Abstract
Although essential hypertension affects a large proportion of the human population and is one of the key drivers of cardiovascular mortality worldwide, we still do not have a complete understanding of its pathophysiology. More than 50 years ago, the immune system has been identified as an important part of the pathogenesis of arterial hypertension. An exceeding variety of recent publications deals with the interplay between the numerous different components of the immune system and mechanisms of arterial hypertension and has substantially contributed to our understanding of the role of immunity and inflammation in the pathogenesis of the disease. In this review, we focus on myeloid cells and anatomical barriers as particular aspects of innate immunity in arterial hypertension. Since it represents a first line of defense protecting against pathogens and maintaining tissue homeostasis, innate immunity provides many mechanistic hinge points in the area of hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arterial hypertension is one of the key drivers of cardiovascular mortality worldwide. Already in 2005, the global burden of disease study predicted a number of 1.56 billion adults with prevalent hypertension in 2025 [1]. Back in 1949, Page introduced the so-called mosaic theory of the pathophysiology of essential hypertension, claiming that not a single factor but a complex interplay of different organs and circumstances is part of the disease [2]. In addition to the endocrine system, kidney, heart, and vasculature, the immune system has been identified to be a major contributor to hypertension. First experimental hints were collected in 1960, when Grollman et al. identified antihypertensive effects of pharmacological immunosuppression in a murine model with partial renal infarction [3]. Since these days, hypertension research increasingly focused on the immune system resulting in the tempting theory of essential hypertension as an autoimmune disease [4, 5].
The first step of classifying the numerous different participants of the immune system is the subdivision into innate and adaptive immunity. Innate immunity represents the first line of defense against pathogens, responsible for maintaining tissue homeostasis and preventing microbe invasion [5]. In this review, we attempt to focus on the role of innate immunity in the mechanisms of essential hypertension discussing briefly the cellular components of innate immunity but also go beyond immune cells, looking at tissue homeostasis, inflammatory microenvironment, and anatomical barriers such as skin and gut. We therefore want to provide space for novel, emerging ideas and unexpected concepts that seem to be promising for further advances in hypertension research.
Murine models of arterial hypertension
As the pathogenesis of essential hypertension remains poorly understood, all of the animal models, which are commonly used in hypertension research, are limited in their translational value for the human disorder. Whereas there are several excellent animal models for primary hypertension, the lack of understanding of the exact pathogenesis of essential hypertension inevitably only allows attempts to mimic aspects of the human disease as good as possible. We want to introduce briefly the two most common inducible models as they form the basis for the largest part of the studies, which are cited in this review. Detailed overviews over all the existing genetic and inducible animal models including their advantages, disadvantages, and translation to human hypertension can be found elsewhere [6,7,8].
Studies using an infusion of the blood pressure hormone angiotensin II (AngII) represent by far the largest share of all murine models. Nearly half of all National Institute of Health–sponsored research projects in the field of arterial hypertension use this model [9]. Patients with essential hypertension show lower blood pressure and improved outcomes when treated with RAAS blockers or inhibitors. However, this clinical observation does not prove the causation that RAAS activation is the most important underlying cause of primary hypertension. Nevertheless, blood pressure elevations seen in animals treated with a continuous infusion of AngII can resemble blood pressure values seen in uncontrolled human hypertension and even induce hypertension-related organ damage [10, 11]. Since it is inducible, the model offers the advantage that different species and genotypes can be used easily for studies and the dose as well as duration of the AngII treatment can be adjusted dependent of the experimental question. As human essential hypertension is clearly not solely depending on AngII, the massive pharmacological challenge of this model only mimics blood pressure effects of a dysregulated RAAS and therefore is not suitable to explain the complete, highly complex pathogenesis of essential hypertension.
In the DOCA-salt model, the combination of mineralocorticoid treatment with deoxycorticosterone (DOCA) and intake of high-salt (e.g., sodium chloride), sometimes extended by uni-nephrectomy, provokes hypertension resembling some features of human low-renin hypertension. Although elevated levels of DOCA can contribute to some rare human forms of hypertension, the most common human form of mineralocorticoid-dependent hypertension involves hyperaldosteronism, which is then one of the subsets of primary and not essential hypertension. The model was often used to mimic salt-dependent hypertension which was explained by salt-driven fluid retention [12]. This concept is more and more questioned [13, 14]; however, despite all legitimate criticism, the model still is valuable, especially for studies with a focus on mineralocorticoid receptor blockage and hyperaldosteronism.
The development and choice of reproducible and translational animal models is still one of the biggest challenges in biomedical research in general and in hypertension research in particular. So far, there is no individual model that can exactly recapitulate all key features of human essential hypertension and reproduce the pathogenesis of this complex disorder. For the interpretation of experiments and publications aiming to contribute to the understanding of essential hypertension, the limits of the studied animal model has always to be taken into consideration to estimate the translational value.
Cell-mediated innate immunity
Since the 1960s, most immune cells in innate and adaptive immunity were described to have contributing roles in essential hypertension [15, 16]. Therefore, a detailed description of the published evidence for every single innate immune cell type would exceed the scope of this review. Nevertheless, we try to provide an overview of the most important recent findings and focus on key pathways of innate immune cells in essential hypertension.
In 2005, de Ciuceis et al. published about the link between innate immune cells and arterial hypertension in mice: They infused osteopetrotic mice (Op/Op)—a mouse strain deficient in macrophage colony-stimulating factor (m-CSF)—with AngII and found reduced systolic blood pressure values as well as a protection from vascular dysfunction in the absence of m-CSF [17]. Their findings indicated that m-CSF-depending cells play a substantial role in AngII-induced hypertension and vascular dysfunction. As m-CSF induces monocyte and macrophage colony formation from bone marrow precursors, these subsets were suspected to participate in the pathogenesis of AngII-induced murine hypertension. Later, we have shown that selective ablation of lysozyme M–positive myeloid cells attenuated AngII-induced endothelial dysfunction, vascular inflammation, and blood pressure increase. This phenotype could be restored by adoptive transfer of monocytes, but not of neutrophils or of monocytes deficient in the AngII receptor type 1 (AT1R) or gp91phox [18].
As tissue-resident macrophages are present in most (if not all) organ systems in the human body [19, 20], they have been under special investigation [21], and the M1-M2 concept of macrophage polarization [22] has been considered to play a role in arterial hypertension. AngII as one of the most prominent hormones in arterial hypertension has been shown to be a potent driver of the M1/M2 ratio towards the pro-inflammatory M1 phenotype [23, 24], In a small cohort of 45 hypertensive and 15 normotensive patients, Ji et al. described more Th1 and Th17 cells in hypertensive patients, which was accompanied by higher IFN-gamma and IL-17 levels [25]. As M1 macrophages are described to promote the induction of Th1 and Th17 cells, macrophage polarization could be a factor in the development of arterial hypertension. This tempting‚ “good cop/bad cop” concept for M1 and M2 macrophages in hypertension was challenged by Moore et al. when they described the accumulation of M2 macrophages in the aortic wall of AngII-infused mice [26] after 14 days of treatment with strong evidence for a shift of the phenotype during the treatment.
Besides monocytes/macrophages, neutrophils and dendritic cells (DCs) are main cellular parts of the innate immune system. Neutrophil counts are elevated in hypertensive patients [27, 28] and in murine models of arterial hypertension [29, 30], but it is not clear yet, if they really drive hypertension themselves or if they are driven by the sterile, low-grade inflammation smoldering in hypertensive individuals. In any case, unequivocal evidence of a decisive role of neutrophils in initiating or promoting hypertension is lacking. Dendritic cells (DCs) exert divergent and important roles, which are required for the orchestrated immune response. They are the most potent of all antigen-presenting cells (APCs), presenting phagocytosed antigens to cells of the adaptive immune system. Antigen presentation and secretion of co-stimulatory cytokines by DCs are crucial for the exact guidance of T cell activation, polarization, and recruitment. This bridging position between innate and adaptive immune system makes DCs an interesting hub in the immunology of hypertension [31].
Kirabo et al. published that proteins modified by oxidation by highly reactive γ-ketoaldehydes (isoketals) found in different murine models of hypertension accumulate in DCs. In their study, this accumulation drives the DC-mediated production of pro-inflammatory cytokines and T cell proliferation resulting in arterial hypertension [32]. DCs also seem to be crucial for the development of a hypertensive response in AngII-infused mice. Lu et al. investigated FLT3L−/− mice lacking classical DCs. After application of AngII, the mice showed reduced blood pressure elevation and lower amounts of inflammatory cells in the kidneys [33].
Taken together, there are several sophisticated studies which focused on monocytes, macrophages, and dendritic cells in experimental hypertension, and many of them could show blood-lowering effects of interfering with one of these leucocyte subsets. Unfortunately, the translational value of these studies for human essential hypertension could neither be proven nor drive a novel therapeutic approach.
Toll-like receptors
As the orchestrated immune response is crucial for host defense, but also for wound healing and maintenance of tissue homeostasis, the simple depletion of myeloid cells is not feasible for humans. Therefore, investigating (dys-)regulations in more specific inter- or intracellular pathways of innate immunity has been the topic of further studies. Fast recognition of invading microorganisms is of utmost priority for the organism. Widely shared molecular structures of pathogens, so-called pathogen-associated molecular patterns (PAMPs), and their direct activation of pattern-recognition receptors (PRRs) of innate immune cells are key components of the early innate immune response [34]. In contrast to the highly rearranged receptors of adaptive immunity, PRRs are limited in their number, germline-encoded, and evolutionary conserved [35].
The mammalian homolog of Drosophila Toll, TLR 4, was described as the first PRR in mice [36] responding to lipopolysaccharide (LPS) [37] as one of the major components of gram-negative bacteria. Until today, 10 different TLRs have been described in humans and 12 in mouse, detecting distinct PAMPs from bacteria, mycobacteria, viruses, fungi, and parasites, whereas one intact pathogen usually contains different PAMPs activating different PRRs [38]. Downstream signaling of TLRs initiates two major signaling pathways: except for TLR 3, which is TRIF-dependent (TIR domain–containing adaptor-inducing interferon-β–dependent), all TLRs recruit myeloid differentiation protein 88 (MyD88)–dependent signaling.
TLR4 exerts different effects in different murine models of arterial hypertension
Enforced TLR-signaling (especially TLR4- and MyD88-dependend pathways) has been described in murine hypertension with a high variability in the effects depending on the investigated model. An overview regarding all different TLRs and their impact on arterial hypertension has been described in detail before [39, 40], so we focus on TLR4 and the partially contradictory findings for its role in murine models of arterial hypertension and discuss the sparse human data.
In genetic models of hypertension, spontaneously hypertensive rats showed higher cardiac TLR4 expression [41] whereas anti-TLR4 antibody treatment decreased blood pressure [42]. These studies provide evidence that enhanced TLR4 expression and TLR4 signaling might be linked to the development and maintenance of hypertension. Same trends could be seen in DOCA-salt and aldosterone-salt hypertensive rats [43]. In mice with NG-nitro-l-arginine methyl ester (l-NAME)–induced hypertension, blood pressure was blunted in TLR4 knockout mice compared with wild-type mice [44], supporting the role of TLR4—at least—in this experimental model of arterial hypertension.
For AngII-infused mice, findings are complex and highly dependent of the studied tissue. Dange et al. published evidence that the blockade of brain TLR4 attenuates blood pressure in the AngII model [45]. In contrast to these findings, Singh et al. found that AngII-induced hypertension was not affected in MyD88−/− but in TRIFmut mice [46] and that a full knockout of TLR4 kept a preserved or even enhanced hypertensive response after the infusion of AngII [47]. This in line with findings of Matsuda et al. who could reproduce the results in TL4-deficient mice [48].
In TLR3-deficient mice, AngII-induced hypertension was abrogated [47] suggesting the TLR3-TRIF pathway as crucial for a blood pressure increase in the AngII model (Fig. 1a). The divergent findings between a full knockout and a brain-specific blockade might indicate either that the investigated tissue is of importance or that the models are so variable and unstable regarding the phenotype that a comparison is not possible. In cardiomyocytes, a different mechanism with a possible interaction between TLR and AngII signaling was published by Han et al. who supposed a MD2-mediated direct activation of TLR-signaling by AngII via TLR4 [49], whereas other experiments with the AT1R-antagonist Valsartan suggest a crosstalk between AT1R- and TLR4-dependent signaling [50] (Fig. 1b). Regarding the human population, Schneider et al. demonstrated that in a cohort of patients with myocardial infarction, older carriers of a TLR4 single-nucleotide polymorphism have a lower systolic blood pressure and pulse pressure indicating that TLR4 signaling influences age-dependent blood pressure increases [51].
Possible crosstalk between angiotensin II (AngII) and Toll-like receptor 4 (TLR 4) in arterial hypertension. a A model proposed by Singh et al. [46, 47] with different effects of TLR 2 and 4 in AngII-induced murine hypertension. The studies propose intrinsic differences between TLR4-TRIF and TLR3-TRIF interactions, where TLR3 directly interacts with TRIF and is required for AngII hypertension, whereas TLR4 activates MyD88, which lowers blood pressure in their studies using AngII-infused mice. b AngII acting through AT1 receptors upregulates TLR4. MyD88-dependent intercellular signaling results in MAP-kinase and NFκB activation with consequent increased production of pro-inflammatory mediators, which in turn contributes to hypertension. Additionally, TLR4 can induce NLRP3 inflammasome by activation of a receptor-interacting protein 1 (RIP1)–FAS-associated death domain protein (FADD)–caspase 8. Furthermore, AngII drives the generation of reactive oxygen species through NADPH oxidase. Downstream of all pathways, TLR4 exerts pro-inflammatory effects which can contribute to arterial hypertension. TLR 4, Toll-like receptor 4; AT1r, Angiotensin II receptor type I, NAPDH, nicotinamide adenine dinucleotide phosphate oxidase; MyD88, Myeloid differentiation primary response 88; ROS, reactive oxygen species; MAPK, mitogen-activated protein kinase; FADD, FAS-associated death domain protein; NFκB, nuclear factor “kappa-light-chain-enhancer” of activated B cells; NLRP3, nucleotide-binding oligomerization domain and leucine-rich repeat-containing receptor 3; ASC, apoptosis-associated Speck-like protein containing a caspase-recruitment domain
Taken together, there is on the one hand promising murine data regarding TLR4-signaling as a potential pharmaceutical target in essential hypertension. On the other hand, the results are strongly depending on the used murine model and could not have been solidly reproduced in humans yet.
NLRP3-inflammasome
Another downstream target of the TLR-4 signaling cascade, the cytosolic NOD-, LRR-, and pyrin domain–containing 3-receptor (NLRP3), has attracted attention within the past years, offering a novel possible pharmacological target for different inflammatory diseases [52]. NLRP3 drives the assembly of the inflammasome, leading to caspase 1–dependent activation of interleukin-1β (IL-1 β) cytokines. Crowley et al. demonstrated that renal IL-α and IL-β levels correlate with systolic blood pressure levels [53] and that IL-1R1 deficiency and blockage lowers blood pressure by reducing sodium re-uptake in the kidneys of AngII-infused animals [54].
Besides potassium-dependent pathways of NLRP3 activation, TLR4 can induce NLRP3-inflammasome by activation of a receptor-interacting protein 1 (RIP1)–FAS-associated death domain protein (FADD)–caspase 8 pathway [55]. Krishnan et al. demonstrated that inflammasome activity is crucial in one-side nephrectomized mice treated with DOCA-high-salt diet [56] and interfering with the NLRP3 inflammasome provided antihypertensive effects [57]. The authors conclude that the observed renal inflammation driven by DOCA/high-salt diet is responsible for arterial hypertension in their model and can be prevented by a pharmacological inhibition of the NLRP3 inflammasome as a novel antihypertensive strategy. These publications are in line with other papers, which link high salt intake with macrophage activation and polarization in other tissues [58, 59]. In the bigger picture of risk factors driving vascular dysfunction and atherosclerosis, targeting NLRP3 might offer a second benefit as well. Christ et al. published strong evidence that western diet triggers NLRP3-dependent innate immune reprogramming [60] and therefore exacerbates pro-inflammatory conditions.
Humoral innate immunity
Antimicrobial peptides and the complement system
Already beyond the anatomical borders, components of the innate immunity defend the integrity of the body against pathogens. Mucus and plasma are enriched with different classes of secreted antimicrobial peptides. Epithelial and circulating cells release defensins, cationic polypeptides with direct and indirect antimicrobial functions. There are still different hypotheses about the exact mechanisms of their microbe-killing capacities, but all focus on interactions between their molecular structure and the membrane of the microbes [61]. Nassar et al. could describe the presence of α-defensin in human coronary arteries and decrease the contraction of smooth muscle cells in response to the vasoconstrictor phenylephrine via the low-density lipoprotein receptor–related protein/α2-macroglobulin receptor, indicating a direct effect of these peptides on vascular tone and a possible role in hypertension as well [62].
Lysozyme, a glycoside hydrolase catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-d-glucosamine, is the most active component of all antimicrobial peptides. Several studies have already investigated a possible interplay between lysozyme and hypertension. With the intention to find a way to measure markers for subclinical atherosclerosis in an easy approachable and non-invasive way, especially salivary levels of lysozyme and other inflammatory molecules have been investigated within the past years. In two different studies, lysozyme in saliva was associated with hypertension. Qvarnstrom et al. suggested in a study with 500 participants that the top quartile of salivary lysozyme levels is significantly associated with prevalent hypertension [63]; similar findings were described in 259 humans by Labat et al. [64] A complementary trend has already been described for salivary lysozyme and coronary heart disease. The authors of the study interpreted these findings in line with the general paradigm that inflammation is an important part of pathogenesis of hypertension and atherosclerosis without defining the relationship as causal.
The complement system is component of the innate immune system. It is a complex humoral cascade consisting of over 30 soluble and membrane-bound molecules which directly sense and destroy microbial invaders [65] via opsonization and phagocytosis or enhance the cellular immune response via induction of chemokine or cytokine production. The complement cascade is mainly found not only in the circulation but also in tissues [66]. Increased plasma levels of different complement proteins in human patients with hypertension have been described. For instance, Eggström et al. and Bao et al. could find a positive association of elevated blood pressure and prehypertension with blood levels of the complement protein C3 [67, 68]. The same trend has also been seen for C3a [69], C4 [68], and C5a [70] indicating that hypertension induces the activation of complement system and production of complement factors.
In murine models, interfering with the complement system has been shown to provide protective mechanisms especially against hypertensive end-organ damage. Pharmacological inhibition of C5a production significantly reduced cardiac inflammation and remodeling in AngII-induced hypertension [70, 71]. Not only for cardiac end-organ damage but also for hypertension-driven pathologic changes in the kidney, different components of the complement system seem to be crucially involved. Chen et al. published that AngII-infused mice deficient for C5a and C3a receptors did not develop arterial hypertension by a decreased renal macrophage and T cell infiltration [72]; moreover, Zhou et al. showed that C3a activates the RAAS system by induction of epithelial-to-mesenchymal transition in renal epithelial cells [73].
Given the promising evidence of the conducted murine studies, the complement system is a likely target in further advances of hypertension research. Promising data regarding the prevention of hypertensive end-organ damage more than possible blood pressure–lowering effects may be at the center of attention in this field.
Anatomical barriers
Visible but often overlooked—the physical barriers gut and skin as the first line of defense in innate immunity
Even though it is the only part of the innate immune system, which is immediately visible for the human eye, skin and mucosal barriers tend to be ignored as innate immune organs. Besides the physical barrier function, the skin and the gut represent the outer interface with the external environment, and are therefore essential organs for the maintenance of physiologic homeostasis and closely related in purpose and function [74]. Within recent years, the skin and the gut have reached growing interest regarding their role in arterial hypertension. More particularly, these additional approaches provide a more physiology-oriented than a molecular biology-oriented approach, which might help to find new treatment strategies for essential hypertension.
The gastrointestinal barrier—human organ or microbiota zoo?
Under healthy conditions, the gastrointestinal barrier exerts an important role in nourishing the organism by digestion of food, influences the development and function of the mucosal immune system, and is an active endocrine organ. The contribution of the gastrointestinal barrier in arterial hypertension has been linked to the effects of gastrointestinal hormones, which were shown to influence systemic blood pressure levels in different murine models. Especially administration of the incretin hormone glucagon-like peptide-1 significantly attenuated the development of hypertension in Dahl salt-sensitive [75] and spontaneously hypertensive rats [76] and antihypertensive capacities of GLP-1 receptor agonists even could provide promising results in human hypertensive diabetics [77].
Within the last years, the gastrointestinal barrier unexpectedly advanced into the closer focus of attention in arterial hypertension—not by the organ function itself but by the fascinating inhabitants it accommodates. In the introduction of their work about the “Human microbiome project,” Turnbaugh et al. described humans as “supraorganisms composed of human and microbial components,” [78] underlining the undeniable close connection between the human organism in health as well as in disease and the microbes that live inside and on us. As the intestine is the organ of the body which is most densely populated with microorganisms, the major part of this emerging research field is dealing with this site, where the microbiome has a deep impact on human body development and contributes to normal physiology and disease developement [79].
Human microbial colonies in the gastro-intestine are described to be generally stable [80], but can be immediately changed and shaped by a large scale of influences. Not surprisingly, the consumed diet is a major factor for the composition and function of gut microbiota [81, 82]. Recent evidence revealed that certain diseases can be transferred via the microbiome: transferring microbiota from genetically obese mice induced higher amounts of body fat in germ-free mice [83]. These findings indicate that not only the diet has an effect on the microbiome but also the microbiome itself can influence host metabolism, which has been proven in further publications [84].
Several studies have linked gut microbiota and dysbiosis of microbiota with arterial hypertension in mice and men [85, 86]. Karbach et al. showed that germ-free-raised mice without a colonializing microbiome do not develop arterial hypertension and vascular dysfunction in response to AngII [87]. The lifelong absence of gut microbiota protected them from AngII-induced arterial hypertension and vascular dysfunction by reduced vascular inflammation and diminished production of reactive oxygen species.
Studies in different murine models of arterial hypertension could show that hypertensive animals have alterations in their microbiome and that it is possible to transfer hypertension by transferring microbiota from hypertensive to formerly normotensive animals [85, 88]. Even more interesting, dietary interventions, which affected the microbial colonization of the gut, modified blood pressure, and protected the animals from hypertension-related end-organ damage in murine models of arterial hypertension [89, 90]. Vice-versa, high-salt diet–driven changes of the gut microbiome induced Th17 cells and caused hypertension in mice, which could successfully be restored by probiotic treatment [91].
Taken together, the studies indicate that the gut microbiome is a fascinating field of research not only to develop a deeper understanding in microbiota host or interactions but also regarding a novel and profitable drug target in arterial hypertension.
Skin, sodium, and sodium-dependent hypertension
Besides its role as an outer barrier, the skin, especially skin glycose-aminoglycans, was detected as large compartment for water-free sodium storage regulating body sodium balance independently of renal function [92, 93]. These findings started to challenge the concept of pressure-natriuresis, an almost 50-year-old theory explaining the causal connection between high-salt intake and high blood pressure.
The pressure-natriuresis approach by Guyton et al. [94] described a first concept of how salt intake regulates blood pressure which led the field for more than half a century. It supposes that direct changes in salt and water intake and the following excretion drive the direction and change in arterial pressure to maintain body salt-water balance. By introducing the skin as the crucial compartment for long-term sodium storage and skin tissue sodium content as a detectable signature which is also non-invasively detectable in human hypertensive patients [95,96,97], Titze et al. indicated that the single-compartment concept of pressure-natriuresis for sodium excretion and blood pressure control might fall short. In a human long-term balance study, they could prove more evidence by detecting continuous fluctuations in sodium excretion, independent of sodium intake, indicating that sodium is stored and released in the skin without a direct impact on parallel water storage or blood pressure increase [98]. Helle et al. contributed further functional aspects of skin sodium storage for the genesis of arterial hypertension by showing that skin arterioles from rats fed a high-salt diet had increased contractility in response to AngII [99].
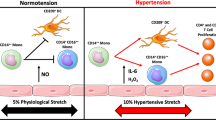
The detected storage of sodium in the skin thereby does not represent a random or incidental process at all. Beyond keratinocytes and extracellular matrix components, the skin barrier accommodates a variety of innate immune cells exerting phagocytic as well as regulatory functions: dendritic cells (DCs), natural killer (NK) cells, innate lymphoid cells (ILCs), mast cells, γδ-T cells, and myelomonocytic cells. These cellular representatives of the innate immune system, in particular tissue-resident macrophages, play a key role in sensing the interstitial, extracellular electrolyte levels in the skin (Fig. 2). Via the expression of tonicity-responsive enhancer binding protein (TONEBP), skin macrophages may modify electrolyte clearance through an extension of the skin lymphatic capillaries, which is part of the body blood pressure regulation [100].
Osmotically inactive Na storage in the skin contributes to body sodium and blood pressure regulation. Interstitial electrolyte balance relies on extrarenal regulatory mechanisms within the skin interstitium. Macrophages act as interstitial osmosensors that regulate local electrolyte composition via a tonicity enhancer binding protein/vascular endothelial growth factor-C (TonEBP/VEGF-C)–dependent mechanism [100]. The subsequent modulation of the lymph capillary network in the skin drives clearance of interstitial electrolytes from the interstitium into the bloodstream for renal clearance. Failure of this physiological extrarenal regulatory mechanism leads to a salt-sensitive blood pressure response. eNOS, endothelial nitric oxide synthase; VEGFR, vascular endothelial growth factor receptor; TonEBP, tonicity enhancer binding protein
Taken together, the skin is a key component regarding the innate immune system and mechanisms of hypertension. At cutaneous tissue level, macrophages modulate the interstitial salt storage and release by changing the lymph capillary network in the skin, which results in a kidney-like lymphatic counter-current system. As the body’s largest reservoir for sodium storage, it is—next to the kidney—a crucial organ for regulating the body salt-water balance and arterial blood pressure.
Conclusion
Innate immune cells are important drivers and modulators in the pathophysiology of hypertension on many levels. It has never been tested specifically, whether a pharmacologic intervention that would impact on anatomical barriers or other components’ innate immune system is effective to treat human arterial hypertension or prevent emergence or exacerbation of high blood pressure. An exception is concomitant autoimmune disease such as psoriasis or rheumatoid arthritis; patients afflicted by these diseases who were treated with mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase and suppressant of T and B cell proliferation [101], or the monoclonal TNF-α-antibody, infliximab [102], had reductions in blood pressure. There seems to be a strong link between genes favoring an enhanced status of inflammasome activation, IL-1β levels, arterial hypertension, and reduced longevity [103]. Whether anti-IL-1β helps to reduce increased blood pressure, however, is unclear. Secondary analyses from the CANTOS trial [104, 105] at least suggest that the IL-1β antibody canakinumab, which was effective to prevent cardiovascular events in patients with established coronary artery disease, was neutral with regard to arterial hypertension [106], although this trial also revealed that hsCRP level increases with increasing tertiles of blood pressure values at baseline. Whether arterial hypertension can specifically be treated by an anti-inflammatory therapy [107] remains to be tested in randomized controlled trials.
Data availability
Not applicable.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365(9455):217–223
Page IH (1967) The mosaic theory of arterial hypertension--its interpretation. Perspect Biol Med 10(3):325–333
White FN, Grollman A (1964) Autoimmune factors associated with infarction of the kidney. Nephron 1:93–102
Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M (2016) Hypertension as an autoimmune and inflammatory disease. Hypertens Res 39(8):567–573
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16(4):343–353
Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, Reckelhoff JF, Iadecola C, Coffman TM (2019) Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 73(6):e87–e120
Dornas WC, Silva ME (2011) Animal models for the study of arterial hypertension. J Biosci 36(4):731–737
Sigmund CD, Carey RM, Appel LJ, Arnett DK, Bosworth HB, Cushman WC, Galis ZS, Green Parker M, Hall JE, Harrison DG, McDonough AA, Nicastro HL, Oparil S, Osborn JW, Raizada MK, Wright JD, Oh YS (2020) Report of the National Heart, Lung, and Blood Institute Working Group on Hypertension: barriers to translation. Hypertension 75(4):902–917
Galis ZS, Thrasher T, Reid DM, Stanley DV, Oh YS (2013) Investing in high blood pressure research: a national institutes of health perspective. Hypertension 61(4):757–761
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204(10):2449–2460
Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM (2008) Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295(2):F515–F524
Lifton RP, Gharavi AG, Geller DS (2001) Molecular mechanisms of human hypertension. Cell 104(4):545–556
Kurtz TW, Dominiczak AF, DiCarlo SE, Pravenec M, Morris RC Jr (2015) Molecular-based mechanisms of Mendelian forms of salt-dependent hypertension: questioning the prevailing theory. Hypertension 65(5):932–941
Titze J, Dahlmann A, Lerchl K, Kopp C, Rakova N, Schroder A, Luft FC (2014) Spooky sodium balance. Kidney Int 85(4):759–767
McMaster WG, Kirabo A, Madhur MS, Harrison DG (2015) Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116(6):1022–1033
Norlander AE, Madhur MS, Harrison DG (2018) The immunology of hypertension. J Exp Med 215(1):21–33
De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL (2005) Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25(10):2106–2113
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T (2011) Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124(12):1370–1381
Davies LC, Taylor PR (2015) Tissue-resident macrophages: then and now. Immunology 144(4):541–548
Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14(10):986–995
Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, Shikatani EA, El-Maklizi M, Williams JW, Robins L, Li C, Lewis B, Yun TJ, Lee JS, Wieghofer P, Khattar R, Farrokhi K, Byrne J, Ouzounian M, Zavitz CC, Levy GA, Bauer CM, Libby P, Husain M, Swirski FK, Cheong C, Prinz M, Hilgendorf I, Randolph GJ, Epelman S, Gramolini AO, Cybulsky MI, Rubin BB, Robbins CS (2016) Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol 17(2):159–168
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164(12):6166–6173
Aki K, Shimizu A, Masuda Y, Kuwahara N, Arai T, Ishikawa A, Fujita E, Mii A, Natori Y, Fukunaga Y, Fukuda Y (2010) ANG II receptor blockade enhances anti-inflammatory macrophages in anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol 298(4):F870–F882
Yamamoto S, Yancey PG, Zuo Y, Ma LJ, Kaseda R, Fogo AB, Ichikawa I, Linton MF, Fazio S, Kon V (2011) Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arterioscler Thromb Vasc Biol 31(12):2856–2864
Ji Q, Cheng G, Ma N, Huang Y, Lin Y, Zhou Q, Que B, Dong J, Zhou Y, Nie S (2017) Circulating Th1, Th2, and Th17 levels in hypertensive patients. Dis Markers 2017:7146290
Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT, Lieu M, Samuel CS, Diep H, Kemp-Harper BK, Tare M, Ricardo SD, Guzik TJ, Sobey CG, Drummond GR (2015) M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am J Physiol Heart Circ Physiol 309(5):H906–H917
Jhuang YH, Kao TW, Peng TC, Chen WL, Li YW, Chang PK, Wu LW (2019) Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9-year cohort study in Taiwan. Hypertens Res 42(8):1209–1214
Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, Wang C, Xia Y, Guo X, Li C, Bao X, Su Q, Sun S, Wang X, Zhou M, Jia Q, Zhao H, Song K, Niu K (2015) Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens 28(11):1339–1346
Karbach S, Croxford AL, Oelze M, Schuler R, Minwegen D, Wegner J, Koukes L, Yogev N, Nikolaev A, Reissig S, Ullmann A, Knorr M, Waldner M, Neurath MF, Li H, Wu Z, Brochhausen C, Scheller J, Rose-John S, Piotrowski C, Bechmann I, Radsak M, Wild P, Daiber A, von Stebut E, Wenzel P, Waisman A, Munzel T (2014) Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol 34(12):2658–2668
Zhang R, Inagawa H, Kazumura K, Tsuchiya H, Miwa T, Morishita N, Uchibori S, Hanashiro J, Masaki T, Kobara H, Soma GI (2018) Evaluation of a hypertensive rat model using peripheral blood neutrophil activity, phagocytic activity and oxidized LDL evaluation. Anticancer Res 38(7):4289–4294
Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A (2017) Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep 21(4):1009–1020
Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG (2014) DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124(10):4642–4656
Lu X, Rudemiller NP, Privratsky JR, Ren J, Wen Y, Griffiths R, Crowley SD (2020) Classical dendritic cells mediate hypertension by promoting renal oxidative stress and fluid retention. Hypertension 75(1):131–138
Akira S (2009) Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci 85(4):143–156
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388(6640):394–397
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162(7):3749–3752
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34(5):637–650
McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC (2014) Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol 306(2):H184–H196
Goulopoulou S, McCarthy CG, Webb RC (2016) Toll-like receptors in the vascular system: sensing the dangers within. Pharmacol Rev 68(1):142–167
Eissler R, Schmaderer C, Rusai K, Kuhne L, Sollinger D, Lahmer T, Witzke O, Lutz J, Heemann U, Baumann M (2011) Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res 34(5):551–558
Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH (2012) Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 122(11):535–543
Zhang Y, Peng W, Ao X, Dai H, Yuan L, Huang X, Zhou Q (2015) TAK-242, a toll-like receptor 4 antagonist, protects against aldosterone-induced cardiac and renal injury. PLoS One 10(11):e0142456
Sollinger D, Eissler R, Lorenz S, Strand S, Chmielewski S, Aoqui C, Schmaderer C, Bluyssen H, Zicha J, Witzke O, Scherer E, Lutz J, Heemann U, Baumann M (2014) Damage-associated molecular pattern activated Toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovasc Res 101(3):464–472
Dange RB, Agarwal D, Masson GS, Vila J, Wilson B, Nair A, Francis J (2014) Central blockade of TLR4 improves cardiac function and attenuates myocardial inflammation in angiotensin II-induced hypertension. Cardiovasc Res 103(1):17–27
Singh MV, Cicha MZ, Meyerholz DK, Chapleau MW, Abboud FM (2015) Dual activation of TRIF and MyD88 adaptor proteins by angiotensin II evokes opposing effects on pressure, cardiac hypertrophy, and inflammatory gene expression. Hypertension 66(3):647–656
Singh MV, Cicha MZ, Nunez S, Meyerholz DK, Chapleau MW, Abboud FM (2019) Angiotensin II-induced hypertension and cardiac hypertrophy are differentially mediated by TLR3- and TLR4-dependent pathways. Am J Physiol Heart Circ Physiol 316(5):H1027–H1038
Matsuda S, Umemoto S, Yoshimura K, Itoh S, Murata T, Fukai T, Matsuzaki M (2015) Angiotensin activates MCP-1 and induces cardiac hypertrophy and dysfunction via toll-like receptor 4. J Atheroscler Thromb 22(8):833–844
Han J, Zou C, Mei L, Zhang Y, Qian Y, You S, Pan Y, Xu Z, Bai B, Huang W, Liang G (2017) MD2 mediates angiotensin II-induced cardiac inflammation and remodeling via directly binding to Ang II and activating TLR4/NF-kappaB signaling pathway. Basic Res Cardiol 112(1):9
Yang J, Jiang H, Yang J, Ding JW, Chen LH, Li S, Zhang XD (2009) Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem 330(1–2):39–46
Schneider S, Koch W, Hoppmann P, Ubrich R, Kemmner S, Steinlechner E, Heemann U, Laugwitz KL, Kastrati A, Baumann M (2015) Association of Toll-like receptor 4 polymorphism with age-dependent systolic blood pressure increase in patients with coronary artery disease. Immun Ageing 12:4
Zhang X, Xu A, Lv J, Zhang Q, Ran Y, Wei C, Wu J (2020) Development of small molecule inhibitors targeting NLRP3 inflammasome pathway for inflammatory diseases. Eur J Med Chem 185:111822
Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P (2010) A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55(1):99–108
Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD (2016) Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 23(2):360–368
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17(8):588–606
Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D, Kett MM, Pinar A, Samuel CS, Vinh A, Arumugam TV, Hewitson TD, Kemp-Harper BK, Robertson AA, Cooper MA, Latz E, Mansell A, Sobey CG, Drummond GR (2016) Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 173(4):752–765
Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, Diep H, Kett MM, Samuel CS, Kemp-Harper BK, Robertson AAB, Cooper MA, Peter K, Latz E, Mansell AS, Sobey CG, Drummond GR, Vinh A (2019) Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res 115(4):776–787
Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, Voelkl J, Schatz V, Linker RA, Lang F, Voehringer D, Wright MD, Hubner N, Dechend R, Jantsch J, Titze J, Muller DN (2015) High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest 125(11):4223–4238
Jantsch J, Schatz V, Friedrich D, Schroder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, Gebhardt M, Heinig M, Neubert P, Fischer F, Teufel S, David JP, Neufert C, Cavallaro A, Rakova N, Kuper C, Beck FX, Neuhofer W, Muller DN, Schuler G, Uder M, Bogdan C, Luft FC, Titze J (2015) Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 21(3):493–501
Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Bassler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag S, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E (2018) Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172(1–2):162–175 e114
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395
Nassar T, Akkawi S, Bar-Shavit R, Haj-Yehia A, Bdeir K, Al-Mehdi AB, Tarshis M, Higazi AA (2002) Human alpha-defensin regulates smooth muscle cell contraction: a role for low-density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Blood 100(12):4026–4032
Qvarnstrom M, Janket S, Jones JA, Nuutinen P, Baird AE, Nunn ME, Van Dyke TE, Meurman JH (2008) Salivary lysozyme and prevalent hypertension. J Dent Res 87(5):480–484
Labat C, Temmar M, Nagy E, Bean K, Brink C, Benetos A, Back M (2013) Inflammatory mediators in saliva associated with arterial stiffness and subclinical atherosclerosis. J Hypertens 31(11):2251–2258 discussion 2258
Zipfel PF, Skerka C (2009) Complement regulators and inhibitory proteins. Nat Rev Immunol 9(10):729–740
Koenderman L, Buurman W, Daha MR (2014) The innate immune response. Immunol Lett 162(2 Pt B):95–102
Bao X, Meng G, Zhang Q, Liu L, Wu H, Du H, Shi H, Xia Y, Guo X, Liu X, Han P, Dong R, Wang X, Li C, Su Q, Gu Y, Fang L, Yu F, Yang H, Kang L, Ma Y, Yu B, Ma X, Sun S, Wang X, Zhou M, Jia Q, Guo Q, Song K, Wang G, Huang G, Niu K (2017) Elevated serum complement C3 levels are associated with prehypertension in an adult population. Clin Exp Hypertens 39(1):42–49
Engstrom G, Hedblad B, Berglund G, Janzon L, Lindgarde F (2007) Plasma levels of complement C3 is associated with development of hypertension: a longitudinal cohort study. J Hum Hypertens 21(4):276–282
Magen E, Mishal J, Paskin J, Glick Z, Yosefy C, Kidon M, Schlesinger M (2008) Resistant arterial hypertension is associated with higher blood levels of complement C3 and C-reactive protein. J Clin Hypertens (Greenwich) 10(9):677–683
Zhang C, Li Y, Wang C, Wu Y, Cui W, Miwa T, Sato S, Li H, Song WC, Du J (2014) Complement 5a receptor mediates angiotensin II-induced cardiac inflammation and remodeling. Arterioscler Thromb Vasc Biol 34(6):1240–1248
Zhang C, Li Y, Wang C, Wu Y, Du J (2014) Antagonist of C5aR prevents cardiac remodeling in angiotensin II-induced hypertension. Am J Hypertens 27(6):857–864
Chen XH, Ruan CC, Ge Q, Ma Y, Xu JZ, Zhang ZB, Lin JR, Chen DR, Zhu DL, Gao PJ (2018) Deficiency of complement C3a and C5a receptors prevents angiotensin II-induced hypertension via regulatory T cells. Circ Res 122(7):970–983
Zhou X, Fukuda N, Matsuda H, Endo M, Wang X, Saito K, Ueno T, Matsumoto T, Matsumoto K, Soma M, Kobayashi N, Nishiyama A (2013) Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am J Physiol Renal Physiol 305(7):F957–F967
O'Neill CA, Monteleone G, McLaughlin JT, Paus R (2016) The gut-skin axis in health and disease: a paradigm with therapeutic implications. Bioessays 38(11):1167–1176
Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ (2003) Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 21(6):1125–1135
Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y (2012) Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension 60(3):833–841
Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, Zhang Y, Quan X, Ji L, Zhan S (2015) Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res Clin Pract 110(1):26–37
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI (2007) The human microbiome project. Nature 449(7164):804–810
Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291(5505):881–884
David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ (2014) Host lifestyle affects human microbiota on daily timescales. Genome Biol 15(7):R89
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107(33):14691–14696
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484):559–563
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122):1027–1031
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341(6150):1241214
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M (2015) Gut dysbiosis is linked to hypertension. Hypertension 65(6):1331–1340
Pevsner-Fischer M, Blacher E, Tatirovsky E, Ben-Dov IZ, Elinav E (2017) The gut microbiome and hypertension. Curr Opin Nephrol Hypertens 26(1):1–8
Karbach SH, Schonfelder T, Brandao I, Wilms E, Hormann N, Jackel S, Schuler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schafer K, Munzel T, Reinhardt C, Wenzel P (2016) Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 5(9):e003698
Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ (2017) Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49(2):96–104
Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM (2017) High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135(10):964–977
Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr, Durgan DJ (2018) Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72(5):1141–1150
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mahler A, Balogh A, Marko L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Fratzer C, Krannich A, Gollasch M, Grohme DA, Corte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Muller DN (2017) Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551(7682):585–589
Hofmeister LH, Perisic S, Titze J (2015) Tissue sodium storage: evidence for kidney-like extrarenal countercurrent systems? Pflugers Arch 467(3):551–558
Titze J, Bauer K, Schafflhuber M, Dietsch P, Lang R, Schwind KH, Luft FC, Eckardt KU, Hilgers KF (2005) Internal sodium balance in DOCA-salt rats: a body composition study. Am J Physiol Renal Physiol 289(4):F793–F802
Guyton AC, Coleman TG, Granger HJ (1972) Circulation: overall regulation. Annu Rev Physiol 34:13–46
Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schofl C, Renz W, Santoro D, Niendorf T, Muller DN, Neininger M, Cavallaro A, Eckardt KU, Schmieder RE, Luft FC, Uder M, Titze J (2012) (23)Na magnetic resonance imaging of tissue sodium. Hypertension 59(1):167–172
Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J (2013) 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61(3):635–640
Laffer CL, Scott RC 3rd, Titze JM, Luft FC, Elijovich F (2016) Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension 68(1):195–203
Rakova N, Juttner K, Dahlmann A, Schroder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, Vassilieva G, Lenkova L, Johannes B, Wabel P, Moissl U, Vienken J, Gerzer R, Eckardt KU, Muller DN, Kirsch K, Morukov B, Luft FC, Titze J (2013) Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab 17(1):125–131
Helle F, Karlsen TV, Tenstad O, Titze J, Wiig H (2013) High-salt diet increases hormonal sensitivity in skin pre-capillary resistance vessels. Acta Physiol (Oxf) 207(3):577–581
Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Muller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J (2013) Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123(7):2803–2815
Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B (2006) Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17(12 Suppl 3):S218–S225
Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T (2014) Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens 28(3):165–169
Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK, Spitzer MH, Douchet I, Daburon S, Moreau JF, Nolan GP, Blanco P, Dechanet-Merville J, Dekker CL, Jojic V, Kuo CJ, Davis MM, Faustin B (2017) Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 23(2):174–184
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101(15):1767–1772
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377(12):1119–1131
Rothman AM, MacFadyen J, Thuren T, Webb A, Harrison DG, Guzik TJ, Libby P, Glynn RJ, Ridker PM (2020) Effects of interleukin-1beta inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension 75(2):477–482
Leslie M (2018) Restraining immunity could lower high blood pressure. Science 359(6379):966–967
Acknowledgments
We thank Susanne Karbach, MD, for critical discussion of the subject matter.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Federal Ministry for Education and Research (BMBF 01EO1503) and the Boehringer-Ingelheim Foundation, and received a grant from the German Research Foundation (DFG WE 4361/7-1).
Author information
Authors and Affiliations
Contributions
Conceptualization: Philip Wenzel; writing - original draft preparation: Johannes Wild; writing - review and editing: Philip Wenzel; funding acquisition: Johannes Wild and Philip Wenzel.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wild, J., Wenzel, P. Myeloid cells, tissue homeostasis, and anatomical barriers as innate immune effectors in arterial hypertension. J Mol Med 99, 315–326 (2021). https://doi.org/10.1007/s00109-020-02019-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-02019-1