Abstract
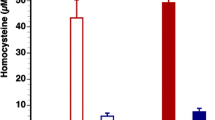
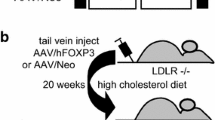
Hyperhomocysteinemia is an independent risk factor for ischemic cardiovascular diseases, but its causal role in atherothrombosis remains controversial. Proatherogenic and/or prothrombotic effects may underlie the potential causal relation between hyperhomocysteinemia and cardiovascular events. Here, the effects of selective lowering of plasma homocysteine, plasma cholesterol, or both on endothelial function and on atherogenesis in male hyperlipidemic and hyperhomocysteinemic C57BL/6 low-density lipoprotein receptor (LDLr)−/−/cystathionine-β-synthase (CBS)+/−-deficient mice were investigated. Second, we evaluated whether selective homocysteine lowering has anti-thrombotic effects in a model of arterial thrombosis. A hyperhomocysteinemic and atherogenic diet was started at the age of 12 weeks. Three weeks later, gene transfer was performed with E1E3E4-deleted adenoviral vectors for hepatocyte-restricted overexpression of CBS (AdCBS) or of the LDLr (AdLDLr), or with the control vector Adnull. In a fourth group, AdCBS and AdLDLr were co-administered. Selective homocysteine lowering but not selective cholesterol lowering restored endothelial function at 6 weeks after gene transfer. Intimal area in the aortic root and in the brachiocephalic artery at 13 weeks was more than 100-fold (p < 0.001) smaller in AdLDLr and AdCBS/AdLDLr mice than in control mice and AdCBS mice. No differences in intimal area were observed between control mice and AdCBS mice. In a model of carotid artery thrombosis, the average time to first occlusion and to stable occlusion were 1.9-fold (p < 0.01) and 2.1-fold longer (p < 0.01), respectively, in AdCBS-treated mice than in control mice. Taken together, these data show that correction of endothelial dysfunction following selective homocysteine lowering has anti-thrombotic but no anti-atherogenic effects.




Similar content being viewed by others
References
Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI et al (2000) Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest 106:483–491
Dayal S, Lentz SR (2008) Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol 28:1596–1605
Undas A, Brozek J, Szczeklik A (2005) Homocysteine and thrombosis: from basic science to clinical evidence. Thromb Haemost 94:907–915
Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR (2001) Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res 88:1203–1209
Hajjar KA (1993) Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest 91:2873–2879
Jacovina AT, Deora AB, Ling Q, Broekman MJ, Almeida D, Greenberg CB, Marcus AJ, Smith JD, Hajjar KA (2009) Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J Clin Invest 119:3384–3394
Homocysteine Studies Collaboration (2002) Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. Jama 288:2015–2022
Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ (Clinical research ed) 325:1202
Marti-Carvajal AJ, Sola I, Lathyris D, Salanti G (2009) Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev: CD006612
Potter K, Hankey GJ, Green DJ, Eikelboom JW, Arnolda LF (2008) Homocysteine or renal impairment: which is the real cardiovascular risk factor? Arterioscler Thromb Vasc Biol 28:1158–1164
Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K (2006) Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 354:1578–1588
Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE (1997) Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 337:230–236
Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R (1985) The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet 37:1–31
Schimke RN, McKusick VA, Huang T, Pollack AD (1965) Homocystinuria. Studies of 20 families with 38 affected members. Jama 193:711–719
McCully KS (1969) Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 56:111–128
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Van Linthout S, Lusky M, Collen D, De Geest B (2002) Persistent hepatic expression of human apo A-I after transfer with a helper-virus independent adenoviral vector. Gene Ther 9:1520–1528
Van Craeyveld E, Gordts SC, Nefyodova E, Jacobs F, De Geest B (2011) Regression and stabilization of advanced murine atherosclerotic lesions: a comparison of LDL lowering and HDL raising gene transfer strategies. J Mol Med 89:555–567
Shipchandler MT, Moore EG (1995) Rapid, fully automated measurement of plasma homocyst(e)ine with the Abbott IMx analyzer. Clin Chem 41:991–994
Peters LL, Cheever EM, Ellis HR, Magnani PA, Svenson KL, Von Smith R, Bogue MA (2002) Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice. Physiol Genomics 11:185–193
Feng Y, Lievens J, Jacobs F, Hoekstra M, Van Craeyveld E, Gordts SC, Snoeys J, De Geest B (2010) Hepatocyte-specific ABCA1 transfer increases HDL cholesterol but impairs HDL function and accelerates atherosclerosis. Cardiovasc Res 88:376–385
Wilson KM, McCaw RB, Leo L, Arning E, Lhotak S, Bottiglieri T, Austin RC, Lentz SR (2007) Prothrombotic effects of hyperhomocysteinemia and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27:233–240
Wang H, Jiang X, Yang F, Gaubatz JW, Ma L, Magera MJ, Yang X, Berger PB, Durante W, Pownall HJ et al (2003) Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood 101:3901–3907
Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X et al (2009) Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation 120:1893–1902
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Fransen P, Van Assche T, Guns PJ, Van Hove CE, De Keulenaer GW, Herman AG, Bult H (2008) Endothelial function in aorta segments of apolipoprotein E-deficient mice before development of atherosclerotic lesions. Pflugers Arch 455:811–818
Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI et al (2000) Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Investig 106:483–491
Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR (2006) Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood 108:2237–2243
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15:551–561
Tabas I, Williams KJ, Boren J (2007) Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116:1832–1844
Steinbrecher UP, Lougheed M (1992) Scavenger receptor-independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arterioscler Thromb 12:608–625
Glaser CB, Morser J, Clarke JH, Blasko E, McLean K, Kuhn I, Chang RJ, Lin JH, Vilander L, Andrews WH (1992) Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J Clin Investig 90:2565–2573
Loscalzo J (2009) Homocysteine-mediated thrombosis and angiostasis in vascular pathobiology. J Clin Invest 119:3203–3205
Sauls DL, Lockhart E, Warren ME, Lenkowski A, Wilhelm SE, Hoffman M (2006) Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry 45:2480–2487
Colucci M, Cattaneo M, Martinelli I, Semeraro F, Binetti BM, Semeraro N (2008) Mild hyperhomocysteinemia is associated with increased TAFI levels and reduced plasma fibrinolytic potential. J Thromb Haemost 6:1571–1577
Acknowledgments
This work was supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (grant G.0533.08). The Center for Molecular and Vascular Biology is supported by the Excellentiefinanciering KU Leuven (EF/05/013). Frank Jacobs is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. Eline Van Craeyveld is a Research Assistant of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. Stephanie C. Gordts is a Research Assistant of the Agentschap voor Innovatie door Wetenschap en Technologie.
Disclosures
None. There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM. 1
(PDF 439 kb)
Rights and permissions
About this article
Cite this article
Jacobs, F., Van Craeyveld, E., Muthuramu, I. et al. Correction of endothelial dysfunction after selective homocysteine lowering gene therapy reduces arterial thrombogenicity but has no effect on atherogenesis. J Mol Med 89, 1051–1058 (2011). https://doi.org/10.1007/s00109-011-0778-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0778-7