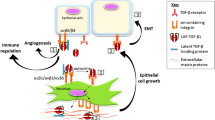
Abstract
Fibrosis is a pathological process characterized by accumulation of fibrous connective tissue in organs, leading to organ malfunction and failure. At the cellular level, tissue injury or cellular stress results in aberrant and/or sustained fibroblast “activation” leading to excessive extracellular matrix (ECM) accumulation and remodeling, as well as abnormal crosstalk with other cell types. Fibroblast functions within the fibrotic milieu are broad and complex, but among the most prominent are regulation of tissue architecture via modulation of ECM deposition and synthesis, and production of, activation of, and response to growth factors. Thus, both integrins and growth factor receptors (GFRs) play critical roles in fibroblast orchestration of tissue remodeling. However, the interplay between integrins and GFRs in this context is not fully understood. Their interaction has been described for other diseases, such as cancer. Here, we review the literature relevant to integrin/GFR interactions in the context of fibrosis, classify the known interactions into broad categories, and discuss research opportunities that may yield novel therapeutic targets for a broad range of debilitating chronic diseases.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Protein and gene information for Fig. 3 were obtained from the UniProt database (https://www.uniprot.org). The software used to develop networking analysis was Cytoscape 3.7 (free source https://cytoscape.org/).
References
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214(2):199–210
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7):1028–1040
Olson AL, Gifford AH, Inase N, Fernández Pérez ER, Suda T (2018) The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev 27(150):180077
Kendall RT, Feghali-Bostwick CA (2014) Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5:123–123
Darby IA, Hewitson TD (2007) Fibroblast differentiation in wound healing and fibrosis, International Review of Cytology, Academic Press, pp. 143–179
Waters DW, Blokland KEC, Pathinayake PS, Burgess JK, Mutsaers SE, Prele CM, Schuliga M, Grainge CL, Knight DA (2018) Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Phys Lung Cell Mol Phys 315(2):L162–l172
Fiore VF, Strane PW, Bryksin AV, White ES, Hagood JS, Barker TH (2015) Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction. J Cell Biol 211(1):173–190
Fiore VF, Wong SS, Tran C, Tan C, Xu W, Sulchek T, White ES, Hagood JS, Barker TH (2018) αvβ3 integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI insight 3(20):e97597
DeLeon-Pennell KY, Barker TH, Lindsey ML (2020) Fibroblasts: the arbiters of extracellular matrix remodeling. Matrix Biol 91-92:1–7
Barker TH, Engler AJ (2017) The provisional matrix: setting the stage for tissue repair outcomes. Matrix Biol 60-61:1–4
Saied EM, Bediwy AS (2011) Expression of epidermal growth factor receptor (EGFR) in the bronchial epithelium of patients with chronic obstructive pulmonary disease (COPD). Eur Respir J 38(Suppl 55):p3823
Vallath S, Hynds RE, Succony L, Janes SM, Giangreco A (2014) Targeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunities. Eur Respir J 44(2):513–522
Henderson NC, Sheppard D (2013) Integrin-mediated regulation of TGFβ in fibrosis. Biochim Biophys Acta 1832(7):891–896
Margadant C, Sonnenberg A (2010) Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 11(2):97–105
Chen X, Whiting C, Borza C, Hu W, Mont S, Bulus N, Zhang M-Z, Harris RC, Zent R, Pozzi A (2010) Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression. Mol Cell Biol 30(12):3048–3058
Ivaska J, Heino J (2011) Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol 27(1):291–320
Takada Y, Takada YK, Fujita M (2017) Crosstalk between insulin-like growth factor (IGF) receptor and integrins through direct integrin binding to IGF1. Cytokine Growth Factor Rev 34:67–72
Thomas JR, Paul NR, Morgan MR (2019) Adhesion and growth factor receptor crosstalk mechanisms controlling cell migration. Essays Biochem 63(5):553–567
Hynes RO (2002) Integrins: bidirectional. Allosteric Signaling Machines, Cell 110(6):673–687
Harburger DS, Calderwood DA (2009) Integrin signalling at a glance. J Cell Sci 122(2):159–163
Kechagia JZ, Ivaska J, Roca-Cusachs P (2019) Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol 20(8):457–473
Hermosilla T, Muñoz D, Herrera-Molina R, Valdivia A, Muñoz N, Nham SU, Schneider P, Burridge K, Quest AF, Leyton L (2008) Direct Thy-1/alphaVbeta3 integrin interaction mediates neuron to astrocyte communication. Biochim Biophys Acta 1783(6):1111–1120
Manninen A, Varjosalo M (2017) A proteomics view on integrin-mediated adhesions. Proteomics 17(3–4)
Humphries JD, Byron A, Humphries MJ (2006) Integrin ligands at a glance. J Cell Sci 119(19):3901–3903
Leyton L, Díaz J, Martínez S, Palacios E, Pérez LA, Pérez RD (2019) Thy-1/CD90 a bidirectional and lateral signaling scaffold. Front Cell Dev Biol 7:132
Maldonado H, Calderon C, Burgos-Bravo F, Kobler O, Zuschratter W, Ramirez O, Härtel S, Schneider P, Quest AF, Herrera-Molina R, Leyton L (2017) Astrocyte-to-neuron communication through integrin-engaged Thy-1/CBP/Csk/Src complex triggers neurite retraction via the RhoA/ROCK pathway. Biochim Biophys Acta, Mol Cell Res 1864(2):243–254
Avalos AM, Valdivia AD, Muñoz N, Herrera-Molina R, Tapia JC, Lavandero S, Chiong M, Burridge K, Schneider P, Quest AF, Leyton L (2009) Neuronal Thy-1 induces astrocyte adhesion by engaging syndecan-4 in a cooperative interaction with alphavbeta3 integrin that activates PKCalpha and RhoA. J Cell Sci 122(Pt 19):3462–3471
Jiao Y, Feng X, Zhan Y, Wang R, Zheng S, Liu W, Zeng X (2012) Matrix metalloproteinase-2 promotes αvβ3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PLoS One 7(7):e41591–e41591
Borrirukwanit K, Lafleur MA, Mercuri FA, Blick T, Price JT, Fridman R, Pereira JJ, Leardkamonkarn V, Thompson EW (2007) The type I collagen induction of MT1-MMP-mediated MMP-2 activation is repressed by alphaVbeta3 integrin in human breast cancer cells. Matrix Biol 26(4):291–305
Morozevich G, Kozlova N, Cheglakov I, Ushakova N, Berman A (2009) Integrin α5β1 controls invasion of human breast carcinoma cells by direct and indirect modulation of MMP-2 collagenase activity. Cell Cycle 8(14):2219–2225
Abdalla M, Thompson L, Gurley E, Burke S, Ujjin J, Newsome R, Somanath PR (2015) Dasatinib inhibits TGFβ-induced myofibroblast differentiation through Src-SRF pathway. Eur J Pharmacol 769:134–142
Lawson C, Schlaepfer DD (2012) Integrin adhesions: who’s on first? What’s on second? Connections between FAK and talin, Cell Adhes Migr 6(4) 302–306
Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M (2012) Turnover of focal adhesions and cancer cell migration. Int J Cell Biol 2012:310616
Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skládal P, Pešl M, Caluori G, Pagliari S, Martino F, Maceckova Z, Hajduch M, Sanz-Garcia A, Pugno NM, Stokin GB, Forte G (2017) YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun 8:15321
Rausch V, Hansen CG (2020) The hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol 30(1):32–48
Balasubramanian S, Quinones L, Kasiganesan H, Zhang Y, Pleasant DL, Sundararaj KP, Zile MR, Bradshaw AD, Kuppuswamy D (2012) β3 integrin in cardiac fibroblast is critical for extracellular matrix accumulation during pressure overload hypertrophy in mouse. PLoS One 7(9):e45076
Chen C, Li R, Ross RS, Manso AM (2016) Integrins and integrin-related proteins in cardiac fibrosis. J Mol Cell Cardiol 93:162–174
Conroy KP, Kitto LJ, Henderson NC (2016) alphav integrins: key regulators of tissue fibrosis. Cell Tissue Res 365(3):511–519
Perrucci GL, Barbagallo VA, Corliano M, Tosi D, Santoro R, Nigro P, Poggio P, Bulfamante G, Lombardi F, Pompilio G (2018) Integrin alphanubeta5 in vitro inhibition limits pro-fibrotic response in cardiac fibroblasts of spontaneously hypertensive rats. J Transl Med 16(1):352
Perrucci GL, Barbagallo VA, Corlianò M, Tosi D, Santoro R, Nigro P, Poggio P, Bulfamante G, Lombardi F, Pompilio G (2018) Integrin ανβ5 in vitro inhibition limits pro-fibrotic response in cardiac fibroblasts of spontaneously hypertensive rats. J Transl Med 16(1):352
Schnittert J, Bansal R, Storm G, Prakash J (2018) Integrins in wound healing, fibrosis and tumor stroma: high potential targets for therapeutics and drug delivery. Adv Drug Deliv Rev 129:37–53
Coelho NM, McCulloch CA (2016) Contribution of collagen adhesion receptors to tissue fibrosis. Cell Tissue Res 365(3):521–538
Heino J (2014) Cellular signaling by collagen-binding integrins, in: D. Gullberg (Ed.), I Domain integrins, Springer Netherlands, Dordrecht, pp. 143–155
Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3(12):a005058
Kagami S, Kondo S, Löster K, Reutter W, Kuhara T, Yasutomo K, Kuroda Y (1999) α1β1 integrin-mediated collagen matrix remodeling by rat mesangial cells is differentially regulated by transforming growth factor-β and platelet-derived growth factor-BB. J Am Soc Nephrol 10(4):779–789
Yang C, Zeisberg M, Lively JC, Nyberg P, Afdhal N, Kalluri R (2003) Integrin α1β1 and α2β1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res 63(23):8312–8317
Gardner H, Broberg A, Pozzi A, Laato M, Heino J (1999) Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci 112(Pt 3):263–272
Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, Polk DB, Singh AB, Harris RC, Zent R, Pozzi A (2007) Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol 27(9):3313–3326
Rubel D, Frese J, Martin M, Leibnitz A, Girgert R, Miosge N, Eckes B, Müller G-A, Gross O (2014) Collagen receptors integrin alpha2beta1 and discoidin domain receptor 1 regulate maturation of the glomerular basement membrane and loss of integrin alpha2beta1 delays kidney fibrosis in COL4A3 knockout mice. Matrix Biol 34:13–21
Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A (2004) Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol 165(2):617–630
Borok Z (2009) Role for alpha3 integrin in EMT and pulmonary fibrosis. J Clin Invest 119(1):7–10
Bansal R, Nakagawa S, Yazdani S, van Baarlen J, Venkatesh A, Koh AP, Song W-M, Goossens N, Watanabe H, Beasley MB, Powell CA, Storm G, Kaminski N, van Goor H, Friedman SL, Hoshida Y, Prakash J (2017) Integrin alpha 11 in the regulation of the myofibroblast phenotype: implications for fibrotic diseases. Exp Mol Med 49(11):e396–e396
Dewidar B, Meyer C, Dooley S, Meindl B, Nadja (2019) TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells 8(11):1419
Giannandrea M, Parks WC (2014) Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 7(2):193–203
Ravanti L, Heino J, López-Otinin C, Kähäri V-M (1999) Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem 274(4):2446–2455
Zigrino P, Drescher C, Mauch C (2001) Collagen-induced proMMP-2 activation by MT1-MMP in human dermal fibroblasts and the possible role of α2β1 integrins. Eur J Cell Biol 80(1):68–77
Brilha S, Chong DLW, Khawaja AA, Ong CWM, Guppy NJ, Porter JC, Friedland JS (2018) Integrin α2β1 expression regulates matrix metalloproteinase-1-dependent bronchial epithelial repair in pulmonary tuberculosis. Front Immunol 9:1348–1348
Hung CF, Wilson CL, Chow Y-H, Schnapp LM (2018) Role of integrin alpha8 in murine model of lung fibrosis. PLoS One 13(5):e0197937–e0197937
Song K-H, Cho S-J, Song J-Y (2016) αvβ1 integrin as a novel therapeutic target for tissue fibrosis. Ann Transl Med 4(20):411–411
Tatler AL, Goodwin AT, Gbolahan O, Saini G, Porte J, John AE, Clifford RL, Violette SM, Weinreb PH, Parfrey H, Wolters PJ, Gauldie J, Kolb M, Jenkins G (2016) Amplification of TGFβ induced ITGB6 gene transcription may promote pulmonary fibrosis. PLoS One 11(8):e0158047–e0158047
Gian L. Bagnato, N. Irrera, G. Pizzino, D. Santoro, William N. Roberts, G. Bagnato, G. Pallio, M. Vaccaro, F. Squadrito, A. Saitta, D. Altavilla, A. Bitto, Dual αvβ3 and αvβ5 blockade attenuates fibrotic and vascular alterations in a murine model of systemic sclerosis, Clin Sci 132(2) (2018) 231–242
McCarty JH (2020) αvβ8 integrin adhesion and signaling pathways in development, physiology and disease. J Cell Sci 133(12):jcs239434
Annes JP, Chen Y, Munger JS, Integrin DBR (2004) αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol 165(5):723–734
Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV Jr, Sheppard D (1996) Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 133(4):921–928
Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF 3rd, Sheppard D, Gardner HA, Violette SM (2007) Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170(1):110–125
Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS (2008) Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177(1):82–90
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96(3):319–328
Bandyopadhyay A, Raghavan S (2009) Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets 10(7):645–652
Liu H, Wu Y, Wang F, Liu Z (2014) Molecular imaging of integrin αvβ6 expression in living subjects. Am J Nucl Med Mol Imaging 4(4):333–345
Nishimura SL (2009) Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol 175(4):1362–1370
Tan C, Jiang M, Wong SS, Espinoza CR, Kim C, Li X, Connors E, Hagood JS (2019) Soluble Thy-1 reverses lung fibrosis via its integrin-binding motif. JCI Insight 4(21)
Khanna D, Tashkin D, Wells A, Goldin J, Lubell M, Wax S, Damian D, Denton C (2017) Randomized controlled trial of abituzumab in systemic sclerosis-associated interstitial lung disease. Eur Respir J 50(suppl 61):OA2930
Hersey P, Sosman J, O’Day S, Richards J, Bedikian A, Gonzalez R, Sharfman W, Weber R, Logan T, Buzoianu M, Hammershaimb L, Kirkwood JM (2010) A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or - dacarbazine in patients with stage IV metastatic melanoma. Cancer 116(6):1526–1534
Ritzenthaler JD, Zhang M, Torres-Gonzalez E, Roman J (2020) The integrin inhibitor cilengitide and bleomycin-induced pulmonary fibrosis : cilengitide and bleomycin-induced pulmonary fibrosis. Lung 198:947–955
Guzy RD, Li L, Smith C, Dorry SJ, Koo HY, Chen L, Ornitz DM (2017) Pulmonary fibrosis requires cell-autonomous mesenchymal fibroblast growth factor (FGF) signaling. J Biol Chem 292(25):10364–10378
Joannes A, Brayer S, Besnard V, Marchal-Somme J, Jaillet M, Mordant P, Mal H, Borie R, Crestani B, Mailleux AA (2016) FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and migration and inhibit myofibroblast differentiation of human lung fibroblasts in vitro. Am J Phys Lung Cell Mol Phys 310(7):L615–L629
Singh B, Carpenter G, Coffey RJ (2016) EGF receptor ligands: recent advances, F1000Res 5
Huang F, Chen Y-G (2012) Regulation of TGF-β receptor activity. Cell Biosci 2:9–9
Guan S, Zhou J (2017) Frizzled-7 mediates TGF-β-induced pulmonary fibrosis by transmitting non-canonical Wnt signaling. Exp Cell Res 359(1):226–234
Huang H-C, Klein PS (2004) The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol 5(7):234–234
Smith ER, Holt SG, Hewitson TD (2017) FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-beta autoinduction. Int J Biochem Cell Biol 92:63–78
Piersma B, R.A. Bank, Boersema M (2015) Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med 2(59)
Gauldie J, Kolb M, Ask K, Martin G, Bonniaud P, Warburton D (2006) Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc Am Thorac Soc 3(8):696–702
Walton KL, Johnson KE, Harrison CA (2017) Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol 8(461)
Liu F, Wang L, Qi H, Wang J, Wang Y, Jiang W, Xu L, Liu N, Zhuang S (2017) Nintedanib, a triple tyrosine kinase inhibitor, attenuates renal fibrosis in chronic kidney disease. Clin Sci 131(16):2125–2143
Wollin L, Maillet I, Quesniaux V, Ryffel B (2013) Nintedanib reduces bleomycin-induced lung inflammation and fibrosis in mice. Eur Respir J 42(Suppl 57):P682
Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M (2015) Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis, Eur Respir J ERJ-01749-2014
Ju W, Zhihong Y, Zhiyou Z, Qin H, Dingding W, Li S, Baowei Z, Xing W, Ying H, An H (2012) Inhibition of α-SMA by the ectodomain of FGFR2c attenuates lung fibrosis. Mol Med 18(1):992–1002
Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S (2013) Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol 183(1):160–172
Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, DePeralta DK, Chen X, Kuroda T, Lanuti M, Schmitt AD, Gupta S, Crenshaw A, Onofrio R, Taylor B, Winckler W, Bardeesy N, Caravan P, Golub TR, Tanabe KK (2014) Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 59(4):1577–1590
Liu N, Guo J-K, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S (2012) Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23(5):854–867
Shu DY, Wojciechowski M, Lovicu FJ (2019) ERK1/2-mediated EGFR-signaling is required for TGFβ-induced lens epithelial-mesenchymal transition. Exp Eye Res 178:108–121
Faress JA, Nethery DE, Kern EFO, Eisenberg R, Jacono FJ, Allen CL, Kern JA (2007) Bleomycin-induced pulmonary fibrosis is attenuated by a monoclonal antibody targeting HER2. J Appl Physiol 103(6):2077–2083
Tzouvelekis A, Ntolios P, Karameris A, Vilaras G, Boglou P, Koulelidis A, Archontogeorgis K, Kaltsas K, Zacharis G, Sarikloglou E, Steiropoulos P, Mikroulis D, Koutsopoulos A, Froudarakis M, Bouros D (2013) Increased expression of epidermal growth factor receptor (EGF-R) in patients with different forms of lung fibrosis. Biomed Res Int 2013:654354
Weiskirchen R, Weiskirchen S, Tacke F (2019) Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Asp Med 65:2–15
de Heus C, Kagie N, Heukers R, van Bergen en Henegouwen PMP, Gerritsen HC (2013) Chapter 16 - analysis of EGF receptor oligomerization by homo-FRET, in: P.M. Conn (Ed.), Methods in cell biology, Academic Press, pp. 305–321
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141(7):1117–1134
Karimizadeh E, Motamed N, Mahmoudi M, Jafarinejad-Farsangi S, Jamshidi A, Faridani H, Gharibdoost F (2015) Attenuation of fibrosis with selective inhibition of c-Abl by siRNA in systemic sclerosis dermal fibroblasts. Arch Dermatol Res 307(2):135–142
Kinoshita K, Aono Y, Azuma M, Kishi J, Takezaki A, Kishi M, Makino H, Okazaki H, Uehara H, Izumi K, Sone S, Nishioka Y (2013) Antifibrotic effects of focal adhesion kinase inhibitor in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 49(4):536–543
Xu S-w, Liu S, Eastwood M, Sonnylal S, Denton CP, Abraham DJ, Leask A (2009) Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One 4(10):e7438–e7438
Andrikopoulos P, Kieswich J, Pacheco S, Nadarajah L, Harwood SM, O’Riordan CE, Thiemermann C, Yaqoob MM (2019) The MEK inhibitor trametinib ameliorates kidney fibrosis by suppressing ERK1/2 and mTORC1 signaling. J Am Soc Nephrol 30(1):33–49
Wen J, Lin X, Gao W, Qu B, Ling Y, Liu R, Yu M (2019) MEK inhibition prevents TGF-β1-induced myofibroblast transdifferentiation in human tenon fibroblasts. Mol Med Rep 19(1):468–476
Madala SK, Edukulla R, Phatak M, Schmidt S, Davidson C, Acciani TH, Korfhagen TR, Medvedovic M, LeCras TD, Wagner K, Hardie WD (2014) Dual targeting of MEK and PI3K pathways attenuates established and progressive pulmonary fibrosis. PLoS One 9(1):e86536
Laplante P, Raymond M-A, Gagnon G, Vigneault N, Sasseville AM-J, Langelier Y, Bernard M, Raymond Y, Hébert M-J (2005) Novel fibrogenic pathways are activated in response to endothelial apoptosis: implications in the pathophysiology of systemic sclerosis. J Immunol 174(9):5740–5749
Hu M, Che P, Han X, Cai G-Q, Liu G, Antony V, Luckhardt T, Siegal GP, Zhou Y, Liu R-m, Desai LP, O’Reilly PJ, Thannickal VJ, Ding Q (2014) Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther 351(1):87–95
Lu Y-Y, Zhao X-K, Yu L, Qi F, Zhai B, Gao C-Q, Ding Q (2017) Interaction of Src and alpha-V integrin regulates fibroblast migration and modulates lung fibrosis in a preclinical model of lung fibrosis. Sci Rep 7:46357–46357
Massip Copiz MM, Santa Coloma TA (2016) c- Src and its role in cystic fibrosis. Eur J Cell Biol 95(10):401–413
Tice DA, Biscardi JS, Nickles AL, Parsons SJ (1999) Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci U S A 96(4):1415–1420
Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ (1995) Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A 92(15):6981–6985
Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E (2011) Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene 30(13):1566–1576
Moro L, Dolce L, Cabodi S, Bergatto E, Erba EB, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P (2002) Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem 277(11):9405–9414
Somanath PR, Malinin NL, Byzova TV (2009) Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis 12(2):177–185
Borges E, Jan Y, Ruoslahti E (2000) Platelet-derived growth factor receptor β and vascular endothelial growth factor receptor 2 bind to the β3 integrin through its extracellular domain. J Biol Chem 275(51):39867–39873
Woodard AS, García-Cardeña G, Leong M, Madri JA, Sessa WC, Languino LR (1998) The synergistic activity of alphavbeta3 integrin and PDGF receptor increases cell migration. J Cell Sci 111(Pt 4):469–478
Mori S, Takada Y (2013) Crosstalk between fibroblast growth factor (FGF) receptor and integrin through direct integrin binding to FGF and resulting integrin-FGF-FGFR ternary complex formation. Medi Sci 1(1):20–36
Mori S, Wu CY, Yamaji S, Saegusa J, Shi B, Ma Z, Kuwabara Y, Lam KS, Isseroff RR, Takada YK, Takada Y (2008) Direct binding of integrin alphavbeta3 to FGF1 plays a role in FGF1 signaling. J Biol Chem 283(26):18066–18075
Avalos AM, Valdivia AD, Muñoz N, Herrera-Molina R, Tapia JC, Lavandero S, Chiong M, Burridge K, Schneider P, Quest AFG, Leyton L (2009) Neuronal Thy-1 induces astrocyte adhesion by engaging syndecan-4 in a cooperative interaction with alphavbeta3 integrin that activates PKCalpha and RhoA. J Cell Sci 122(Pt 19):3462–3471
Hagood JS (2019) Thy-1 as an integrator of diverse extracellular signals. Front Cell Dev Biol 7:26–26
Leyton L, Díaz J, Martínez S, Palacios E, Pérez LA, Pérez RD (2019) Thy-1/CD90 a bidirectional and lateral signaling scaffold. Front Cell Dev Biol 7(132)
Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M (2005) Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol 167(2):365–379
Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS (2009) Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122(Pt 2):227–232
Ning Y, Buranda T, Hudson LG (2007) Activated epidermal growth factor receptor induces integrin alpha2 internalization via caveolae/raft-dependent endocytic pathway. J Biol Chem 282(9):6380–6387
Paul NR, Thomas JR, Maldonado H, Wolanska KI, Koper EJ, Humphries JD, Byron A, George A, Allen N, Prior IA, Streuli CH, Humphries MJ, Morgan MR (2018) Eps8 is a convergence point integrating EGFR and integrin trafficking and crosstalk, bioRxiv 405043
Lindsay AJ, Hendrick AG, Cantalupo G, Senic-Matuglia F, Goud B, Bucci C, McCaffrey MW (2002) Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem 277(14):12190–12199
Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC (2008) Rab-coupling protein coordinates recycling of α5β1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 183(1):143–155
Funding
This review was supported by The UNC Catalyst for Rare Disease, Eshelman School of Pharmacy at the University of North Carolina at Chapel Hill Foundation (to J.S.H.).
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Horacio Maldonado and James Hagood. The first draft of the manuscript was written by Horacio Maldonado and James Hagood; both authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maldonado, H., Hagood, J.S. Cooperative signaling between integrins and growth factor receptors in fibrosis. J Mol Med 99, 213–224 (2021). https://doi.org/10.1007/s00109-020-02026-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-02026-2