Abstract
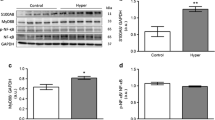
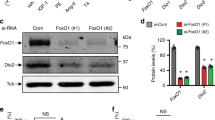
Recent studies have evidenced the involvement of inflammation-related pathways to the development of cardiac hypertrophy and other consequences on the cardiovascular system, including the calcium-binding protein S100A8. However, this has never been investigated in the thyroid hormone (TH)-prompted cardiac hypertrophy. Thus, we aimed to test whether S100A8 and related signaling molecules, myeloid differentiation factor-88 (MyD88) and nuclear factor kappa B (NF-қB), could be associated with the cardiomyocyte hypertrophy induced by TH. Our results demonstrate that the S100A8/MyD88/NF-қB signaling pathway is activated in cardiomyocytes following TH stimulation. The knockdown of S100A8 and MyD88 indicates the contribution of those molecules to cardiomyocyte hypertrophy in response to TH, as evaluated by cell surface area, leucine incorporation assay, and gene expression. Furthermore, S100A8 and MyD88 are crucial mediators of NF-қB activation, which is also involved in the hypertrophic growth of TH-treated cardiomyocytes. Supporting the in vitro data, the contribution of NF-қB for TH-induced cardiac hypertrophy is confirmed in vivo, by using transgenic mice with cardiomyocyte-specific suppression of NF-қB. These data identify a novel pathway regulated by TH that mediates cardiomyocyte hypertrophy. However, the potential role of this new pathway in short and long-term cardiac effects of TH remains to be further investigated.
Key messages
-
Inflammation-related signaling is activated by T3 in cardiomyocytes.
-
S100A8 and MyD88 have a crucial role in cardiomyocyte hypertrophy by T3.
-
S100A8 and MyD88 mediate NF-қB activation by T3.
-
NF-қB contributes to T3-induced cardiac hypertrophy in vitro and in vivo







Similar content being viewed by others
References
Dillmann W (2010) Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev 15:125–132
Kenessey A, Ojamaa K (2006) Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem 281:20666–20672
Dahl P, Danzi S, Klein I (2008) Thyrotoxic cardiac disease. Curr Heart Fail Rep 5:170–176
Elnakish MT, Schultz EJ, Gearinger RL, Saad NS, Rastogi N, Ahmed AA, Mohler PJ, Janssen PM (2015) Differential involvement of various sources of reactive oxygen species in thyroxin-induced hemodynamic changes and contractile dysfunction of the heart and diaphragm muscles. Free Radic Biol Med 83:252–261
Weltman NY, Wang D, Redetzke RA, Gerdes AM (2012) Longstanding hyperthyroidism is associated with normal or enhanced intrinsic cardiomyocyte function despite decline in global cardiac function. PLoS One 7:e46655
Diniz GP, Carneiro-Ramos MS, Barreto-Chaves ML (2009) Angiotensin type 1 receptor mediates thyroid hormone-induced cardiomyocyte hypertrophy through the Akt/GSK-3β/mTOR signaling pathway. Basic Res Cardiol 104:653–667
Iordanidou A, Hadzopoulou-Cladaras M, Lazou A (2010) Non-genomic effects of thyroid hormone in adult cardiac myocytes: relevance to gene expression and cell growth. Mol Cell Biochem 340:291–300
Takano AP, Diniz GP, Barreto-Chaves ML (2013) AMPK signaling pathway is rapidly activated by T3 and regulates the cardiomyocyte growth. Mol Cell Endocrinol 376:43–50
Nemska S, Monassier L, Gassmann M, Frossard N, Tavakoli R (2016) Kinetic mRNA profiling in a rat model of left-ventricular hypertrophy reveals early expression of chemokines and their receptors. PLoS One 11:e0161273
Mann DL (2015) Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 116:1254–1268
Imbalzano E, Mandraffino G, Casciaro M, Quartuccio S, Saitta A, Gangemi S (2016) Pathophysiological mechanism and therapeutic role of S100 proteins in cardiac failure: a systematic review. Heart Fail Rev 21:463–473
Foell D, Frosch M, Sorg C, Roth J (2004) Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta 344:37–51
Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, auto-immunity, and cancer. J Leukoc Biol 86:557–566
Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, Van Zoelen MA, Nacken W, Foell D, Van Der Poll T, Sorg C et al (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13:1042–1049
Schiopu A (2013) Cotoi OS (2013) S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediat Inflamm 2013:828354
Boyd JH, Kan B, Roberts H, Wang Y, Walley KR (2008) S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res 102:1239–1246
Wu Y, Li Y, Zhang C, A X, Wang Y, Cui W, Li H, Du J (2014) S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II-induced cardiac inflammation and injury. Hypertension 63:1241–1250
Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A (2001) Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci U S A 98:6668–6673
Gupta S, Purcell NH, Lin A, Sen S (2002) Activation of nuclear factor-kappaB is necessary for myotrophin-induced cardiac hypertrophy. J Cell Biol 159:1019–1028
Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL et al (2005) Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med 39:1570–1580
Ha T, Hua F, Li Y, Ma J, Gao X, Kelley J, Zhao A, Haddad GE, Williams DL, Browder IW et al (2006) Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 290:H985–H994
Li T, Wang Y, Liu C, Hu Y, Wu M, Li J, Guo L, Chen L, Chen Q, Ha T et al (2009) MyD88-dependent nuclear factor-kappaB activation is involved in fibrinogen-induced hypertrophic response of cardiomyocytes. J Hypertens 27:1084–1093
Gao W, Wang H, Zhang L, Cao Y, Bao JZ, Liu ZX, Wang LS, Yang Q, Lu X (2016) Retinol-binding protein 4 induces cardiomyocyte hypertrophy by activating TLR4/MyD88 pathway. Endocrinology 157:2282–2293
Barreto-Chaves ML, Heimann A, Krieger JE (2000) Stimulatory effect of dexamethasone on angiotensin-converting enzyme in neonatal rat cardiac myocytes. Braz J Med Biol Res 33:661–664
Watkins SJ, Borthwick GM, Arthur HM (2011) The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim 47:125–131
Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, Hahn H, Feldman AM, Jones WK (2005) Cardiac-specific blockade of NF-қB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am J Physiol Heart Circ Physiol 289:H466–H476
Averill MM, Kerkhoff C, Bornfeldt KE (2012) S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol 32:223–229
Liu Q, Chen Y, Auger-Messier M, Molkentin JD (2012) Interaction between NFκB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res 110:1077–1086
Tsoporis JN, Marks A, Haddad A, O’Hanlon D, Jolly S, Parker TG (2005) S100A6 is a negative regulator of the induction of cardiac genes by trophic stimuli in cultured rat myocytes. Exp Cell Res 303:471–481
Schneider M, Kostin S, Strøm CC, Aplin M, Lyngbaek S, Theilade J, Grigorian M, Andersen CB, Lukanidin E, Lerche Hansen J et al (2007) S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res 75:40–50
Yan L, Mathew L, Chellan B, Gardner B, Earley J, Puri TS, Hofmann Bowman MA (2014) S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner. Arterioscler Thromb Vasc Biol 34:1399–1411
Wei X, Wu B, Zhao J, Zeng Z, Xuan W, Cao S, Huang X, Asakura M, Xu D, Bin J et al (2015) Myocardial hypertrophic preconditioning attenuates cardiomyocyte hypertrophy and slows progression to heart failure through upregulation of S100A8/A9. Circulation 131:1506–1517
Rahimi F, Hsu K, Endoh Y, Geczy CL (2005) FGF-2, IL-1beta and TGF-beta regulate fibroblast expression of S100A8. FEBS J 272:2811–2827
McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL (2005) S100A8 and S100A9 in human arterial wall: implications for atherogenesis. J Biol Chem 280:41521–41529
Gordon JW, Shaw JA, Kirshenbaum LA (2011) Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res 108:1122–1132
Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME (2012) MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol 52:1135–1144
O’Neill LA (2006) How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol 18:3–9
Singh MV, Abboud FM (2014) Toll-like receptors and hypertension. Am J Physiol Regul Integr Comp Physiol 307:R501–R504
Hua F, Ha T, Ma J, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C (2005) Blocking the MyD88-dependent pathway protects the myocardium from ischemia/reperfusion injury in rat hearts. Biochem Biophys Res Commun 338:1118–1125
Alverdi V, Hetrick B, Joseph S, Komives EA (2014) Direct observation of a transient ternary complex during IκBα-mediated dissociation of NF-κB from DNA. Proc Natl Acad Sci U S A 111:225–230
Jones WK, Brown M, Ren X, He S, McGuinness M (2003) NF-kappaB as an integrator of diverse signaling pathways: the heart of myocardial signaling? Cardiovasc Toxicol 3:229–254
Reale C, Iervolino A, Scudiero I, Ferravante A, D'Andrea LE, Mazzone P, Zotti T, Leonardi A, Roberto L, Zannini M et al (2016) NF-κB essential modulator (NEMO) is critical for thyroid function. J Biol Chem 291:5765–5773
Shin HM, Kim MH, Kim BH, Jung SH, Kim YS, Park HJ, Hong JT, Min KR, Kim Y (2004) Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-kappaB without affecting IkappaB degradation. FEBS Lett 571:50–54
Hu LW, Benvenuti LA, Liberti EA, Carneiro-Ramos MS, Barreto-Chaves ML (2003) Thyroxine-induced cardiac hypertrophy: influence of adrenergic nervous system versus renin-angiotensin system on myocyte remodeling. Am J Physiol Regul Integr Comp Physiol 285:R1473–R1480
Fujiu K, Nagai R (2014) Fibroblast-mediated pathways in cardiac hypertrophy. J Mol Cell Cardiol 70:64–73
Gupta S, Young D, Sen S (2005) Inhibition of NF-kappaB induces regression of cardiac hypertrophy, independent of blood pressure control, in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 289:H20–H29
Javan H, Szucsik AM, Li L, Schaaf CL, Salama ME, Selzman CH (2015) Cardiomyocyte p65 nuclear factor-κB is necessary for compensatory adaptation to pressure overload. Circ Heart Fail 8:109–118
Acknowledgements
The authors acknowledge that the 3M mice were generously provided by Dr. W. Keith Jones at Loyola University, Chicago. The authors also thank Marina R. Fevereiro for valuable technical assistance. This work was supported by Sao Paulo Research Foundation (FAPESP), grants #2011/23352-4, #2013/22480-4, and #2015/01166-5, and in part by the start-up funds from Texas A&M Health Science Center, College of Medicine and American Heart Association Grant-in-Aid (0835227 N) to S.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The experiments were performed in accordance with NIH guidelines and were approved by the Biomedical Sciences Institute/USP Ethics Committee for Animal Research and the Institutional Animal Care and Use Committee at the Texas A&M Health Science Center and Scott & White Hospital.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Takano, A.P.C., Munhoz, C.D., Moriscot, A.S. et al. S100A8/MYD88/NF-қB: a novel pathway involved in cardiomyocyte hypertrophy driven by thyroid hormone. J Mol Med 95, 671–682 (2017). https://doi.org/10.1007/s00109-017-1511-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1511-y