Abstract
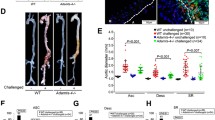
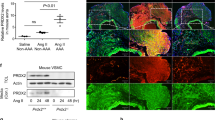
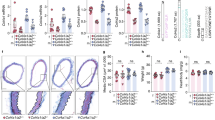
Thoracic aortic aneurysm and dissection (TAAD) is a life-threatening cardiovascular disease with severe extracellular matrix (ECM) remodeling that lacks efficient early stage diagnosis and nonsurgical therapy. A disintegrin and metalloproteinase with thrombospondin motif 7 (ADAMTS-7) is recognized as a novel locus for human coronary artery atherosclerosis. Previous work by us and others showed that ADAMTS-7 promoted atherosclerosis, postinjury neointima formation, and vascular calcification. However, whether ADAMTS-7 is involved in TAAD pathogenesis is unknown. We aimed to explore the alterations in ADAMTS-7 expression in human and mouse TAAD, and investigate the role of ADAMTS-7 in TAAD formation. A case–control study of TAAD patients (N = 86) and healthy participants (N = 88) was performed. The plasma ADAMTS-7 levels were markedly increased in TAAD patients within 24 h and peaked in 7 days. A TAAD mouse model was induced with 0.5% β-aminopropionitrile (BAPN) in drinking water. ELISA analysis of mouse plasma, Western blotting, and immunohistochemical staining of aorta showed an increase in ADAMTS-7 in the early stage of TAAD. Moreover, ADAMTS-7-deficient mice exhibited significantly attenuated TAAD formation and TAAD rupture-related mortality in both male and female mice, which was accompanied by reduced artery dilation and inhibited elastin degradation. ADAMTS-7 deficiency caused repressed inflammatory response and complement system activation during TAAD formation. An increase in plasma ADAMTS-7 is a novel biomarker for human TAAD. ADAMTS-7 deficiency attenuates BAPN-induced murine TAAD. ADAMTS-7 is a potential novel target for TAAD diagnosis and therapy.
Key messages
-
A case-control study revealed increased plasma ADAMTS-7 is a risk factor for TAAD.
-
ADAMTS-7 was elevated in plasma and aorta at early stage of mouse TAAD.
-
ADAMTS-7 knockout attenuated mouse TAAD formation and mortality in both sexes.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V (2014) Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 64(16):1725–1739. https://doi.org/10.1016/j.jacc.2014.08.025
Nienaber CA, Clough RE (2015) Management of acute aortic dissection. Lancet (London, England) 385(9970):800–811. https://doi.org/10.1016/S0140-6736(14)61005-9
Gawinecka J, Schönrath F, von Eckardstein A (2017) Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly 147:w14489. https://doi.org/10.4414/smw.2017.14489
Ren W, Liu Y, Wang X, Piao C, Ma Y, Qiu S, Jia L, Chen B, Wang Y, Jiang W et al (2018) The complement C3a-C3aR axis promotes development of thoracic aortic dissection via regulation of MMP2 expression. J Immunol (Baltimore, Md. : 1950) 200(5):1829–1838. https://doi.org/10.4049/jimmunol.1601386
Sakai LY, Keene DR, Renard M, De Backer J (2016) FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene 591(1):279–291. https://doi.org/10.1016/j.gene.2016.07.033
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science (New York, N.Y.) 326(5957):1216–1219. https://doi.org/10.1126/science.1176009
Ma Z, Mao C, Jia Y, Fu Y, Kong W (2020) Extracellular matrix dynamics in vascular remodeling. Am J Physiol Cell Physiol 319(3):C481–C499. https://doi.org/10.1152/ajpcell.00147.2020
Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, Burnett MS, Devaney JM, Knouff CW, Thompson JR et al (2011) Identification of ADAMTS-7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet (London, England) 377(9763):383–392. https://doi.org/10.1016/S0140-6736(10)61996-4
Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C et al (2011) Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 43(4):333–338. https://doi.org/10.1038/ng.784
Bauer RC, Tohyama J, Cui J, Cheng L, Yang J, Zhang X, Ou K, Paschos GK, Zheng XL, Parmacek MS et al (2015) Knockout of ADAMTS-7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation 131(13):1202–1213. https://doi.org/10.1161/CIRCULATIONAHA.114.012669
Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, Zhu Y, Wang N, Kong W, Wang X (2009) ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res 104(5):688–698. https://doi.org/10.1161/CIRCRESAHA.108.188425
Kessler T, Zhang L, Liu Z, Yin X, Huang Y, Wang Y, Fu Y, Mayr M, Ge Q, Xu Q et al (2015) ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation 131(13):1191–1201. https://doi.org/10.1161/CIRCULATIONAHA.114.014072
Du Y, Gao C, Liu Z, Wang L, Liu B, He F, Zhang T, Wang Y, Wang X, Xu M et al (2012) Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler Thromb Vasc Biol 32(11):2580–2588. https://doi.org/10.1161/ATVBAHA.112.300206
Bengtsson E, Hultman K, Dunér P, Asciutto G, Almgren P, Orho-Melander M, Melander O, Nilsson J, Hultgårdh-Nilsson A, Gonçalves I (2017) ADAMTS-7 is associated with a high-risk plaque phenotype in human atherosclerosis. Sci Rep 7(1):3753. https://doi.org/10.1038/s41598-017-03573-4
Fu Y, Huang Y, Yang Z, Chen Y, Zheng J, Mao C, Li Z, Liu Z, Yu B, Li T et al (2021) Cartilage oligomeric matrix protein is an endogenous β-arrestin-2-selective allosteric modulator of AT1 receptor counteracting vascular injury. Cell Res 31(7):773–790. https://doi.org/10.1038/s41422-020-00464-8
Ren W, Liu Y, Wang X, Jia L, Piao C, Lan F, Du J (2016) β-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci Rep 6:28149. https://doi.org/10.1038/srep28149
Zhao G, Fu Y, Cai Z, Yu F, Gong Z, Dai R, Hu Y, Zeng L, Xu Q, Kong W (2017) Unspliced XBP1 Confers VSMC Homeostasis and Prevents Aortic Aneurysm Formation via FoxO4 Interaction. Circ Res 121(12):1331–1345. https://doi.org/10.1161/CIRCRESAHA.117.311450
Le S, Zhang H, Huang X, Chen S, Wu J, Chen S, Ding X, Chen S, Zhao J, Xu H et al (2020) PKM2 activator TEPP-46 attenuates thoracic aortic aneurysm and dissection by inhibiting NLRP3 inflammasome-mediated IL-1β secretion. J Cardiovasc Pharmacol Ther 25(4):364–376. https://doi.org/10.1177/1074248420919966
Xu H, Du S, Fang B, Li C, Jia X, Zheng S, Wang S, Li Q, Su W, Wang N et al (2019) VSMC-specific EP4 deletion exacerbates angiotensin II-induced aortic dissection by increasing vascular inflammation and blood pressure. Proc Natl Acad Sci USA 116(17):8457–8462. https://doi.org/10.1073/pnas.1902119116
Lian G, Li X, Zhang L, Zhang Y, Sun L, Zhang X, Liu H, Pang Y, Kong W, Zhang T et al (2019) Macrophage metabolic reprogramming aggravates aortic dissection through the HIF1α-ADAM17 pathway. EBioMedicine 49:291–304. https://doi.org/10.1016/j.ebiom.2019.09.041
Wang X, Zhang H, Ge Y, Cao L, He Y, Sun G, Jia S, Ma A, Liu J, Rong D et al (2021) AT1R regulates macrophage polarization through YAP and regulates aortic dissection incidence. Front Physiol 12:644903. https://doi.org/10.3389/fphys.2021.644903
Baliga RR, Nienaber CA, Bossone E, Oh JK, Isselbacher EM, Sechtem U, Fattori R, Raman SV, Eagle KA (2014) The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging 7(4):406–424. https://doi.org/10.1016/j.jcmg.2013.10.015
Wen D, Zhou XL, Li JJ, Hui RT (2011) Biomarkers in aortic dissection. Clin Chim Acta Int J Clin Chem 412(9–10):688–695. https://doi.org/10.1016/j.cca.2010.12.039
Genest J (2010) C-reactive protein: risk factor, biomarker and/or therapeutic target? Canadian J Cardiol 26 Suppl A:41A-44A. https://doi.org/10.1016/s0828-282x(10)71061-8
Nazerian P, Mueller C, Soeiro AM, Leidel BA, Salvadeo S, Giachino F, Vanni S, Grimm K, Oliveira MT Jr, Pivetta E et al (2018) Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes: the ADvISED prospective multicenter study. Circulation 137(3):250–258. https://doi.org/10.1161/CIRCULATIONAHA.117.029457
Wang Y, Tan X, Gao H, Yuan H, Hu R, Jia L, Zhu J, Sun L, Zhang H, Huang L et al (2018) Magnitude of Soluble ST2 as a novel biomarker for acute aortic dissection. Circulation 137(3):259–269. https://doi.org/10.1161/CIRCULATIONAHA.117.030469
Cui H, Chen Y, Li K et al (2021) Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur Heart J 42(42):4373–4385. https://doi.org/10.1093/eurheartj/ehab605
Postnov A, Suslov A, Sobenin I, Chairkin I, Sukhorukov V, Ekta MB, Khotina V, Afanasiev M, Chumachenko P, Orekhov A (2021) Thoracic aortic aneurysm: blood pressure and inflammation as key factors in the development of aneurysm dissection. Curr Pharm Des 27(28):3122–3127. https://doi.org/10.2174/1381612827666210210142200
Doshida Y, Sano H, Iwabuchi S, Aigaki T, Yoshida M, Hashimoto S, Ishigami A (2020) Age-associated changes in the transcriptomes of non-cultured adipose-derived stem cells from young and old mice assessed via single-cell transcriptome analysis. PloS One 15(11):e0242171. https://doi.org/10.1371/journal.pone.0242171
Isselbacher EM, Lino Cardenas CL, Lindsay ME (2016) Hereditary influence in thoracic aortic aneurysm and dissection. Circulation 133(24):2516–2528. https://doi.org/10.1161/CIRCULATIONAHA.116.009762
Maguire EM, Pearce S, Xiao R, Oo AY, Xiao Q (2019) Matrix metalloproteinase in abdominal aortic aneurysm and aortic dissection. Pharmaceuticals (Basel, Switzerland) 12(3):118. https://doi.org/10.3390/ph12030118
Liu O, Xie W, Qin Y, Jia L, Zhang J, Xin Y, Guan X, Li H, Gong M, Liu Y et al (2016) MMP-2 gene polymorphisms are associated with type A aortic dissection and aortic diameters in patients. Medicine 95(42):e5175. https://doi.org/10.1097/MD.0000000000005175
Shen M, Lee J, Basu R, Sakamuri SS, Wang X, Fan D, Kassiri Z (2015) Divergent roles of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler Thromb Vasc Biol 35(4):888–898. https://doi.org/10.1161/ATVBAHA.114.305115
Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH, Pharmaceutical Aneurysm Stabilisation Trial Study Group (2013) Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann Intern Med 159(12):815–823. https://doi.org/10.7326/0003-4819-159-12-201312170-00007
Zeng T, Yuan J, Gan J, Liu Y, Shi L, Lu Z, Xue Y, Xiong R, Huang M, Yang Z et al (2019) Thrombospondin 1 is increased in the aorta and plasma of patients with acute aortic dissection. Can J Cardiol 35(1):42–50. https://doi.org/10.1016/j.cjca.2018.11.008
Yang H, Zhou T, Sorenson CM, Sheibani N, Liu B (2020) Myeloid-derived TSP1 (Thrombospondin-1) contributes to abdominal aortic aneurysm through suppressing tissue inhibitor of metalloproteinases-1. Arterioscler Thromb Vasc Biol 40(12):e350–e366. https://doi.org/10.1161/ATVBAHA.120.314913
Liu Z, Morgan S, Ren J, Wang Q, Annis DS, Mosher DF, Zhang J, Sorenson CM, Sheibani N, Liu B (2015) Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res 117(2):129–141. https://doi.org/10.1161/CIRCRESAHA.117.305262
Krishna SM, Seto SW, Jose RJ, Biros E, Moran CS, Wang Y, Clancy P, Golledge J (2015) A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler Thromb Vasc Biol 35(2):389–398. https://doi.org/10.1161/ATVBAHA.114.304732
Colige A, Monseur C, Crawley J, Santamaria S, de Groot R (2019) Proteomic discovery of substrates of the cardiovascular protease ADAMTS-7. J Biol Chem 294(20):8037–8045. https://doi.org/10.1074/jbc.RA119.007492
MacDonald BT, Keshishian H, Mundorff CC, Arduini A, Lai D, Bendinelli K, Popp NR, Bhandary B, Clauser KR, Specht H et al (2022) TAILS identifies candidate substrates and biomarkers of ADAMTS-7, a therapeutic protease target in coronary artery disease. Mol Cell Proteom 21(4):100223. https://doi.org/10.1016/j.mcpro.2022.100223
Lee VS, Halabi CM, Hoffman EP, Carmichael N, Leshchiner I, Lian CG, Bierhals AJ, Vuzman D, Medicine BG, Mecham RP et al (2016) Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci USA 113(31):8759–8764. https://doi.org/10.1073/pnas.1601442113
Chai T, Tian M, Yang X, Qiu Z, Lin X, Chen L (2022) Association of circulating cathepsin B levels with blood pressure and aortic dilation. Front Cardiovasc Med 9:762468. https://doi.org/10.3389/fcvm.2022.762468
Mao C, Ma Z, Jia Y, Li W, Xie N, Zhao G, Ma B, Yu F, Sun J, Zhou Y et al (2021) Nidogen-2 maintains the contractile phenotype of vascular smooth muscle cells and prevents neointima formation via bridging jagged1-Notch3 signaling. Circulation 144(15):1244–1261. https://doi.org/10.1161/CIRCULATIONAHA.120.053361
Sun X, Malandraki-Miller S, Kennedy T, Bassat E, Klaourakis K, Zhao J, Gamen E, Vieira JM, Tzahor E, Riley PR (2021) The extracellular matrix protein agrin is essential for epicardial epithelial-to-mesenchymal transition during heart development. Development (Cambridge, England) 148(9):dev197525. https://doi.org/10.1242/dev.197525
Kim RY, Robertson EJ, Solloway MJ (2001) Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol 235(2):449–466. https://doi.org/10.1006/dbio.2001.0284
Qin W, Cao Y, Li L, Chen W, Chen X (2017) Upregulation of ADAMTS-7 and downregulation of COMP are associated with aortic aneurysm. Mol Med Rep 16(4):5459–5463. https://doi.org/10.3892/mmr.2017.7293
Mizoguchi T, MacDonald BT, Bhandary B, Popp NR, Laprise D, Arduini A, Lai D, Zhu QM, Xing Y, Kaushik VK et al (2021) Coronary disease association with ADAMTS7 is due to protease activity. Circ Res 129(4):458–470. https://doi.org/10.1161/CIRCRESAHA.121.319163
Pu X, Xiao Q, Kiechl S, Chan K, Ng FL, Gor S, Poston RN, Fang C, Patel A, Senver EC et al (2013) ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet 92(3):366–374. https://doi.org/10.1016/j.ajhg.2013.01.012
Pu X, Chan K, Yang W, Xiao Q, Zhang L, Moore AD, Liu C, Webb TR, Caulfield MJ, Samani NJ et al (2020) Effect of a coronary-heart-disease-associated variant of ADAMTS7 on endothelial cell angiogenesis. Atherosclerosis 296:11–17. https://doi.org/10.1016/j.atherosclerosis.2020.01.015
Acknowledgements
We thank Mr. Hao Liu and Dr. Xueyuan Yang from Peking University for kindly helping collecting clinical records in human study.
Funding
This research was supported by funding from the National Natural Science Foundation of China (NSFC, 81921001, 81730010, 31930056, 82100436 and 82230010), and the National Key R&D Program of China (2019YFA 0801600, 2022YFA 1302900).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Human study was designed by Wei Kong and Qingbian Ma and performed by Ze Gong and Daidai Wang. Animal study was designed by Wei Kong, Yi Fu, and Ze Gong. Material preparation, data collection, and analysis were performed by Ze Gong, Jiaqi Huang, Zihan Ma, and Shiyu Yang. The first draft of the manuscript was written by Ze Gong and Jiaqi Huang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The human case–control study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Peking University Third Hospital (M2020410). Informed consent was obtained from all participants. All animal experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee of Peking University Health Science Center.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
109_2023_2284_MOESM1_ESM.pdf
Supplementary file1 (PDF 1840 KB) Supplementary Figure 1. Ex vivo images of aortas from all eight-group mice. Four groups of 3-week-old mice (BAPN: WT-Male, N=15; TS7KO-Male, N=16; WT-Female, N=17; TS7KO-Female, N=18) were treated with 0.5% BAPN in drinking water for 28 days. And another four groups of 3-week-old mice (CTL: WT-Male, N=6; TS7KO-Male, N=6; WT-Female, N=6; TS7KO-Female, N=6) were fed by normal water as control (one aorta from WT male mice in BAPN group which 19 / 30 died at day 28 failed to collect). For those mice that died from TAAD rupture, death date was marked in red under the image. And Day 28 in orange means those mice with TAAD alive till sacrifice, while Day 28 in black means mice without TAAD. Scale bar = 1 cm, as shown in the image.
109_2023_2284_MOESM2_ESM.pdf
Supplementary file2 (PDF 111 KB) Supplementary Figure 2. ADAMTS-7 knockout inhibited BAPN-induced artery dilation in female mice. (A-D) Two groups of 3-week-old female mice (BAPN: WT, N=17; TS7KO, N=18) were treated with 0.5% BAPN in drinking water for 28 days. And another two groups of 3-week-old female mice (CTL: WT, N=6; TS7KO, N=6) were fed by normal water as control. Maximum diameter of aorta was measured ex vivo at four segments, including ascending aorta (Asc, A), aortic arch (Arch, B), descending aorta (Des, C) and abdominal aorta (Abd, D). *P<0.05 by two-way ANOVA with Sidak’s multiple comparisons test.
109_2023_2284_MOESM3_ESM.pdf
Supplementary file3 (PDF 102 KB) Supplementary Figure 3. Unedited image for western blotting. Edited image and its corresponding unedited image for Figure 2A. Lane samples were described in the image.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, Z., Huang, J., Wang, D. et al. ADAMTS-7 deficiency attenuates thoracic aortic aneurysm and dissection in mice. J Mol Med 101, 237–248 (2023). https://doi.org/10.1007/s00109-023-02284-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02284-w