Abstract
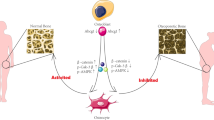
Chaperone-mediated autophagy (CMA) plays multiple roles in cell metabolism. We found that lysosome-associated membrane protein type 2A (LAMP2A), a crucial protein of CMA, plays a key role in the control of mesenchymal stem cell (MSC) adipo-osteogenesis. We identified a differentially expressed CMA gene (LAMP2) in GEO datasets (GSE4911 and GSE494). Further, we performed co-expression analyses to define the relationships between CMA components genes and other relevant genes including Col1a1, Runx2, Wnt3 and Gsk3β. Mouse BMSCs (mMSCs) exhibiting Lamp2a gene knockdown (LA-KD) and overexpression (LA-OE) were created using an adenovirus system; then we investigated LAMP2A function in vitro by Western blot, Oil Red staining, ALP staining, ARS staining and Immunofluorescence analysis. Next, we used a modified mouse model of tibial fracture to investigate LAMP2A function in vivo. LAMP2A knockdown in mMSCs decreased the levels of osteogenic-specific proteins (COL1A1 and RUNX2) and increased those of the adipogenesis markers PPARγ and C/EBPα; LAMP2A overexpression had the opposite effects. The active-β-catenin and phospho-GSK3β (Ser9) levels were upregulated by LAMP2A overexpression and downregulated by LAMP2A knockdown. In the mouse model of tibial fracture, mMSC-overexpressing LAMP2A improved bone healing, as demonstrated by microcomputed tomography and histological analyses. In summary, LAMP2A positively regulates mMSC osteogenesis and suppresses adipo-osteogenesis, probably via Wnt/β-catenin/GSK3β signaling. LAMP2A promoted fracture-healing in the mouse model of tibial fracture.
Key messages
• LAMP2 positively regulates the mBMSCs osteogenic differentiation.
• LAMP2 negatively regulates the mBMSCs adipogenic differentiation.
• LAMP2 regulates mBMSCs osteogenesis via Wnt/β-catenin/GSK3β signaling pathway.
• LAMP2 overexpression mBMSCs promote the fracture healing.










Similar content being viewed by others
Data availability statement
The gene expression databases of mice wildtype and RUNX2 knockout humeri (GSE4911-GPL83), human non-union skeletal fracture samples and normal samples (GSE494-GPL92) were downloaded from Gene Expression Omnibus (GEO) datasets. The raw expression data of LAMP2, HSPA8, COL1A1, RUNX2, WNT3 and GSK3B genes were downloaded from the Cancer Cell Line Encyclopedia (CCLE) project (https://portals.broadinstitute.org/ccle), The Cancer Genome Atlas (TCGA) project (http://cancergenome.nih.gov/) and the Genotype Tissue Expression (GTEx) project (https://www.gtexportal.org/).
Abbreviations
- BMSCs:
-
bone marrow mesenchymal stem cells
- mMSCs:
-
mouse bone marrow mesenchymal stem cells
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
differentially expressed genes
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene Ontology
- BP:
-
biological process
- CCLE:
-
the Cancer Cell Line Encyclopedia project
- TCGA:
-
The Cancer Genome Atlas project
- GTEx:
-
the Genotype Tissue Expression project
- IHC:
-
immunohistochemistry
- ALP:
-
alkaline phosphatase
- ARS:
-
alizarin red staining
- CMA:
-
chaperone-mediated autophagy
- LAMP2A:
-
lysosome-associated membrane protein type 2Α
- HSPA8:
-
heat shock protein A8
- COL1A1:
-
collagen type I alpha 1 chain
- RUNX2:
-
runt-related transcription factor 2
- OPN:
-
osteopontin
- PPARγ:
-
peroxisome proliferator-activated receptor gamma
- C/EBPα:
-
CCAAT enhancer binding protein alpha
- WNT3:
-
wnt family member 3
- GSK3β:
-
glycogen synthase kinase 3 beta
References
Rui P, Kang K (2017) National Hospital Ambulatory Medical Care Survey: 2017 emergency department summary tables. Natl Ambul Med Care Surv 37
Zura R, Xiong Z, Einhorn T et al (2016) Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg 151:e162775. https://doi.org/10.1001/jamasurg.2016.2775
Hak DJ, Fitzpatrick D, Bishop JA et al (2014) Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 45:S3–S7. https://doi.org/10.1016/j.injury.2014.04.002
Pajarinen J, Lin T, Gibon E et al (2019) Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 196:80–89. https://doi.org/10.1016/j.biomaterials.2017.12.025
Polimeni G, Xiropaidis AV, Wikesjö UME (2006) Biology and principles of periodontal wound healing/regeneration. Periodontol 2000 41:30–47. https://doi.org/10.1111/j.1600-0757.2006.00157.x
Wu AC, Raggatt LJ, Alexander KA, Pettit AR (2013) Unraveling macrophage contributions to bone repair. Bonekey Rep 2:373. https://doi.org/10.1038/bonekey.2013.107
Wang Y, Liu Y, Chen E, Pan Z (2020) The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res 382:457–462. https://doi.org/10.1007/s00441-020-03272-z
Pei L, Tontonoz P (2004) Fat’s loss is bone’s gain. J Clin Invest 113:805–6. https://doi.org/10.1172/JCI21311
Picke A-K, Campbell GM, Blüher M et al (2018) Thy-1 (CD90) promotes bone formation and protects against obesity. Sci Transl Med 10. https://doi.org/10.1126/scitranslmed.aao6806
Horwitz EM, Prockop DJ, Fitzpatrick LA et al (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5:309–13. https://doi.org/10.1038/6529
Kaushik S, Massey AC, Cuervo AM (2006) Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J 25:3921–33. https://doi.org/10.1038/sj.emboj.7601283
Kaushik S, Cuervo AM (2012) Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol 22:407–17. https://doi.org/10.1016/j.tcb.2012.05.006
Cuervo AM, Wong E (2014) Chaperone-mediated autophagy: Roles in disease and aging. Cell Res 24:92–104. https://doi.org/10.1038/cr.2013.153
Cuervo AM, Dice JF (2000) Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci 113(Pt 24):4441–50. https://doi.org/10.1242/jcs.113.24.4441
Cuervo AM, Dice JF (2000) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1:570–83. https://doi.org/10.1034/j.1600-0854.2000.010707.x
Notomi S, Ishihara K, Efstathiou NE et al (2019) Genetic LAMP2 deficiency accelerates the age-associated formation of basal laminar deposits in the retina. Proc Natl Acad Sci USA 116:23724–23734. https://doi.org/10.1073/pnas.1906643116
Murphy KE, Gysbers AM, Abbott SK et al (2015) Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov Disord 30:1639–1647. https://doi.org/10.1002/mds.26141
Endo Y, Furuta A, Nishino I (2015) Danon disease: a phenotypic expression of LAMP-2 deficiency. Acta Neuropathol 129:391–398. https://doi.org/10.1007/s00401-015-1385-4
Arad M, Maron BJ, Gorham JM et al (2005) Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med 352:362–372. https://doi.org/10.1056/NEJMoa033349
Zhao K, Hao H, Liu J et al (2015) Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced β-cell injury through modulation of autophagy. Cell Death Dis 6:e1885. https://doi.org/10.1038/cddis.2015.230
Jansen IDC, Tigchelaar-Gutter W, Hogervorst JMA et al (2020) LAMP-2 Is Involved in Surface Expression of RANKL of Osteoblasts In Vitro. Int J Mol Sci 21:1–17. https://doi.org/10.3390/ijms21176110
Chaudhary SC, Kuzynski M, Bottini M et al (2016) Phosphate induces formation of matrix vesicles during odontoblast-initiated mineralization in vitro. Matrix Biol 52–54:284–300. https://doi.org/10.1016/j.matbio.2016.02.003
Akel N, MacLeod RS, Berryhill SB et al (2022) Loss of chaperone-mediated autophagy is associated with low vertebral cancellous bone mass. Sci Rep 12:3134. https://doi.org/10.1038/s41598-022-07157-9
Ritchie ME, Phipson B, Wu D et al (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. https://doi.org/10.1093/nar/gkv007
Hang K, Ye C, Xu J et al (2019) Apelin enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly through Wnt/β-catenin signaling pathway. Stem Cell Res Ther 10:1–10. https://doi.org/10.1186/s13287-019-1286-x
Pajares M, Rojo AI, Arias E et al (2018) Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy 14:1310–1322. https://doi.org/10.1080/15548627.2018.1474992
Harry LE, Sandison A, Paleolog EM et al (2008) Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res 26:1238–1244. https://doi.org/10.1002/jor.20649
Glass GE, Chan JK, Freidin A et al (2011) TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci USA 108:1585–90. https://doi.org/10.1073/pnas.1018501108
Bouxsein ML, Boyd SK, Christiansen BA et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. https://doi.org/10.1002/jbmr.141
Gremse F, Stärk M, Ehling J et al (2016) Imalytics Preclinical: Interactive Analysis of Biomedical Volume Data. Theranostics 6:328–41. https://doi.org/10.7150/thno.13624
Dong S, Wang Q, Kao Y-R et al (2021) Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature 591:117–123. https://doi.org/10.1038/s41586-020-03129-z
Xu Y, Zhang Y, García-Cañaveras JC et al (2020) Chaperone-mediated autophagy regulates the pluripotency of embryonic stem cells. Science 369:397–403. https://doi.org/10.1126/science.abb4467
Chaudhry N, Sica M, Surabhi S et al (2022) Lamp1 mediates lipid transport, but is dispensable for autophagy in Drosophila. Autophagy 00:1–16. https://doi.org/10.1080/15548627.2022.2038999
Xu J, Wang Y, Hsu C-Y et al (2020) Lysosomal protein surface expression discriminates fat- from bone-forming human mesenchymal precursor cells. Elife 9:. https://doi.org/10.7554/eLife.58990
Hatem CL, Gough NR, Fambrough DM (1995) Multiple mRNAs encode the avian lysosomal membrane protein LAMP-2, resulting in alternative transmembrane and cytoplasmic domains. J Cell Sci 108(Pt 5):2093–2100. https://doi.org/10.1242/jcs.108.5.2093
Gough NR, Hatem CL, Fambrough DM (1995) The family of LAMP-2 proteins arises by alternative splicing from a single gene: characterization of the avian LAMP-2 gene and identification of mammalian homologs of LAMP-2b and LAMP-2c. DNA Cell Biol 14:863–867. https://doi.org/10.1089/dna.1995.14.863
Konecki DS, Foetisch K, Zimmer KP et al (1995) An alternatively spliced form of the human lysosome-associated membrane protein-2 gene is expressed in a tissue-specific manner. Biochem Biophys Res Commun 215:757–767. https://doi.org/10.1006/bbrc.1995.2528
Eskelinen E-L, Cuervo AM, Taylor MRG et al (2005) Unifying nomenclature for the isoforms of the lysosomal membrane protein LAMP-2. Traffic 6:1058–1061. https://doi.org/10.1111/j.1600-0854.2005.00337.x
Chi C, Leonard A, Knight WE et al (2019) LAMP-2B regulates human cardiomyocyte function by mediating autophagosome–lysosome fusion. Proc Natl Acad Sci USA 116:556–565. https://doi.org/10.1073/pnas.1808618116
Manso AM, Hashem SI, Nelson BC et al (2020) Systemic AAV9.LAMP2B injection reverses metabolic and physiologic multiorgan dysfunction in a murine model of Danon disease. Sci Transl Med 12:1–13. https://doi.org/10.1126/scitranslmed.aax1744
Pérez L, McLetchie S, Gardiner GJ et al (2016) LAMP-2C Inhibits MHC Class II Presentation of Cytoplasmic Antigens by Disrupting Chaperone-Mediated Autophagy. J Immunol 196:2457–2465. https://doi.org/10.4049/jimmunol.1501476
Pérez L, Sinn AL, Sandusky GE et al (2018) Melanoma LAMP-2C Modulates Tumor Growth and Autophagy. Front cell Dev Biol 6:101. https://doi.org/10.3389/fcell.2018.00101
Lee W, Kim HY, Choi Y-J et al (2022) SNX10-mediated degradation of LAMP2A by NSAIDs inhibits chaperone-mediated autophagy and induces hepatic lipid accumulation. Theranostics 12:2351–2369. https://doi.org/10.7150/thno.70692
Gong Y, Li Z, Zou S et al (2021) Vangl2 limits chaperone-mediated autophagy to balance osteogenic differentiation in mesenchymal stem cells. Dev Cell 56:2103-2120.e9. https://doi.org/10.1016/j.devcel.2021.06.011
Augello A, De Bari C (2010) The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther 21:1226–1238. https://doi.org/10.1089/hum.2010.173
Lefterova MI, Zhang Y, Steger DJ et al (2008) PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev 22:2941–2952. https://doi.org/10.1101/gad.1709008
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3:379–389. https://doi.org/10.1111/j.1474-9728.2004.00127.x
Meunier P, Aaron J, Edouard C, Vignon G (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80:147–54. https://doi.org/10.1097/00003086-197110000-00021
Deng Z, Sharff KA, Tang N et al (2008) Regulation of osteogenic differentiation during skeletal development. Front Biosci 13:2001–21. https://doi.org/10.2741/2819
Bennett CN, Ross SE, Longo KA et al (2002) Regulation of Wnt signaling during adipogenesis. J Biol Chem 277:30998–31004. https://doi.org/10.1074/jbc.M204527200
Yuan Z, Li Q, Luo S et al (2016) PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr Stem Cell Res Ther 11:216–25. https://doi.org/10.2174/1574888x10666150519093429
Song B, Chi Y, Li X et al (2015) Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/mTOR Pathway. Cell Physiol Biochem 36:1991–2002. https://doi.org/10.1159/000430167
Fontaine C, Cousin W, Plaisant M et al (2008) Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells 26:1037–46. https://doi.org/10.1634/stemcells.2007-0974
Ding VW, Chen RH, McCormick F (2000) Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem 275:32475–32481. https://doi.org/10.1074/jbc.M005342200
Wu D, Pan W (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35:161–168. https://doi.org/10.1016/j.tibs.2009.10.002
Hur E-M, Zhou F-Q (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11:539–51. https://doi.org/10.1038/nrn2870
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–92. https://doi.org/10.1038/nm.3074
Gustafson B, Smith U (2010) Activation of canonical wingless-type MMTV integration site family (Wnt) signaling in mature adipocytes increases beta-catenin levels and leads to cell dedifferentiation and insulin resistance. J Biol Chem 285:14031–41. https://doi.org/10.1074/jbc.M110.102855
Chen J-R, Lazarenko OP, Wu X et al (2010) Obesity reduces bone density associated with activation of PPARγ and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS One 5:e13704. https://doi.org/10.1371/journal.pone.0013704
Reggio A, Rosina M, Palma A et al (2020) Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/β-catenin axis. Cell Death Differ 27:2921–2941. https://doi.org/10.1038/s41418-020-0551-y
Acknowledgements
We thank the clients who have offered assistance to this study from Clinical Research Center of the second affiliated hospital, Zhejiang university, including Xing Zhang, Meirong Yu, Sicong Chen, Liya Lin, Yunlu Chen, et al. We also appreciate the general help of Linlin Zhang and Shoumin Feng from Orthopedics Research Institute of Zhejiang University.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81874007; 82172404) and the Natural Science Foundation of Zhejiang Province (Y21H070011).
Author information
Authors and Affiliations
Contributions
XG, DTX and ZJP designed the research; YBW, KH and LY performed the in vitro experiments; YBW, KH, XYW, WJZ, LJL and JWB performed the in vivo experiments; YBW and HK analyzed the data; YBW, KH and XYW wrote the paper; DTX and ZJP revised the paper. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were in accordance to the Animal Care and Use Committee guidelines of Zhejiang University. All experimental procedures were in accordance with the and Institutional Animal Care Use Committee at the Second Affiliated Hospital, School of Medicine, Zhejiang University.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Hang, K., Ying, L. et al. LAMP2A regulates the balance of mesenchymal stem cell adipo-osteogenesis via the Wnt/β-catenin/GSK3β signaling pathway. J Mol Med 101, 783–799 (2023). https://doi.org/10.1007/s00109-023-02328-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02328-1