Abstract
A panel of 10 IgG enzyme-linked immunosorbent assays (ELISAs) were developed for the detection of anti-microbial immune responses in the cerebrospinal fluid (CSF) of patients with demyelinating diseases (DD). The anti-microbial ELISA assays follow on prior human brain tissue RNA sequencing studies that established multiple sclerosis (MS) microbial candidates. Lysates included in the ELISA panel were derived from Akkermansia muciniphila, Atopobium vaginae, Bacteroides fragilis, Lactobacillus paracasei, Odoribacter splanchnicus, Pseudomonas aeruginosa, Cutibacterium (Propionibacterium) acnes, Fusobacterium necrophorum, Porphyromonas gingivalis, and Streptococcus mutans. CSF responses from patients with demyelinating diseases (DD, N = 14) were compared to those with other neurological diseases (OND, N = 8) and controls (N = 13). Commercial positive and negative control CSF specimens were run with each assay. ELISA index values were derived for each specimen against each of the 10 bacterial lysates. CSF reactivity was significantly higher in the DD group compared to the controls against Akkermansia, Atopobium, Bacteroides, Lactobacillus, Odoribacter, and Fusobacterium. Four of the 11 tested DD group subjects had elevated antibody indexes against at least one of the 10 bacterial species, suggesting intrathecal antibody production. This CSF serological study supports the hypothesis that several of the previously identified MS candidate microbes contribute to demyelination in some patients.
Key messages
-
A panel of 10 IgG enzyme-linked immunosorbent assays (ELISAs) were developed for the detection of anti-microbial immune responses in the cerebrospinal fluid (CSF) of patients with demyelinating diseases, including multiple sclerosis and acute disseminated encephalomyelitis.
-
CSF reactivity was significantly higher in the demyelination group compared to the controls against the bacteria Akkermansia, Atopobium, Bacteroides, Lactobacillus, Odoribacter, and Fusobacterium.
-
Several of the demyelination subjects had elevated antibody indexes against at least one of the 10 antigens, suggesting at least limited intrathecal production of anti-bacterial antibodies.
-
This CSF serological study supports the hypothesis that several of the previously identified MS candidate microbes contribute to demyelination in some patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of infections of the central nervous system (CNS) can lead to demyelination, including distemper (dogs), measles (humans), JC virus (human), influenza (humans), and now possibly SARS-CoV-2 [1, 2]. The underlying risk factors and mechanisms for microbe-driven demyelination are poorly understood, since it occurs unpredictably and at variable rates.
Microbes, particularly herpes viruses such as EBV and HHV6, have long been suspected as causative agents of the prototypic demyelinating disease multiple sclerosis (MS), based on the epidemiology of the disease including geographic patterns, isolated outbreaks, and migration studies [3, 4]. However, this field is contentious with much conflicting evidence. Establishment of causal relationships is complicated by the prolonged time interval (years to decades) between initial exposure, likely in puberty, and the development of MS, most often in young adulthood. Researchers in the 1970s and 1980s made extensive efforts to find and isolate possible viral pathogens from fresh autopsy brain tissue [5]. These efforts included inoculation of diseased human brain tissue into cell cultures, live animals, and even eggs, without any evidence of replicating viruses. However, bacterial and fungal cultures were not performed, and ex vivo viral culture techniques included the use of antibiotics in the culture medium. While these efforts to find viruses responsible for MS were certainly ambitious and laudable, this strategy likely would not have detected most bacteria, fungi, or protists within the affected brain tissue.
We recently utilized next-generation unbiased RNA sequencing of demyelinated human brain samples from living subjects as a method for defining possible microbial contributors to MS and related demyelinating diseases (e.g., acute disseminated encephalomyelitis, ADEM; neuromyelitis optica, NMO) [6]. RNA sequencing of fixed, paraffinized brain biopsy specimens from these research subjects was performed in comparison to control epilepsy brain samples, including blank samples to rigorously control for environmental and reagent contamination. This work showed significantly more mapped microbial sequences in the demyelinated brain samples than in the controls. Stringent computational analysis of the sequencing data resulted in a list of 29 differentially expressed MS microbial candidates, mostly anaerobic bacteria which are often difficult to culture. Some of these bacterial species are derived from the physiological microbiota of the human gut and vaginal epithelium.
Collectively, these findings suggest that both exogenous bacterial pathogens and commensal species could directly or indirectly contribute to the development and progression of demyelinating disease. This study was designed to determine if some MS candidate microbes, discovered by sequencing of brain tissue, are also eliciting an immune response in the CSF of patients with demyelinating diseases, including MS. Ten organisms were selected for further study. Bacterial lysates were used to develop a panel of 10 novel dedicated ELISA assays. The ELISA panel was applied to CSF from patients with demyelinating disease (DD), other neurologic disease (OND), and controls. The data demonstrates significantly higher CSF reactivity among DD subjects against several of the panel components compared to controls.
Materials and methods
Subjects, CSF, and serum collections
The study was approved by the University of Utah Health Sciences Institutional Review Board (IRB). Each subject either signed an IRB-approved informed consent or had a legally authorized representative sign for them. CSF was obtained from 14 patients with MS or other demyelinating diseases (DD) during the course of their regular clinical care. CSF was also collected from 8 patients with other neurologic diseases (OND) and 13 controls. Most of the OND subjects were enrolled in the study for suspicion of demyelinating disease, but the final neurologic diagnosis was something else (e.g., vasculitis). The controls were patients undergoing spinal fluid shunt placements or exchanges who did not have active infection at the time of collection. These control subjects did not have clinical tests performed on their CSF samples, but were expected to have normal CSF based on their stable clinical condition. Paired serum samples taken around the time of CSF collections were obtained from 11 DD and 8 OND subjects. Serum was not collected on the control cohort. Oligoclonal band OCB testing and albumin index determinations were performed (ARUP Laboratories) as part of regular clinical care in some of the DD and OND subjects. CSF and serum IgG concentrations were determined by nephelometry, also at ARUP laboratories.
Criteria for microbial selection and inclusion
Selection of microbial candidates was guided by the MS microbial candidate list [6]. MS candidate microorganisms were selected for optimization if they were as follows: (1) readily available from ATCC or clinical sources, (2) culture conditions were not too demanding or dangerous, and (3) they were widely distributed among the contributing microbial families or were of particular interest. Characteristics of ATCC product numbers and justification for the microbes selected for the CSF serology panel are displayed in Table 1. The organisms were grown in the appropriate conditions, washed, diluted to optical density (OD) of approximately 0.5 or 1.0 (~ 10,000 cells/ml), and sonicated. Microbes selected for the CSF serology panel include Akkermansia muciniphila, Atopobium vaginae, Bacteroides fragilis, Cutibacterium (Propionibacterium) acnes, Lactobacillus paracasei, Odoribacter splanchnicus, Porphyromonas gingivalis, Pseudomonas aeruginosa, Streptococcus mutans, and Fusobacterium necrophorum.
Bacterial lysate preparation
Bacterial strains were obtained from ATCC as freeze-dried, lyophilized cultures. Cultures were rehydrated using the appropriate medium and incubation conditions specified on the ATCC product sheets. Organisms were allowed to come out of the lyophilized state, and once within the exponential phase of growth, stock cultures were stored at -80 °C in 10% glycerol. The cultured bacteria were harvested during the optimal growth phase as recommended by ATCC. Bacterial cells from broth cultures were washed in PBS at least twice before proceeding. Bacteria obtained from both plate and broth cultures were diluted in PBS to obtain a final OD of 1.0, equivalent to 3.9 × 108 bacterial cells per milliliter and 1.93 × 107 bacterial cells per well in a flat-bottom 96-well plate (Corning Life Sciences Product 9018). Plates were read on a Gen5 2.08 ELISA plate reader at 600 nm. Cells were sonicated for 10 s with cycle time continuous, a duty cycle of 40%, and at position 4.5 with a Branson Sonifier 250. The sonicated bacterial lysates were aliquoted and stored at -80 °C for future use.
ELISA procedure
Flat-bottom 96-well ELISA plates were coated with 50 μl/well of harvested lysate (sonicated bacteria), covered, and rocked overnight at 4 °C. The plates were washed with phosphate buffered saline with 0.1% Tween 20 (PBST), blocked with Starting Blocker for 1 h at room temperature, and washed again before applying the appropriate primary antibody for 2 h. Secondary antibodies were applied for 1 h. The plates were developed with TMB for 5 min and stopped with 2 N HCL. Absorbance was read on a Gen5 2.08 ELISA plate reader at 450 nm.
A series of steps were taken to optimize the 10 separate ELISA assays. Four commercial blocking agents were assessed for blocking efficiency including 5X ELISA/ELISPOT Diluent (Invitrogen), Blocker BLOTTO in Tris-buffered saline (ThermoScientific), Blocker Casein (ThermoScientific), and Starting Blocker (ThermoScientific). Starting Blocker proved to be optimal for most of the assays and was adopted for use with all 10 bacterial species. Three primary anti-bacterial antibodies were assessed as positive controls for assay performance validation: mouse monoclonal IgG3 anti-peptidoglycan (MAB995, Sigma-Aldrich), mouse monoclonal IgG2b anti-LPS (ab35654, Abcam), and rabbit polyclonal anti-pseudomonas (PA1-73116, Thermo Fisher Scientific). These positive control primary Abs were titrated to yield strong and weak (just above the background) positive signals. The polyclonal anti-Pseudomonas Ab PA1-73116 proved to be the best primary positive control antibody against all the 10 bacteria tested. This positive control Ab was used at dilutions between 1:200 and 1:6400 as strong (OD ~ 1.0) and between 1:400 and 1:102,400 as weak (OD > background) positive controls for each of the 10 assays. Secondary antibodies used in the study included anti-mouse IgG (Vector PI-2000, 1:3000), anti-rabbit IgG (Vector PI-1000, 1:3000), and anti-human (Jackson ImmunoResearch, 1:10,000 or 1:20,000). The antibodies and dilutions used for the study are detailed in Table S1.
Commercial human CSF (Randox Laboratories Ltd, UK; ([IgG] 10 mg/dl) was used as a positive control. IgG-depleted commercial human CSF (Randox Laboratories Ltd, Crumlin, UK) was utilized as a negative (calibration) control. The “cut-off calibrator” method was chosen for each of the assays [7] [8, 9]. This involved making calibrator (negative) control CSF samples depleted of virtually all IgG. Effective IgG depletion was performed with two elutions of commercial CSF (Randox®) through the HiTrap Protein G HP kit (GE Healthcare, Product 29-0485-81). IgG depletion was verified with Human Total IgG Platinum ELISA kit (Affymetrix eBioscience). The final depleted IgG measured concentration was 5.25 ng/ml. Other negative controls run with each assay included PBS only (blank) and (no-primary) secondary Ab only wells.
Analysis of ELISA data
CSF and diluted serum samples were run in duplicates. An average OD was taken for each experimental and control sample and divided by the cutoff calibrator to obtain an ELISA index (EI) value for each of the samples. Matched serum samples were collected from as many of the DD and OND subjects as possible. CSF and serum IgG levels were obtained from clinical data, and/or from nephelometry determinations, all performed at ARUP Laboratories (Salt Lake City, Utah, USA). The serum samples were diluted in PBS to match each sample’s CSF IgG concentration. This allowed the direct assessment of intrathecal IgG synthesis using the EICSF:EIserum ratio or antibody index (AI). An AI greater than one shows excess Ab in the CSF compared to serum, suggesting that intrathecal Ab synthesis has taken place [10, 11].
Statistical analysis
The ages of the subjects in the DD, OND, and control groups were compared by one-way ANOVA, and sex distributions of the subjects were compared using the 2 × 3 Fishers exact test [12]. EI values among the groups were screened with an unweighted one-way ANOVA [12, 13]. Differences in EI values between the groups were compared by using 2-tailed Mann-Whitney nonparametric testing [14]. EI’s and albumin index values were compared by linear regression, performed within the Prism 9.0 computer application [13].
Linear regression was also used to investigate the relationship between EI, the assay types, and the subject groups in a combined analysis. Because the EI is defined as a standardized ratio, we modeled its logarithm as a linear function of the covariates as this transformation is more in line with the assumption in linear regression that the errors are normally distributed. In order to correct for age and sex effects, we included these in our baseline model. To assess the effects of assay type and subject group, we augmented the baseline model by adding first a main effect for assay type (bacterial lysate) and then a main effect for subject group. Finally, to determine whether the subject group EI levels followed the same pattern across the different assays or showed different patterns for different assays, we added an assay type by subject group interaction term. The statistical analyses are summarized in Table S2. The results and conclusions of the study were reviewed by a professional statistician (see “Acknowledgements” section).
Results
Study population
The characteristics of the study population are shown in Table 2. Of the 14 patients that were diagnosed with demyelinating disease (DD), 2 had relapsing-remitting MS, 1 had a clinically isolated syndrome, 3 had tumefactive MS, 3 were classified as untyped or neurodegenerative MS, 4 had ADEM, and 1 had anti-MOG syndrome. OND subjects had CNS Lyme disease, vasculitis, rhombencephalitis, neurosyphilis, migraine, spina bifida, atypical stroke, CSF shunt infection with C. acnes, or pudendal neuralgia. Median ages of the subjects were 43 (DD), 42 (OND), and 62 (control), not significantly different (p = 0.23). The sex distributions of the groups were also not significantly different (p = 0.24).
Clinical findings in the MS and OND groups
Six of the 14 DD subjects had positive OCB testing (Table 2). Two of the DD patients who had negative OCB tests had CSF collected within 3 days of their disease onset (DD-80, DD-82), while 2 others had matched bands in serum and CSF (DD-17, DD-72), and one had only a single band in CSF. It is possible that some of these subjects might have developed OCBs later (e.g., DD-80, DD-82), but, unfortunately, CSF was not recollected in these subjects after their initial hospitalizations and enrollment in the study. Three subjects had CSF collected after many years of stable disease (DD-17, DD-19, DD-21). Six of the eight OND subjects also had OCB testing. Among these, one was positive, which was a subject with proven CNS Lyme disease (OND-81).
Ten of the 14 DD subjects had albumin index determinations, a measure of blood-brain barrier (BBB) intactness. Five of 10 albumin index determinations were normal (< 9.0), providing evidence for an intact BBB in half the DD subjects.
Spinal fluid IgG reactivity against the 10 MS candidate bacteria
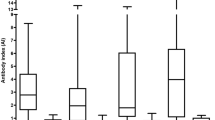
All the DD, OND, and control specimens were tested for the CSF IgG responses to each organism, expressed as ELISA index (EI). The microbes selected for study, based on the prior human brain RNA sequencing study, are shown in Table 1. The CSF serology data is displayed in Fig. 1 and tabulated in Tables S3 and S4. CSF reactivity (EI) was significantly higher in the DD group compared to the controls against 6 of the 10 microbial candidates: Akkermansia, Atopobium, Bacteroides, Lactobacillus, Odoribacter, and Fusobacterium. This is the core finding of the study. Measured CSF seroreactivity (mean EI) was greatest against Cutibacterium, Atopobium, and Lactobacillus and lowest against Fusobacterium and Porphyromonas. CSF seroreactivity was similar between the DD and OND groups.
ELISA index values are increased in the DD group compared with controls for 6 of the 10 candidate bacteria. Indirect ELISA was performed on CSF from subjects with definite demyelinating disease, other neurologic diseases (OND), and controls. Statistical comparisons were made using the Mann-Whitney nonparametric test. The bars indicate mean values ± standard deviation. See Table S2 for additional information
Linear regression analysis showed that both assay type (bacterial lysate) (p < 0.0001) and subject group (p < 0.0001) are strong predictors of log(EI). This effect is primarily due to control group EI being lower than those for the other groups. There is also evidence (p = 0.048) for a group by assay interaction term, from which we infer that the pattern of subject group EI levels varies across the different (bacterial lysate) ELISA assays. There are four assays where the control group levels are below the other two (Akkermansia, Atopobium, Lactobacillus, and Odoribacter), three assays where the DD levels appear to be intermediate between the controls and OND (Bacteroides, Fusobacterium, Pseudomonas), and three assays where there is no evidence for group differences (Cutibacterium, Porphyromonas, Streptococcus).
Within the DD group, subject DD-03, diagnosed with ADEM, had measurable reactivity in the CSF against 9 of the 10 bacterial lysates and was strongly reactive against 5 bacterial species. The other ADEM subject, DD-13, displayed some reactivity against all 6 bacteria tested, with strong reactivity against 3 of the 6.
Evidence for intrathecal antibody synthesis
Antibody indexes (AIs) were determined in 11 subjects from the DD group and 4 subjects from the OND group (Table 3). Serum was generally not collected from the control subjects, precluding calculation of AIs in this group. Four of the 11 tested DD group subjects had AIs > 1.0 against at least one of the 10 bacteria, suggesting intrathecal production of anti-bacterial antibodies [11]. One subject, DD-82 with anti-MOG (NMO spectrum disease) had elevated AIs against 5 of the 10 bacteria. Elevated AIs within the DD group were only modestly increased, ranging from 1 to 15% above the matched serum EI determinations. CSF from subject OND-78, a 42-year-old man with CNS vasculitis and normal albumin ration, had a much higher AI (3.61) measured against Odoribacter. The assay passed quality control measures, and duplicate determinations within the assay were similar. Unfortunately, this CSF specimen was limited, so this value could not be confirmed with retesting.
The data allowed comparison of the measured EI’s with BBB intactness (albumin index) for each of the subjects (Fig. S1). This is considered to be an exploratory analysis that to our knowledge, has not been attempted before. Positive slopes (EI/AI) were observed for 5 of the 10 tested bacteria, suggesting that higher anti-bacterial Ab responses correlated with less intact BBB (higher albumin index). Data points above the expected EI (line) for a given albumin index (e.g., subjects DD-10 and DD-72 in the anti-Akkermansia plot) are interpreted as evidence for intrathecal antibody synthesis. For example, subject DD-82 had higher than expected EIs against Bacteroides, Lactobacillus, and Odoribacter (Fig. S1), partially corresponding to elevated AIs against Atopobium, Lactobacillus, Odoribacter, Pseudomonas, and Streptococcus (Table 3). This data suggests intrathecal IgG antibody synthesis against these 6 different MS microbial candidates.
Comparison with the updated sequencing findings
DD subjects 03, 17, 19, 21, and 72 all had surgically obtained brain tissue sequenced and analyzed using the methods previously described [6]. Updated sequencing results are reported in Table S5. Comparisons of EI values and sequencing results are shown in Table 4. The intervals between brain biopsy and CSF collections were highly variable, ranging from 1 week to 10 years. Many but not all of the CSF EI values are supported by sequencing reads. For instance, in subject DD-03, the most abundant sequence mappings are to Lactobacillus, Streptococcus, and Cutibacterium. CSF EI values are highest for this subject against Lactobacillus and Cutibacterium, with less reactivity against Streptococcus. All the 5 sequenced subjects’ CSF samples were reactive against Akkermansia, and there were at least a few sequencing reads mapping to this bacterium among these subjects. In contrast, all 5 subjects’ CSF samples were also reactive against Odoribacter, but only 2 of 5 corresponding brain tissue samples had reads that mapped to this bacterium. Discrepancies between sequencing and serologic results may be ascribed to the sometimes prolonged intervals between the brain biopsies and CSF collections, or to cross reactivity to other bacteria among the ELISA assays.
Discussion
We conducted a study of CSF reactivity against 10 MS microbial candidates previously identified by RNA sequencing of diseased brain tissue. CSF reactivity was significantly higher in the demyelination group compared to the controls against 6 of the bacteria: Akkermansia, Atopobium, Bacteroides, Lactobacillus, Odoribacter, and Fusobacterium. Four of the 11 tested DD group subjects had elevated antibody indexes against at least one of the 10 bacteria, suggesting at least limited intrathecal production of anti-bacterial antibodies. This study clearly shows that there are antibacterial IgG antibodies against several MS candidate bacteria in most of the CSF specimens tested in the DD and OND groups. The findings are consistent with the hypothesis that MS candidate microbes trigger demyelination in some of these patients.
The experiments and analysis were performed in the most straightforward manner possible—direct comparisons between undiluted CSF in all the groups, and serum dilutions matched exactly to CSF IgG concentrations for the determinations of AI. The authors understand that there will be some leakage of antibodies from serum into the CSF and that this is part of the pathologic process that defines the disease. Blood-brain barrier dysfunction in MS is well established, also reflected by IgG access to the parenchyma, a parameter used by neuropathologists to identify sites with barrier breach. However, not all the subjects in this study had BBB dysfunction according to clinical measurement of the albumin index.
The current study does have some limitations. First, the reference specimens were taken from patients who had CSF shunts placed for hydrocephalus and related conditions. Therefore, these control specimens are not from completely healthy volunteers. Since CSF is not generally available from healthy donors, the uninfected shunt procedure samples were selected as the best reference group readily available.
Second, many of the MS and DD patients received some disease-modifying therapy (DMT). Therefore, we cannot exclude some drug effects on the anti-microbial responses in DD subjects, although most of the DD antibacterial EIs were higher than the controls, not lower as would be expected with immunosuppressive DMT.
Third, since it was not feasible to assess all 29 MS candidate microbes derived from the sequencing data, a rational and pragmatic selection of 10 major candidates was made [6]. For instance, the top candidate identified from the sequencing data based on both mRNA load and prevalence, Nitrosospira, simply could not be cultivated in sufficient quantity to develop an ELISA, despite extensive efforts over several months.
Fourth, we focused this study on the detection of the human IgG isotype and its four subclasses. Recent elegant studies emphasize that gut-educated IgA B cells and plasma cells contribute to surveillance of the meningeal venous sinuses and can limit CNS inflammation in EAE mouse models for MS [15] [16]. We did not assay for IgA or IgM in the CSF because IgG is the dominant form of immunoglobulin in human CSF with concentrations approximately 10-fold higher that IgA or IgM [17]. However, IgA is present in abundance in the human body, where there is an important interaction with the gut microbiome, promoting the presence of some bacteria (e.g., Bacteroides), and discouraging the presence of others [18]. And IgA deficiency is associated with several autoimmune diseases including lupus and rheumatoid arthritis, but not MS. Alterations in IgA responses to the MS candidate bacteria could conceivably promote or protect from demyelinating disease.
Based on the commercial polyclonal and monoclonal positive control antibodies employed, the novel ELISAs demonstrated good specificity and sensitivity. Randox® CSF was chosen as a reference for patient CSF because it is commercially available and relatively well characterized. This commercial CSF preparation containing 10 mg/dl IgG was used as a positive control for each of the assays. Randox® CSF was also IgG-depleted for use as the negative or calibration control in each of the assays [7,8,9]. This method was chosen as a reliable and reproducible method for determining a baseline level of CSF antibody reactivity between assays.
Within these ELISA assays, we suspect that there is considerable cross-reactivity, similar to that seen with the commercial polyclonal anti-Pseudomonas aeruginosa antibody used as a positive control in all the assays. Cross-reactivity between Lyme and syphilis testing is well recognized among clinicians who employ these anti-bacterial serologic tests in clinical practice [19]. The results from the neurologic Lyme disease subject (OND-81) demonstrate cross-reactivity, where her CSF was strongly reactive against 3 of the MS bacterial lysates and moderately reactive to 5 others. However, there was no elevation in AI for this subject against any of the 10 bacteria included in this study. Borrelia burgdorferi was not included as a candidate bacterium in this study, but clinical diagnostic testing showed clear intrathecal production of anti-B. burgdorferi antibodies. This patient did have an elevated albumin index suggesting a compromised BBB, so some anti-bacterial antibodies could have leaked into the CNS from the serum. Cross-reactivity could also be due to the commonly shared features of bacterial organisms such as the lipopolysaccharide (LPS), lipoteichoic acid (LTA), or peptidoglycan (PGN).
We argue that the data presented here supports a pathogenic role for several of the organisms in some of the subjects. That is, there is evidence of intrathecal antibody production against several of the MS candidate microbes in several of the subjects. Specifically, there were AI’s > 1.0, evidence for intrathecal antibacterial antibody synthesis, in DD subjects 19, 80, 82, and 83. Among these, subjects DD-19, DD-82, and DD-83 were relatively young women at the time of diagnosis, the most common demographic for the development of MS and related diseases. Comparisons of EI’s with the albumin index analysis (Fig. S1) suggested that subjects DD-72 and DD-13 may also have intrathecal production of antibodies against some of the bacteria tested. Elevated AI’s were observed against 5 bacterial lysates in subject DD-82, a young woman with anti-MOG myelitis. Interestingly, tumefactive MS developed in subject DD-83 about 3 weeks after brain surgery performed for intractable epilepsy. She did not develop any signs of infection at the parietal surgical site, and demyelination occurred both near this site and distant in the cerebellum. She had no evidence of MS prior to the demyelination event. The AI was elevated only against Bacteroides, a gut anaerobe that is seldom associated with neurosurgical infections.
Testing of CSF from subject DD-03, a relatively young Idaho rancher, proved to be uniquely interesting. This subject and his family have been outspoken about his disease, both in the local media and as the subject of an ESPN feature video [20]. A possible inciting event was believed to be an attempted winter rescue of a distressed newborn calf, possibly leading to a prolonged aerosol exposure within an enclosed pickup truck. This was followed by a brief febrile illness and then progressive neurologic dysfunction over the course of several weeks, with ADEM as the final neurologic diagnosis. Sequencing of a small diagnostic brain biopsy of this patient revealed lactobacillus as the most abundantly mapped microbe at the genus level. Lactobacillus is found in the female genital tract of many mammals, including humans, where it is dominant, and in cattle including newborn calves [21, 22]. This subject’s CSF was highly reactive against 5 of the 10 tested bacteria, with the highest EI against Lactobacillus paracasei among all the subjects tested. However, the AI against lactobacillus (0.86) was not elevated in this subject, arguing against intrathecal antibody production. However, intrathecal antibody production is notoriously difficult to prove in individual subjects, methods vary, and a patient with compatible clinical findings should be considered to have the disease being considered, with or without elevated AI [11, 23]. In sum, we argue that it is plausible that this subject’s ADEM was triggered by microbes, including L. paracasei, encountered from prolonged exposure to a newborn calf.
OCBs are a common clinical diagnostic feature of MS. For the DD cases, CSF was taken about a week after DD-03 s onset of neurologic symptoms, but DD-017, DD-019, and DD-021 had their CSF taken years after disease onset without any intervening attacks. DD-082 had her CSF collected only a few days after onset of disease, so it is possible that OCBs did not have time to develop. Additional CSF collections were not feasible in our population because, in general, the treating neurologists did not believe that they were necessary or helpful for clinical care. It would be ideal to collect CSF at the time of attack, again a few months later, and around the time of any MS relapses, to assess if the antibacterial CSF responses become more focused over time.
Previously, one of the current authors (J.D.L.) demonstrated anti-peptidoglycan antibodies in CSF collected from patients with active MS [24]. This group also hypothesizes that bacterial cell wall peptidoglycan is involved in the development of CNS autoimmunity [25, 26]. Another group has demonstrated that peptidoglycan contributes to demyelinating autoimmunity by engaging NOD1 and NOD2 in dendritic cells leading to downstream RIPK1 activation (a.k.a. RIP2 and RICK), promoting T-helper 17 (Th17) responses [27].
Similarly, for Guillain-Barré syndrome (GBS), serological studies demonstrated that most GBS patients who had anti-GBS antibodies also had anti-Campylobacter jejuni antibodies [28]. GBS is an acute inflammatory polyradiculoneuropathy that typically develops following a gastrointestinal infection, with some reports suggesting a more severe form of the disease after C. jejuni infection [29,30,31,32]. The cell wall of C. jejuni contains polysaccharides that resemble glycoconjugates of the human nerve tissues resulting in misguided attack on myelin and axons. Certain types of this disorder involve an immune response against gangliosides, which is suspected to originate due to molecular mimicry between gangliosides and lipopolysaccharides of C. jejuni [33].
Due to the importance of the gut microbiome and immune function, Janji et al. assessed the gut microbiome within MS patients [34]. They demonstrated that Akkermansia muciniphila was enriched in the stool of MS patients when compared to healthy controls and, when using a murine model for MS, altering the gut microbiome modulates CNS autoimmunity. A French group recently demonstrated that anti-Akkermansia muciniphila immunoglobulin G (IgG) was increased in CSF from people with MS compared to controls, congruent with our findings [35]. They also used indirect ELISA. However, this group did not find elevations in anti-Fusobacterium necrophorum or anti-Bacteroides fragilis antibodies in their CSF samples, while we did show an elevation in EIs in the DD group against these bacterial lysates.
In conclusion, the current study demonstrates that antibacterial antibodies can be detected in the CSF of subjects with demyelinating disease and that CSF reactivity in the DD and OND groups exceeds reactivity in the controls for several bacterial species. This supports, but does not prove, the hypothesis that microbes contribute to demyelination in persons with MS and related diseases.
Data availability
The primary datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Atkins G, McQuaid S, Morris-Downes M, Galbraith S, Amor S, Cosby S, Sheahan B (2000) Transient virus infection and multiple sclerosis. Rev Med Virol 10:291–303
Zanin, L, G Saraceno, PP Panciani, G Renisi, L Signorini, K Migliorati, and MM Fontanella.
Kurtzke J (2005) Epidemiology and etiology of multiple sclerosis. Phys Med Rehabil Clin N Am 16:327–349
Kurtzke JF (2000) Multiple sclerosis in time and space--geographic clues to cause. J Neuro-Oncol 6(Suppl 2):S134–S140
Gilden D, Devlin M, Burgoon M, Owens G (1996) The search for virus in multiple sclerosis brain. Mult Scler J 2(4):179–183
Kriesel JD, Bhetariya P, Wang ZM, Renner D, Palmer C, Fischer KF (2019) Spectrum of microbial sequences and a bacterial cell wall antigen in primary demyelination brain specimens obtained from living patients. Sci Rep 9:1387
Marrero-Santos KM, Beltran M, Carrion-Lebron J, Sanchez-Vegas C, Hamer DH, Barnett ED, Santiago LM, Hunsperger EA (2013) Optimization of the cutoff value for a commercial anti-dengue virus IgG immunoassay. Clin Vaccine Immunol 20(3):358–362
Captia HSV 2 Type Specific IgG [package insert]. Ref 2323900 and 2323901. Wicklow. Ireland: Trinity Biotech Corp
Adenovirus IgG ELISA Kit [package insert]. Catalog Number KA3273. Taipei, Taiwan: Abnova Corporation.
Halperin JJ, Luft BJ, Anand AK, Roque CT, Alvarez O, Volkman DJ, Dattwyler RJ (1989) Lyme neuroborreliosis: central nervous system manifestations. Neurology 39(6):753–759
Theel ES, Aguero-Rosenfeld ME, Pritt B, Adem PV, Wormser GP (2019) Limitations and confusing aspects of diagnostic testing for neurologic Lyme disease in the United States. J Clin Microbiol 57(1):57
Lowry, R. VassarStats: Website for statistical computation. 2020; Available from: http://vassarstats.net.
Ivashchenko R, Bilogurova T, Bykov I, Dolgaya L, Iegorov V, Malko T, Protsenko A, Prism 9 for macOS (2020) GraphPad Software. LLC, San Diego
Stangroom J (2018) Mann-Whitney U test calculator. Social Science Statistics Available from: https://www.socscistatistics.com/tests/mannwhitney/default.aspx
Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, Tuong ZK, Negro-Demontel ML, Kumar N, Suchanek O, Tajsic T, Harcourt K, Scott K, Bashford-Rogers R, Helmy A, Reich DS, Belkaid Y, Lawley TD, Gavern DBM, Clatworthy MR (2020) Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 587(7834):472–476
Rojas OL, Pröbstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, Leung LYT, Najafi G, Khaleghi K, Garcillán B, Li A, Besla R, Naouar I, Cao EY, Chiaranunt P, Burrows K, Robinson HG, Allanach JR, Yam J, Luck H, Campbell DJ, Allman D, Brooks DG, Tomura M, Baumann R, Zamvil SS, Bar-Or A, Horwitz MS, Winer DA, Mortha A, Mackay F, Prat A, Osborne LC, Robbins C, Baranzini SE, Gommerman JL (2019) Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 176(3):610–624.e18
Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O (1998) Reference distributions for immunoglobulins A, G, and M: a comparison of a large cohort to the world's literature. J Clin Lab Anal 12(6):371–377
Huus KE, Petersen C, Finlay BB (2021) Diversity and dynamism of IgA-microbiota interactions. Nat Rev Immunol. https://doi.org/10.1038/s41577-021-00506-1
Halperin JJ (2017) Neuroborreliosis. J Neurol 264(6):1292–1297
ESPN. E:60 - Made in Dietrich: the life of Acey Shaw. 2016; Available from: https://www.espn.com/video/clip/_/id/12786742.
Swartz JD, Lachman M, Westveer K, O’Neill T, Geary T, Kott RW, Berardinelli JG, Hatfield PG, Thomson JM, Roberts A, Yeoman CJ (2014) Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of lactobacilli and near-neutral pH. Front Vet Sci 1:19
Wang Y, Ametaj BN, Ambrose DJ, Gänzle MG (2013) Characterisation of the bacterial microbiota of the vagina of dairy cows and isolation of pediocin-producing Pediococcus acidilactici. BMC Microbiol 13:19
Blanc F, Jaulhac B, Fleury M, de Seze J, de Martino SJ, Remy V, Blaison G, Hansmann Y, Christmann D, Tranchant C (2007) Relevance of the antibody index to diagnose Lyme neuroborreliosis among seropositive patients. Neurology 69(10):953–958
Schrijver IA, van Meurs M, Melief MJ, Ang CW, Buljevac D, Ravid R, Hazenberg MP, Laman JD (2001) Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain 124(Pt 8):1544–1554
Visser L, de Heer HJ, Boven LA, van Riel D, van Meurs M, Melief MJ, Zahringer U, van Strijp J, Lambrecht BN, Nieuwenhuis EE, Laman JD (2005) Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol 174(2):808–816
Visser L, Melief MJ, van Riel D, van Meurs M, Sick EA, Inamura S, Bajramovic JJ, Amor S, Hintzen RQ, Boven LA, t Hart BA, Laman JD (2006) Phagocytes containing a disease-promoting Toll-like receptor/Nod ligand are present in the brain during demyelinating disease in primates. Am J Pathol 169(5):1671–1685
Shaw PJ, Barr MJ, Lukens JR, McGargill MA, Chi H, Mak TW, Kanneganti TD (2011) Signaling via the RIP2 adaptor protein in central nervous system-infiltrating dendritic cells promotes inflammation and autoimmunity. Immunity 34(1):75–84
Jacobs BC, van Doorn PA, Schmitz PI, Tio-Gillen AP, Herbrink P, Visser LH, Hooijkass H, van der Meché FG (1996) Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann Neurol 40(2):181–187
Kaldor J, Speed BR (1984) Guillain-Barré syndrome and Campylobacter jejuni: a serological study. Br Med J (Clin Res Ed) 288(6434):1867–1870
Winer JB, Hughes RA, Anderson MJ, Jones DM, Kangro H, Watkins RP (1988) A prospective study of acute idiopathic neuropathy. II. Antecedent events. J Neurol Neurosurg Psychiatry 51(5):613–618
Yu RK, Usuki S, Ariga T (2006) Ganglioside molecular mimicry and its pathological roles in Guillain-Barré syndrome and related diseases. Infect Immun 74(12):6517–6527
Yuki N, Yoshino H, Sato S, Miyatake T (1990) Acute axonal polyneuropathy associated with anti-GM1 antibodies following Campylobacter enteritis. Neurology 40(12):1900–1902
Moran AP, Annuk H, Prendergast MM (2005) Antibodies induced by ganglioside-mimicking Campylobacter jejuni lipooligosaccharides recognise epitopes at the nodes of Ranvier. J Neuroimmunol 165(1-2):179–185
Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topcuolu BD, Holden J, Kivisakk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7:12015
Vallino A, Dos Santos A, Mathé CV, Garcia A, Morille J, Dugast E, Shah SP, Héry-Arnaud G, Guilloux CA, Gleeson PJ, Monteiro RC, Soulillou JP, Harb J, Bigot-Corbel E, Michel L, Wiertlewski S, Nicot AB, Laplaud DA, Berthelot L (2020) Gut bacteria Akkermansia elicit a specific IgG response in CSF of patients with MS. Neurol Neuroimmunol Neuroinflamm 7(3). https://doi.org/10.1212/nxi.0000000000000688
Acknowledgements
The authors would like to thank Professor Alun Thomas from the Study Design and Biostatistics Center, University of Utah School of Medicine, for his review of and contribution to the final statistical analysis of the data.
Code availability
Not applicable.
Funding
The study was supported by funds raised through the University of Utah School of Medicine. Author J.D.L. is supported by the Dutch MS Research Foundation (Voorschoten, the Netherlands) and the Zabawas Foundation (Den Haag, the Netherlands).
Author information
Authors and Affiliations
Contributions
Conceptualization and Planning: EE, JDL, JDK, KFF. Methods: EE, JDK, BL, TBM, HRH. Microbiology: EE, BL. Manuscript Draft: EE. Manuscript Editing: JDL, JDK, KFF, HRH. Manuscript Final: JDK.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the University of Utah Health Sciences Institutional Review Board (IRB), protocol IRB_47316.
Consent to participate
Each subject either signed an IRB-approved informed consent or had a legally authorized representative sign for them.
Consent for publication
Research subject DD-03 and his wife have specifically consented to the publication of his case details, including the identifying information provided in reference #20. The consent document was provided by and then approved by the University of Utah.
Conflict of interest
The authors declare no competing interests. The work is protected by United States Provisional Patent Application #62785377, “Compositions and Methods Useful in Detecting and Treating Multiple Sclerosis and Other Demyelinating Diseases” filed by the University of Utah, December 28, 2018.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eckman, E., Laman, J.D., Fischer, K.F. et al. Spinal fluid IgG antibodies from patients with demyelinating diseases bind multiple sclerosis-associated bacteria. J Mol Med 99, 1399–1411 (2021). https://doi.org/10.1007/s00109-021-02085-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-021-02085-z