Abstract
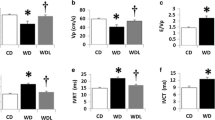
Plasma homocysteine levels predict heart failure incidence in prospective epidemiological studies. We evaluated whether selective homocysteine-lowering gene transfer beneficially affects cardiac remodeling and function in a model of pressure overload-induced cardiomyopathy induced by transverse aortic constriction (TAC). Female C57BL/6 low-density lipoprotein receptor (Ldlr −/−) cystathionine-β-synthase (Cbs +/−) mice were fed standard chow (control mice) or a folate-depleted, methionine-enriched diet to induce hyperhomocysteinemia (diet mice). Three weeks after initiation of thisdiet, mice were intravenously injected with 5 × 1010 viral particles of an E1E3E4-deleted hepatocyte-specific adenoviral vector expressing Cbs (AdCBS), with the same dose of control vector, or with saline buffer. TAC or sham operation was performed 2 weeks later. AdCBS gene transfer resulted in 86.4 % (p < 0.001) and 84.6 % (p < 0.001) lower homocysteine levels in diet sham mice and diet TAC mice, respectively. Mortality rate was significantly reduced in diet AdCBS TAC mice compared to diet TAC mice during a follow-up period of 8 weeks (hazard ratio for mortality 0.495, 95 % CI 0.249 to 0.985). Left ventricular hypertrophy (p < 0.01) and interstitial myocardial fibrosis (p < 0.001) were strikingly lower in control TAC mice and diet AdCBS TAC mice compared to diet TAC mice. Diastolic function in diet AdCBS TAC mice was similar to that of control TAC mice and was significantly improved compared to diet TACmice. AdCBS gene transfer potently reduced oxidative stress as evidenced by a reduction of plasma TBARS and a reduction of myocardial 3-nitrotyrosine-positive area (%). In conclusion, selective homocysteine lowering potently attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress.
Key message
-
Plasma homocysteine levels predict heart failure incidence in epidemiological studies.
-
Transverse aortic constriction (TAC) induces pressure overload.
-
Selective homocysteine-lowering gene therapy reduces mortality after TAC.
-
Selective homocysteine lowering attenuates cardiac hypertrophy and fibrosis after TAC.
-
Decreased homocysteine levels enhance diastolic function and lower oxidative stress.




Similar content being viewed by others
References
Sundstrom J, Sullivan L, Selhub J, Benjamin EJ, D’Agostino RB, Jacques PF, Rosenberg IH, Levy D, Wilson PW, Vasan RS (2004) Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J 25(6):523–530
Nasir K, Tsai M, Rosen BD, Fernandes V, Bluemke DA, Folsom AR, Lima JA (2007) Elevated homocysteine is associated with reduced regional left ventricular function: the Multi-Ethnic Study of Atherosclerosis. Circulation 115(2):180–187
Ruhui L, Jinfa J, Jiahong X, Wenlin M (2014) Influence of hyperhomocysteinemia on left ventricular diastolic function in Chinese patients with hypertension. Herz. doi:10.1007/s00059-014-4098-x
Washio T, Nomoto K, Watanabe I, Tani S, Nagao K, Hirayama A (2011) Relationship between plasma homocysteine levels and congestive heart failure in patients with acute myocardial infarction. Homocysteine and congestive heart failure. Int Heart J 52(4):224–228
Vasan RS, Beiser A, D’Agostino RB, Levy D, Selhub J, Jacques PF, Rosenberg IH, Wilson PW (2003) Plasma homocysteine and risk for congestive heart failure in adults without prior myocardial infarction. JAMA 289(10):1251–1257
May HT, Alharethi R, Anderson JL, Muhlestein JB, Reyna SP, Bair TL, Horne BD, Kfoury AG, Carlquist JF, Renlund DG (2007) Homocysteine levels are associated with increased risk of congestive heart failure in patients with and without coronary artery disease. Cardiology 107(3):178–184
Wang X, Cui L, Joseph J, Jiang B, Pimental D, Handy DE, Liao R, Loscalzo J (2012) Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38 MAPK-mediated increase in oxidant stress. J Mol Cell Cardiol 52(3):753–760
Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, Patibandla PK, Tyagi N, Rai J, Metreveli N et al (2008) Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol 295(2):H890–H897
Raaf L, Noll C, Cherifi Mel H, Samuel JL, Delcayre C, Delabar JM, Benazzoug Y, Janel N (2011) Myocardial fibrosis and TGFB expression in hyperhomocysteinemic rats. Mol Cell Biochem 347(1–2):63–70
Kundu S, Kumar M, Sen U, Mishra PK, Tyagi N, Metreveli N, Lominadze D, Rodriguez W, Tyagi SC (2009) Nitrotyrosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/CBS double knockout mice. J Cell Biochem 106(1):119–126
Muthuramu I, Jacobs F, Singh N, Gordts SC, De Geest B (2013) Selective homocysteine lowering gene transfer improves infarct healing, attenuates remodelling, and enhances diastolic function after myocardial infarction in mice. PLoS One 8(5), e63710. doi:10.1371/journal.pone.0063710
Buys ES, Raher MJ, Blake SL, Neilan TG, Graveline AR, Passeri JJ, Llano M, Perez-Sanz TM, Ichinose F, Janssens S et al (2007) Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol 293(1):H620–H627
Jacobs F, Van Craeyveld E, Muthuramu I, Gordts SC, Emmerechts J, Hoylaerts M, Herijgers P, De Geest B (2011) Correction of endothelial dysfunction after selective homocysteine lowering gene therapy reduces arterial thrombogenicity but has no effect on atherogenesis. J Mol Med 89(10):1051–1058
Jacobs F, Snoeys J, Feng Y, Van Craeyveld E, Lievens J, Armentano D, Cheng SH, De Geest B (2008) Direct comparison of hepatocyte-specific expression cassettes following adenoviral and nonviral hydrodynamic gene transfer. Gene Ther 15(8):594–603
Van Linthout S, Lusky M, Collen D, De Geest B (2002) Persistent hepatic expression of human apo A-I after transfer with a helper-virus independent adenoviral vector. Gene Ther 9(22):1520–1528
Gordts SC, Van Craeyveld E, Muthuramu I, Singh N, Jacobs F, De Geest B (2012) Lipid lowering and HDL raising gene transfer increase endothelial progenitor cells, enhance myocardial vascularity, and improve diastolic function. PLoS One 7(10), e46849. doi:10.1371/journal.pone.0046849
Van Craeyveld E, Jacobs F, Gordts SC, De Geest B (2012) Low-density lipoprotein receptor gene transfer in hypercholesterolemic mice improves cardiac function after myocardial infarction. Gene Ther 19(8):860–871
Van Craeyveld E, Gordts SC, Nefyodova E, Jacobs F, De Geest B (2011) Regression and stabilization of advanced murine atherosclerotic lesions: a comparison of LDL lowering and HDL raising gene transfer strategies. J Mol Med 89(6):555–567
Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE (2003) Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol 285(3):H1261–H1269
Yasuno S, Kuwahara K, Kinoshita H, Yamada C, Nakagawa Y, Usami S, Kuwabara Y, Ueshima K, Harada M, Nishikimi T et al (2013) Angiotensin II type 1a receptor signalling directly contributes to the increased arrhythmogenicity in cardiac hypertrophy. Br J Pharmacol 170(7):1384–1395
Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56(1):56–64
Cohn JN (1993) Post-MI remodeling. Clin Cardiol 16(5 Suppl 2):II21–II24
Barrick CJ, Dong A, Waikel R, Corn D, Yang F, Threadgill DW, Smyth SS (2009) Parent-of-origin effects on cardiac response to pressure overload in mice. Am J Physiol Heart Circ Physiol 297(3):H1003–H1009
Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7(8):589–600
Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA (2002) Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 105(1):85–92
Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF et al (2000) The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19(23):6341–6350
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH (1991) Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114(5):345–352
Kupari M, Turto H, Lommi J (2005) Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J 26(17):1790–1796
Herrmann W, Herrmann M, Joseph J, Tyagi SC (2007) Homocysteine, brain natriuretic peptide and chronic heart failure: a critical review. Clin Chem Lab Med 45(12):1633–1644
Gueant Rodriguez RM, Spada R, Pooya S, Jeannesson E, Moreno Garcia MA, Anello G, Bosco P, Elia M, Romano A, Alberto JM et al (2013) Homocysteine predicts increased NT-pro-BNP through impaired fatty acid oxidation. Int J Cardiol 167(3):768–775
Sirker A, Zhang M, Murdoch C, Shah AM (2007) Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am J Nephrol 27(6):649–660
McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ (1993) Evidence of oxidative stress in chronic heart failure in humans. Eur Heart J 14(11):1493–1498
Sorescu D, Griendling KK (2002) Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 8(3):132–140
Handy DE, Zhang Y, Loscalzo J (2005) Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem 280(16):15518–15525
Suematsu N, Ojaimi C, Kinugawa S, Wang Z, Xu X, Koller A, Recchia FA, Hintze TH (2007) Hyperhomocysteinemia alters cardiac substrate metabolism by impairing nitric oxide bioavailability through oxidative stress. Circulation 115(2):255–262
Kolling J, Scherer EB, da Cunha AA, da Cunha MJ, Wyse AT (2011) Homocysteine induces oxidative-nitrative stress in heart of rats: prevention by folic acid. Cardiovasc Toxicol 11(1):67–73
Dayal S, Arning E, Bottiglieri T, Boger RH, Sigmund CD, Faraci FM, Lentz SR (2004) Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke; J Cereb Circ 35(8):1957–1962
Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI et al (2000) Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest 106(4):483–491
Mishra PK, Tyagi N, Kundu S, Tyagi SC (2009) MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochem Biophys 55(3):153–162
Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A (2003) Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol 23(3):418–424
Xie LH, Chen F, Karagueuzian HS, Weiss JN (2009) Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104(1):79–86
Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N et al (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133(3):462–474
Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D (2005) NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97(9):900–907
Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y (2008) Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem 317(1-2):43–50
Zhao QD, Viswanadhapalli S, Williams P, Shi Q, Tan C, Yi X, Bhandari B, Abboud HE (2015) NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation 131(7):643–655
Dayal S, Rodionov RN, Arning E, Bottiglieri T, Kimoto M, Murry DJ, Cooke JP, Faraci FM, Lentz SR (2008) Tissue-specific downregulation of dimethylarginine dimethylaminohydrolase in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol 295(2):H816–H825
Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP (1999) Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 99(24):3092–3095
Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP (2002) Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106(8):987–992
Stuhlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP (2001) Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104(21):2569–2575
Acknowledgments
This work was supported by Onderzoekstoelagen grant OT/13/090 of the KU Leuven and by grant G0A3114N of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Conflict of interest
None of the authors has a conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Institutional Animal Care and Research Advisory Committee of the Catholic University of Leuven; approval number P154/2013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 25051 kb)
Rights and permissions
About this article
Cite this article
Muthuramu, I., Singh, N., Amin, R. et al. Selective homocysteine-lowering gene transfer attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress. J Mol Med 93, 609–618 (2015). https://doi.org/10.1007/s00109-015-1281-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1281-3