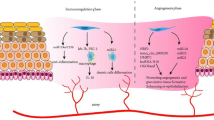
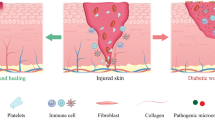
Abstract
As time goes by, the morbidity of diabetes mellitus continues to rise, and the economic burden of diabetic foot ulcers as a common and serious complication of diabetes is increasing. However, currently there is no unified clinical treatment strategy for this complication, and the therapeutic efficacy is unsatisfactory. Recent studies have revealed that biological effects of exosomes involved in multiple stages of the process of wound closure are similar to source cells. Compared with source cells, exosomes possess lowly immunogenicity, highly stability and easily stored, etc. Accumulating evidence confirmed that exosomes promote diabetic wound healing through various pathways such as promoting angiogenesis, collagen fiber deposition, and inhibiting inflammation. The superior therapeutic efficacy of exosomes in accelerating diabetic cutaneous wound healing has attracted an increasing attention. Notably, the molecular mechanisms of exosomes vary among different sources in the chronic wound closure of diabetes. This review focuses on the specific roles and mechanisms of different cell- or tissue-derived exosomes relevant to wound healing. Additionally, the paper provides an overview of the current pre-clinical and clinical applications of exosomes, illustrates their special advantages in wound repair. Furthermore, we discuss the potential obstacles and various solutions for future research on exosomes in the management of diabetic foot ulcer. The aim is to offer novel insights and approaches for the treatment of diabetic foot ulcer.


Similar content being viewed by others
Data availability
Not applicable.
References
Armstrong DG, Boulton AJM, Bus SA (2017) Diabetic foot ulcers and their recurrence. N Engl J Med 376(24):2367–2375. https://doi.org/10.1056/NEJMra1615439
Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ (2016) Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med 33(11):1493–1498. https://doi.org/10.1111/dme.13054
Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Schaper N (2007) High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 50(1):18–25. https://doi.org/10.1007/s00125-006-0491-1
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289. https://doi.org/10.2741/1184
Chang M, Nguyen TT (2021) Strategy for treatment of infected diabetic foot ulcers. Acc Chem Res 54(5):1080–1093. https://doi.org/10.1021/acs.accounts.0c00864
Chudzik W, Kaczorowska B, Przybyla M, Chudzik B, Galka M (2007) Diabetic neuropathy (Neuropatia cukrzycowa). Pol Merkur Lekarski 22(127):66–69. https://www.ncbi.nlm.nih.gov/pubmed/17477095
Kanter JE, Averill MM, Leboeuf RC, Bornfeldt KE (2008) Diabetes-accelerated atherosclerosis and inflammation. Circ Res 103(8):e116-117. https://doi.org/10.1161/CIRCRESAHA.108.182642
Salazar JJ, Ennis WJ, Koh TJ (2016) Diabetes medications: impact on inflammation and wound healing. J Diabetes Complications 30(4):746–752. https://doi.org/10.1016/j.jdiacomp.2015.12.017
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019) Wound healing: a cellular perspective. Physiol Rev 99(1):665–706. https://doi.org/10.1152/physrev.00067.2017
Guan Y, Niu H, Liu Z, Dang Y, Shen J, Zayed M, Guan J (2021) Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv 7(35). https://doi.org/10.1126/sciadv.abj0153
Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD (2004) Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 363(9411):783–784. https://doi.org/10.1016/S0140-6736(04)15695-X
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Lyden D (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527(7578):329–335. https://doi.org/10.1038/nature15756
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY (2018) New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact 292:76–83. https://doi.org/10.1016/j.cbi.2018.07.008
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478). https://doi.org/10.1126/science.aau6977
Li P, Kaslan M, Lee SH, Yao J, Gao Z (2017) Progress in exosome isolation techniques Theranostics 7(3):789–804. https://doi.org/10.7150/thno.18133
Lin J, Wang Z, Huang J, Tang S, Saiding Q, Zhu Q, Cui W (2021) Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small 17(11):e2007235. https://doi.org/10.1002/smll.202007235
Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Xu W (2017) hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther 25(2):465–479. https://doi.org/10.1016/j.ymthe.2016.11.019
Zheng D, Huo M, Li B, Wang W, Piao H, Wang Y, Liu K (2020) The role of exosomes and exosomal microRNA in cardiovascular disease. Front Cell Dev Biol 8:616161. https://doi.org/10.3389/fcell.2020.616161
Li D, Wu N (2022) Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res Clin Pract 187:109882. https://doi.org/10.1016/j.diabres.2022.109882
He C, Zheng S, Luo Y, Wang B (2018) Exosome theranostics: biology and translational medicine. Theranostics 8(1):237–255. https://doi.org/10.7150/thno.21945
Zhao R, Zhao T, He Z, Cai R, Pang W (2021) Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte 10(1):587–604. https://doi.org/10.1080/21623945.2021.1983242
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Wang T (2020) Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics 10(8):3684–3707. https://doi.org/10.7150/thno.41580
Momen-Heravi F (2017) Isolation of extracellular vesicles by ultracentrifugation. Methods Mol Biol 1660:25–32. https://doi.org/10.1007/978-1-4939-7253-1_3
Shu S, Yang Y, Allen CL, Hurley E, Tung KH, Minderman H, Ernstoff MS (2020) Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles 9(1):1692401. https://doi.org/10.1080/20013078.2019.1692401
Jiawei S, Zhi C, Kewei T, Xiaoping L (2022) Magnetic bead-based adsorption strategy for exosome isolation. Front Bioeng Biotechnol 10:942077. https://doi.org/10.3389/fbioe.2022.942077
Keshtkar S, Azarpira N, Ghahremani MH (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9(1):63. https://doi.org/10.1186/s13287-018-0791-7
Wu P, Zhang B, Shi H, Qian H, Xu W (2018) MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy 20(3):291–301. https://doi.org/10.1016/j.jcyt.2017.11.002
Li J, Komatsu H, Poku EK, Olafsen T, Huang KX, Huang LA, Kandeel FR (2022) Biodistribution of intra-arterial and intravenous delivery of human umbilical cord mesenchymal stem cell-derived extracellular vesicles in a rat model to guide delivery strategies for diabetes therapies. Pharmaceuticals (Basel) 15(5). https://doi.org/10.3390/ph15050595
Jiang S, Xu L (2020) Exosomes from gingival mesenchymal stem cells enhance migration and osteogenic differentiation of pre-osteoblasts. Pharmazie 75(11):576–580. https://doi.org/10.1691/ph.2020.0652
Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, Liu G (2021) Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology 19(1):150. https://doi.org/10.1186/s12951-021-00894-5
Lee HD, Kim YH, Kim DS (2014) Exosomes derived from human macrophages suppress endothelial cell migration by controlling integrin trafficking. Eur J Immunol 44(4):1156–1169. https://doi.org/10.1002/eji.201343660
Mi B, Chen L, Xiong Y, Yan C, Xue H, Panayi AC, Liu G (2020) Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J Nanobiotechnology 18(1):68. https://doi.org/10.1186/s12951-020-00624-3
Park GH, Kwon HH, Seok J, Yang SH, Lee J, Park BC, Park KY (2023) Efficacy of combined treatment with human adipose tissue stem cell-derived exosome-containing solution and microneedling for facial skin aging: A 12-week prospective, randomized, split-face study. J Cosmet Dermatol. https://doi.org/10.1111/jocd.15872
Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Guo X (2017) GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol 8:904. https://doi.org/10.3389/fphys.2017.00904
Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ (2017) Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med 6(3):736–747. https://doi.org/10.5966/sctm.2016-0275
Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Han W (2015) LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med 13:308. https://doi.org/10.1186/s12967-015-0642-6
Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, Xiao Z (2021) Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology 19(1):202. https://doi.org/10.1186/s12951-021-00942-0
Wang R, Ji Q, Meng C, Liu H, Fan C, Lipkind S, Xu Q (2020) Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int Immunopharmacol 81:106030. https://doi.org/10.1016/j.intimp.2019.106030
Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z, Qiu G (2020) Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe3O4 nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomedicine 15:7979–7993. https://doi.org/10.2147/IJN.S275650
Yang J, Chen Z, Pan D, Li H, Shen J (2020) Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine 15:5911–5926. https://doi.org/10.2147/IJN.S249129
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Xu W (2015) HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33(7):2158–2168. https://doi.org/10.1002/stem.1771
Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Wang Y (2015) Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med 13:49. https://doi.org/10.1186/s12967-015-0417-0
Zhang X, Jiang Y, Huang Q, Wu Z, Pu H, Xu Z, Peng Z (2021) Exosomes derived from adipose-derived stem cells overexpressing glyoxalase-1 protect endothelial cells and enhance angiogenesis in type 2 diabetic mice with limb ischemia. Stem Cell Res Ther 12(1):403. https://doi.org/10.1186/s13287-021-02475-7
Zhang Y, Yan J, Liu Y, Chen Z, Li X, Tang L, Zhang G (2021) Human amniotic fluid stem cell-derived exosomes as a novel cell-free therapy for cutaneous regeneration. Front Cell Dev Biol 9:685873. https://doi.org/10.3389/fcell.2021.685873
Zhao B, Li X, Shi X, Shi X, Zhang W, Wu G, Hu D (2018) Exosomal microRNAs derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int 2018:5420463. https://doi.org/10.1155/2018/5420463
Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Li M (2018) Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med 50(4):1–14. https://doi.org/10.1038/s12276-018-0058-5
Ma T, Fu B, Yang X, Xiao Y, Pan M (2019) Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/beta-catenin signaling in cutaneous wound healing. J Cell Biochem 120(6):10847–10854. https://doi.org/10.1002/jcb.28376
Shiekh PA, Singh A, Kumar A (2020) Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 249:120020. https://doi.org/10.1016/j.biomaterials.2020.120020
Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Mao C (2019) Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 9(1):65–76. https://doi.org/10.7150/thno.29766
Zhou Y, Zhao B, Zhang XL, Lu YJ, Lu ST, Cheng J, Zhang J (2021) Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther 12(1):257. https://doi.org/10.1186/s13287-021-02287-9
Zhang L, Ouyang P, He G, Wang X, Song D, Yang Y, He X (2021) Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med 25(4):2148–2162. https://doi.org/10.1111/jcmm.16192
Shen K, Wang XJ, Liu KT, Li SH, Li J, Zhang JX, Wang HT, Hu DH (2022) Effects of exosomes from human adipose-derived mesenchymal stem cells on inflammatory response of mouse RAW264. 7 cells and wound healing of full-thickness skin defects in mice. Zhonghua Shao Shang Za Zhi 38(3):215–226
Liu D, Cao Y, Jiang P, Wang Y, Lu Y, Ji Z, Liu W (2023) Tough, Transparent, and Slippery PVA Hydrogel Led by Syneresis. Small 19(14):e2206819. https://doi.org/10.1002/smll.202206819
Xin Zhou WY, Zhang X (2021) Preparation of mesenchymal stem cell exosome-thermosensitive hydrogel and its application in epidermal wound repair. Chin J Pharm 52(09):1199–1207 (in Chinese)
Kwon HH, Yang SH, Lee J, Park BC, Park KY, Jung JY, Park GH (2020) Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: a 12-week prospective, double-blind, randomized, split-face study. Acta Derm Venereol 100(18):adv00310. https://doi.org/10.2340/00015555-3666
Tutuianu R, Rosca AM, Iacomi DM, Simionescu M, Titorencu I (2021) Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int J Mol Sci 22(12). https://doi.org/10.3390/ijms22126239
Yu M, Liu W, Li J, Lu J, Lu H, Jia W, Liu F (2020) Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther 11(1):350. https://doi.org/10.1186/s13287-020-01824-2
Ding J, Wang X, Chen B, Zhang J, Xu J (2019) Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int 2019:9742765. https://doi.org/10.1155/2019/9742765
Liu J, Yan Z, Yang F, Huang Y, Yu Y, Zhou L, Yan Y (2021) Exosomes derived from human umbilical cord mesenchymal stem cells accelerate cutaneous wound healing by enhancing angiogenesis through delivering angiopoietin-2. Stem Cell Rev Rep 17(2):305–317. https://doi.org/10.1007/s12015-020-09992-7
Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y, Liu G (2022) Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis. Front Bioeng Biotechnol 10:829868. https://doi.org/10.3389/fbioe.2022.829868
Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Xing X (2016) Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Transl Med 5(10):1425–1439. https://doi.org/10.5966/sctm.2015-0367
Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X (2021) Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl 120:111671. https://doi.org/10.1016/j.msec.2020.111671
Ye C, Zhang Y, Su Z, Wu S, Li Y, Yi J, Zheng Y (2022) hMSC exosomes as a novel treatment for female sensitive skin: an in vivo study. Front Bioeng Biotechnol 10:1053679. https://doi.org/10.3389/fbioe.2022.1053679
Mathew SA, Naik C, Cahill PA, Bhonde RR (2020) Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol Life Sci 77(2):253–265. https://doi.org/10.1007/s00018-019-03268-1
Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, Morita I (2017) Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther 8(1):219. https://doi.org/10.1186/s13287-017-0660-9
Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE (2013) Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 8(7):e68451. https://doi.org/10.1371/journal.pone.0068451
Du W, Zhang K, Zhang S, Wang R, Nie Y, Tao H, Li Z (2017) Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 133:70–81. https://doi.org/10.1016/j.biomaterials.2017.04.030
Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, Aguila-Diaz C, Fernandez A, Figueroa FE, Khoury M (2015) Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther 6:32. https://doi.org/10.1186/s13287-015-0013-5
Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E (2019) Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med 13(4):555–568. https://doi.org/10.1002/term.2799
Fawzy El-Sayed KM, Dorfer CE (2016) Gingival mesenchymal stem/progenitor cells: a unique tissue engineering gem. Stem Cells Int 2016:7154327. https://doi.org/10.1155/2016/7154327
Burgess DJ (2014) Signalling: vesicle vehicles of genetic information. Nat Rev Genet 15(8):514. https://doi.org/10.1038/nrg3780
Vendramin FS, Franco D, Nogueira CM, Pereira MS, Franco TR (2006) Platelet-rich plasma and growth factors: processing technique and application in plastic surgery. Revista do Colégio Brasileiro de Cirurgiões 33(1)
Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ (2017) Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 7(1):81–96. https://doi.org/10.7150/thno.16803
Xu N, Wang L, Guan J, Tang C, He N, Zhang W, Fu S (2018) Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol 117:102–107. https://doi.org/10.1016/j.ijbiomac.2018.05.066
Li X, Jiang C, Zhao J (2016) Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications 30(6):986–992. https://doi.org/10.1016/j.jdiacomp.2016.05.009
Yi M, Wu Y, Long J, Liu F, Liu Z, Zhang YH, Li J (2019) Exosomes secreted from osteocalcin-overexpressing endothelial progenitor cells promote endothelial cell angiogenesis. Am J Physiol Cell Physiol 317(5):C932–C941. https://doi.org/10.1152/ajpcell.00534.2018
Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, Wang Y (2016) Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci 12(12):1472–1487. https://doi.org/10.7150/ijbs.15514
Yu Q, Liu L, Zhang X, Chang H, Ma S, Xie Z, Zhang Q (2022) MiR-221–3p targets HIPK2 to promote diabetic wound healing. Microvasc Res 140:104306. https://doi.org/10.1016/j.mvr.2021.104306
Kim S, Lee SK, Kim H, Kim TM (2018) Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci 19(10). https://doi.org/10.3390/ijms19103119
Bo Y, Yang L, Liu B, Tian G, Li C, Zhang L, Yan Y (2022) Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing through miR-762 mediated promotion of keratinocytes and endothelial cells migration. J Nanobiotechnology 20(1):291. https://doi.org/10.1186/s12951-022-01504-8
Wgealla M, Liang H, Chen R, Xie Y, Li F, Qin M, Zhang X (2022) Amniotic fluid derived stem cells promote skin regeneration and alleviate scar formation through exosomal miRNA-146a-5p via targeting CXCR4. J Cosmet Dermatol 21(10):5026–5036. https://doi.org/10.1111/jocd.14956
Balbi C, Piccoli M, Barile L, Papait A, Armirotti A, Principi E, Bollini S (2017) First characterization of human amniotic fluid stem cell extracellular vesicles as a powerful paracrine tool endowed with regenerative potential. Stem Cells Transl Med 6(5):1340–1355. https://doi.org/10.1002/sctm.16-0297
Wgealla M, Liang H, Chen R, Xie Y, Li F, Qin M, Zhang X (2022) Amniotic fluid derived stem cells promote skin regeneration and alleviate scar formation through exosomal miRNA-146a-5p via targeting CXCR4. J Cosmet Dermatol. https://doi.org/10.1111/jocd.14956
Zhang D, Wei G, Li P, Zhou X, Zhang Y (2014) Urine-derived stem cells: A novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis 1(1):8–17. https://doi.org/10.1016/j.gendis.2014.07.001
Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y, Xie H (2018) Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics 8(6):1607–1623. https://doi.org/10.7150/thno.22958
Dunnwald M, Tomanek-Chalkley A, Alexandrunas D, Fishbaugh J, Bickenbach JR (2001) Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp Dermatol 10(1):45–54. https://doi.org/10.1034/j.1600-0625.2001.100106.x
Redvers RP, Li A, Kaur P (2006) Side population in adult murine epidermis exhibits phenotypic and functional characteristics of keratinocyte stem cells. Proc Natl Acad Sci USA 103(35):13168–13173. https://doi.org/10.1073/pnas.0602579103
Walmsley GG, Maan ZN, Wong VW, Duscher D, Hu MS, Zielins ER, Longaker MT (2015) Scarless wound healing: chasing the holy grail. Plast Reconstr Surg 135(3):907–917. https://doi.org/10.1097/PRS.0000000000000972
Wang P, Theocharidis G, Vlachos IS, Kounas K, Lobao A, Shu B,Veves A (2022) Exosomes derived from epidermal stem cells improve diabetic wound healing. J Invest Dermatol 142(9):2508–2517. https://doi.org/10.1016/j.jid.2022.01.030
Duan M, Zhang Y, Zhang H, Meng Y, Qian M, Zhang G (2020) Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-beta1 in wound healing. Stem Cell Res Ther 11(1):452. https://doi.org/10.1186/s13287-020-01971-6
Jia R, Li J, Rui C, Ji H, Ding H, Lu Y, Sun L (2015) Comparative proteomic profile of the human umbilical cord blood exosomes between normal and preeclampsia pregnancies with high-resolution mass spectrometry. Cell Physiol Biochem 36(6):2299–2306. https://doi.org/10.1159/000430193
Kim S, Kim Y, Hyun YS, Choi H, Kim SY, Kim TG (2021) Exosomes from human cord blood plasma accelerate cutaneous wound healing by promoting fibroblast function, angiogenesis, and M2 macrophage differentiation. Biomater Sci 9(8):3028–3039. https://doi.org/10.1039/d0bm01801e
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, Xie H (2018) Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 8(1):169–184. https://doi.org/10.7150/thno.21234
Zhao D, Yu Z, Li Y, Wang Y, Li Q, Han D (2020) GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J Mol Histol 51(3):251–263. https://doi.org/10.1007/s10735-020-09877-6
Yuan M, Liu K, Jiang T, Li S, Chen J, Wu Z, Chen Z (2022) GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J Nanobiotechnology 20(1):147. https://doi.org/10.1186/s12951-022-01354-4
Zhang Q, Lai D (2020) Application of human amniotic epithelial cells in regenerative medicine: a systematic review. Stem Cell Res Ther 11(1):439. https://doi.org/10.1186/s13287-020-01951-w
Farhadihosseinabadi B, Farahani M, Tayebi T, Jafari A, Biniazan F, Modaresifar K, Niknejad H (2018) Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol 46(sup2):431–440. https://doi.org/10.1080/21691401.2018.1458730
Zhao B, Wu GF, Zhang YJ, Zhang W, Yang FF, Xiao D, Hu DH (2017) Effects of human amniotic epithelial stem cells-derived exosomes on healing of wound with full-thickness skin defect in rats. Zhonghua Shao Shang Za Zhi 33(1):18–23. https://doi.org/10.3760/cma.j.issn.1009-2587.2017.01.005
Zhao B, Zhang Y, Han S, Zhang W, Zhou Q, Guan H, Hu D (2017) Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol 48(2):121–132. https://doi.org/10.1007/s10735-017-9711-x
Wei P, Zhong C, Yang X, Shu F, Xiao S, Gong T, Xia Z (2020) Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma 8:tkaa020. https://doi.org/10.1093/burnst/tkaa020
Landen NX, Li D, Stahle M (2016) Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci 73(20):3861–3885. https://doi.org/10.1007/s00018-016-2268-0
Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y (2019) Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Biotechnol 47(1):3793–3803. https://doi.org/10.1080/21691401.2019.1669617
Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH (2019) Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci (Weinh) 6(20):1900513. https://doi.org/10.1002/advs.201900513
Han X, Wu P, Li L, Sahal HM, Ji C, Zhang J, Xu W (2021) Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the Akt/beta-catenin pathway. Cell Cycle 20(5–6):616–629. https://doi.org/10.1080/15384101.2021.1894813
Thulabandu V, Chen D, Atit RP (2018) Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol 7(2). https://doi.org/10.1002/wdev.307
Chen L, Qin L, Chen C, Hu Q, Wang J, Shen J (2021) Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol Int 45(9):1976–1985. https://doi.org/10.1002/cbin.11627
Li Y, Yu Y, Xie Z, Ye X, Liu X, Xu B, Mao J (2021) Serum-derived exosomes accelerate scald wound healing in mice by optimizing cellular functions and promoting Akt phosphorylation. Biotechnol Lett 43(8):1675–1684. https://doi.org/10.1007/s10529-021-03148-4
Han Y, Jia L, Zheng Y, Li W (2018) Salivary exosomes: emerging roles in systemic disease. Int J Biol Sci 14(6):633–643. https://doi.org/10.7150/ijbs.25018
Liu M, Liu Z, Chen Y, Peng S, Yang J, Chen C, He W (2022) Dendritic epidermal T cells secreting exosomes promote the proliferation of epidermal stem cells to enhance wound re-epithelialization. Stem Cell Res Ther 13(1):121. https://doi.org/10.1186/s13287-022-02783-6
Geiger A, Walker A, Nissen E (2015) Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem Biophys Res Commun 467(2):303–309. https://doi.org/10.1016/j.bbrc.2015.09.166
Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Qian H (2018) Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving beta-cell destruction. ACS Nano 12(8):7613–7628. https://doi.org/10.1021/acsnano.7b07643
Man K, Brunet MY, Jones MC, Cox SC (2020) Engineered extracellular vesicles: tailored-made nanomaterials for medical applications. Nanomaterials (Basel) 10(9). https://doi.org/10.3390/nano10091838
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P (2020) Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine 15:6917–6934. https://doi.org/10.2147/IJN.S264498
Jahangir MA, Khan R, Sarim Imam S (2018) Formulation of sitagliptin-loaded oral polymeric nano scaffold: process parameters evaluation and enhanced anti-diabetic performance. Artif Cells Nanomed Biotechnol 46(sup1):66–78. https://doi.org/10.1080/21691401.2017.1411933
Funding
This work was supported by Nantong Grant from the Key special science and technology plan projects of COVID-19 for People's livelihood (No. H202136).
Author information
Authors and Affiliations
Contributions
All authors contributed to the review. All authors were involved in literature search and writing of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and agreed to the submitted version of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Yu, Jianxin Qin, and Jiajun Xing contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, L., Qin, J., Xing, J. et al. The mechanisms of exosomes in diabetic foot ulcers healing: a detailed review. J Mol Med 101, 1209–1228 (2023). https://doi.org/10.1007/s00109-023-02357-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02357-w