N, Zhou J et al (2022) Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis 13(1):64
Gao Z, Han X, Zhu Y et al (2021) Drug-resistant cancer cell-derived exosomal EphA2 promotes breast cancer metastasis via the EphA2-Ephrin A1 reverse signaling. Cell Death Dis 12(5):414
Li T, Tao Z, Zhu Y et al (2021) Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer. Cell Death Dis 12(7):684
Wang D, Zhao C, Xu F et al (2021) Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics 11(6):2860–2875
Hu YB, Yan C, Mu L et al (2019) Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene 38(11):1951–1965
Melo SA, Luecke LB, Kahlert C et al (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523(7559):177–182
Goncharova EA, Farkas L (2022) Stem cell-derived nanovesicles for the treatment of pulmonary hypertension: are we there yet?. Am J Respir Cell Mol Biol 67(1):3–5
Liu C, Yan X, Zhang Y et al (2022) Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology 20(1):206
Ma Y, Zhang Y, Han R et al (2022) A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials 282:121384
Zhang J, Ji C, Zhang H et al (2022) Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv 8(2):eabj8207
Fan M, Liu H, Yan H et al (2022) A CAR T-inspiring platform based on antibody-engineered exosomes from antigen-feeding dendritic cells for precise solid tumor therapy. Biomaterials 282:121424
Wu T, Liu Y, Cao Y et al (2022) Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater 34(15):e2110364
Taghikhani A, Farzaneh F, Sharifzad F et al (2020) Engineered tumor-derived extracellular vesicles: potentials in cancer immunotherapy. Front Immunol 11:221
Acknowledgements
We thank all our collaborators and colleagues for their comments and suggestions on this review. Figures were created with BioRender (https://biorender.com/).
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82072728 and 82272858) and R&D Program of Guangzhou Laboratory (No. SRPG22-019).
Author information
Authors and Affiliations
Contributions
J.G. and S.W. conceptualized and supervised the project. H.L. and C.Z. wrote the manuscript. F.W. and F.J. proofread the manuscript draft. Q.Z. and X.Y. critically reviewed the manuscript. All the authors have read and approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Ethics approval
N/A.
Informed consent
N/A.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liao, H., Zhang, C., Wang, F. et al. Tumor-derived extracellular vesicle proteins as new biomarkers and targets in precision oncology. J Mol Med 102, 961–971 (2024). https://doi.org/10.1007/s00109-024-02452-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-024-02452-6