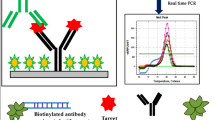
Abstract
Bacterial intoxications represent a substantial public health concern with enterotoxins produced by Staphylococcus aureus among the most common causes of food poisoning. In addition to their role in the pathogenicity of food poisoning, staphylococcal enterotoxins have profound effects on the immune system as members of the family of pyrogenic toxin superantigens. As the classical diagnostic bioassays as well as the routinely used immunological methods are hampered by several drawbacks regarding sensitivity, specificity, and practicability, there is a need for the timely identification of toxins by highly sensitive and specific methods. To combine the versatility of an enzyme immunoassay (EIA) with the amplification power of the PCR, a quantitative real-time immuno-PCR (qRT-iPCR) was developed for the detection of staphylococcal enterotoxins A and B and compared to a commercially available EIA. A broadly applicable tool for signal amplification of pre-formed immunocomplexes was established by covalent binding of a reporter DNA to secondary detection antibodies. Therefore, the amino-modified reporter DNA was coupled successfully to N-succinimidyl-S-actyl-thioacetate-activated secondary detection antibodies. The qRT-iPCR was able to detect highly reproducibly as low as approximately 0.6 to 6 pg (4 to 40 amol/μl) of staphylococcal enterotoxin B and staphylococcal enterotoxin A, respectively. In conclusion, the qRT-iPCR approach was shown to overcome clearly the sensitivity limit of traditional immunological detection procedures for bacterial toxins, as demonstrated in this study for staphylococcal enterotoxins. The development of a stable antibody–DNA conjugate providing a universal signal amplification offers a versatile as well as a highly sensitive and specific tool for diagnostic and research purposes generally applicable for pre-formed antibody–antigen complexes.




Similar content being viewed by others
References
Todd EC (1989) Costs of acute bacterial foodborne disease in Canada and the United States. Int J Food Microbiol 9:313–326
Henghold WB (2004) Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol Clin 22:257–262
Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34
Llewelyn M, Cohen J (2002) Superantigens: microbial agents that corrupt immunity. Lancet Infect Dis 2:156–162
Wang Q, Yu H, Zhang L, Ju D, Pan J, Xia D, Yao H, Zhang W, Wang J, Cao X (2002) Adenovirus-mediated intratumoral lymphotactin gene transfer potentiates the antibody-targeted superantigen therapy of cancer. J Mol Med 80:585–594
Su Y-C, Wong ACL (1997) Current perspectives on detection of staphylococcal enterotoxins. J Food Prot 60:195–202
Vernozy-Rozand C, Mazuy-Cruchaudet C, Bavai C, Richard Y (2004) Comparison of three immunological methods for detecting staphylococcal enterotoxins from food. Lett Appl Microbiol 39:490–494
Wieneke AA (1991) Comparison of four kits for the detection of staphylococcal enterotoxin in foods from outbreaks of food poisoning. Int J Food Microbiol 14:305–312
Becker K, Roth R, Peters G (1998) Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol 36:2548–2553
Becker K, Friedrich AW, Peters G, von Eiff C (2004) Systematic survey on the prevalence of genes coding for staphylococcal enterotoxins SElM, SElO, and SElN. Mol Nutr Food Res 48:488–495
Omoe K, Ishikawa M, Shimoda Y, Hu DL, Ueda S, Shinagawa K (2002) Detection of seg, seh, and sei genes in Staphylococcus aureus isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, seh, or sei genes. J Clin Microbiol 40:857–862
Blaiotta G, Ercolini D, Pennacchia C, Fusco V, Casaburi A, Pepe O, Villani F (2004) PCR detection of staphylococcal enterotoxin genes in Staphylococcus spp. strains isolated from meat and dairy products. Evidence for new variants of seG and seI in S. aureus AB-8802. J Appl Microbiol 97:719–730
Monday SR, Bohach GA (1999) Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J Clin Microbiol 37:3411–3414
Wagener C (1997) Molecular diagnostics. J Mol Med 75:728–744
Sano T, Smith CL, Cantor CR (1992) Immuno-PCR: very sensitive antigen detection by means of specific antibody-DNA conjugates. Science 258:120–122
Joerger RD, Truby TM, Hendrickson ER, Young RM, Ebersole RC (1995) Analyte detection with DNA-labeled antibodies and polymerase chain reaction. Clin Chem 41:1371–1377
Niemeyer CM, Adler M, Wacker R (2005) Immuno-PCR: high sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol 23:208–216
Becker K, Friedrich AW, Lubritz G, Weilert M, Peters G, von Eiff C (2003) Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J Clin Microbiol 41:1434–1439
Hu DL, Omoe K, Saleh MH, Ono K, Sugii S, Nakane A, Shinagawa K (2001) Analysis of the epitopes on staphylococcal enterotoxin A responsible for emetic activity. J Vet Med Sci 63:237–241
Niemeyer CM, Wacker R, Adler M (2003) Combination of DNA-directed immobilization and immuno-PCR: very sensitive antigen detection by means of self-assembled DNA-protein conjugates. Nucleic Acids Res 31:e90
Bergdoll MS (1991) Staphylococcus aureus. J Assoc Off Anal Chem 74:706–710
Bergdoll MS (1990) Staphylococcal food poisoning. In: Cliver DO (ed) Foodborne diseases. Academic, San Diego, pp 85–106
Bergdoll MS (1995) Importance of staphylococci that produce nanogram quantities of enterotoxin. Zentralbl Bakteriol 282:1–6
Dolman CE, Wilson RJ, Cockroft WH (1936) A new method of detecting staphylococcal enterotoxin. Can J Public Health 27:489–493
Surgalla MJ, Bergdoll MS, Dack GM (1953) Some observations on the assay of staphylococcal enterotoxin by the monkey-feeding test. J Lab Clin Med 41:782–788
Becker K (2005) Staphylococcus aureus-toxin detection. In: Fuchs J, Podda M (eds) Encyclopedia of diagnostic genomics and proteomics. Marcel Dekker, New York, pp 1230–1235
Poli MA, Rivera VR, Neal D (2002) Sensitive and specific colorimetric ELISAs for Staphylococcus aureus enterotoxins A and B in urine and buffer. Toxicon 40:1723–1726
Peruski AH, Johnson LH III, Peruski LF Jr. (2002) Rapid and sensitive detection of biological warfare agents using time-resolved fluorescence assays. J Immunol Methods 263:35–41
Ruan C, Zeng K, Varghese OK, Grimes CA (2004) A staphylococcal enterotoxin B magnetoelastic immunosensor. Biosens Bioelectron 20:585–591
Kricka LJ (2000) Interferences in immunoassay—still a threat. Clin Chem 46:1037–1038
Klee GG (2004) Interferences in hormone immunoassays. Clin Lab Med 24:1–18
Acknowledgment
We would like to thank Martina Schulte for excellent experimental assistance. The study was supported in part by research grants from the German Federal Ministry for Education and Science (BMBF) to Karsten Becker, Christof von Eiff, and Georg Peters in the context of the Pathogenomic Network (BMBF 0313134), from the Deutsche Forschungsgemeinschaft (EI 247/7-1) to Christof von Eiff, and to Karsten Becker, Thorsten Kuczius, and Georg Peters in the context of the project BMBF 0312733 and of the project ZM760374 from the Ministry for Education, Science and Research of Nordrhine–Westfalia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, A., von Eiff, C., Kuczius, T. et al. A quantitative real-time immuno-PCR approach for detection of staphylococcal enterotoxins. J Mol Med 85, 461–469 (2007). https://doi.org/10.1007/s00109-006-0142-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-006-0142-5