Abstract
The aim of this study was to compare the antioxidant capacity of hydrophilic extracts of different tissues of wet-hearted silver fir (Abies alba Mill.) logs. Wet heart is a wood defect of silver fir and lowers the value of wood. The exploitation of extractives could increase utilization and financial returns. The bark, sapwood, heartwood and knotwood were investigated by using two green extraction methods: ultrasound assisted extraction (US) and accelerated solvent extraction (ASE), with aqueous ethanol as solvent. Total extractive content (TEC), total polyphenol content (TPC) and antioxidant capacities (FRAP, DPPH, ABTS) from the extracts were determined and compared. The highest contents of total hydrophilic extractives were measured in knotwood (23.07%, ASE) and bark (10.31%, ASE), and the lowest values were determined for sapwood (2.00%, ASE) and heartwood (3.56%, ASE). The ASE method resulted in significantly higher TPC (0.65–10.58%) than the US (0.46–9.19%) method. Nevertheless, the simplicity of instrumentation and costs can make US also a potential candidate for future extraction and utilization. The highest antioxidant capacities were measured in knotwood (FRAP: 159.75 mg AAE/g, ABTS: 316.15 mg TE/g, DPPH: 189.23 mg TE/g) and bark (FRAP: 159.75 mg AAE/g, ABTS: 126.81 mg TE/g, DPPH: 74.52 mg TE/ g) extracts, prepared with ASE, which complements well the existing literature data on silver fir extractives. The knotwood and bark of wet-hearted silver fir is an abundant source of antioxidant polyphenols, whereas sapwood and heartwood are poor in these extractives and potentially unsuitable for the valorization by the extraction of natural antioxidants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The European Green Deal strategy (https://commission.europa.eu) encompasses the concepts of green transition and a circular economy, which give high priority to recycling and the sustainable use of raw materials, especially those of natural origin. In this context, the development of new side-streams and their treatment as new and important value chains is a challenge for all industries. It is well known that the side-streams of the forestry and wood processing industries hold great untapped potential (Verkasalo et al. 2019). Forest and wood processing residues have already been shown to be rich and environmentally friendly feedstock for various bioproducts (Holmbom 2011; Oleson and Schwartz 2016; Ragauskas et al. 2006). Nevertheless, wood residues and bark are mostly used in wood processing plants only as a source of energy. Although the amount of non-structural constituents in wood and bark from trees (generally referred to simply as extractives) is relatively small (about 5–15%, w/w), some of these compounds, e.g., stilbenes, flavonoids and condensed tannins, have been reported to possess antifungal, antimicrobial and antioxidant properties and they therefore have great potential for various applications (Belt et al. 2017; Lu et al. 2016; Valette et al. 2017; Välimaa et al. 2007). According to forecasts, the global market for polyphenols will grow significantly by 2030 (grandviewresearch 2022). The high demand for plant polyphenols with antioxidant, anti-inflammatory and antimicrobial properties is evidence of the increasing use of these plant products in the field of dietary supplements and nutraceuticals, cosmetics, “green” preservatives etc.
One of the negative consequences of climate change is its effect on the distribution of tree species (Gričar et al. 2015; Humar et al. 2021; Vítková et al. 2017). The latest model projections for Central Europe show that tree species of major industrial importance, i.e., spruce and Scots pine, will be most affected, and they are predicted to migrate almost entirely from Central Europe to northern countries (with the exception of the colder Alpine regions) (Dyderski et al. 2018). According to a study by Dyderski et al. (2018), silver fir is identified as a “climate winner”, together with some autochthonous broadleaf species and also alien tree species. Silver fir will thus be one of the conifers that will gain new potential distribution areas in Europe in the future due to climate changes. This could give silver fir even greater economic importance for forest owners and the wood processing industry (Hanewinkel et al. 2013; Humar et al. 2022). Silver fir is already an economically valuable coniferous species in Europe (Dobrowolska et al. 2017). At the same time, silver fir logs and their wood often experience a sharp decrease in value due to the formation of so-called wet heart or wet wood, in the place of the normal heartwood (Torelli et al. 2005, 2006). Wet heart of silver fir is described as wood tissue with compact, increased moisture content at the site of the heartwood (Torelli 1990). Wet heart is subjected to mechanical anomalies involving cracking and ring shakes (Shigo 1984; Torelli et al. 2006, 2007). In fact, the frequency and intensity of wet heart formation in silver fir critically increases with tree age, dimensions and frequent injuries, e.g., from broken/dropped branches. Wet heart in mature silver trees is therefore actually a normal and age-related phenomenon (Torelli et al. 2005). It is clear that not much attention is paid to the presence of wet heart in the existing literature on the extractive compounds of silver fir, even though this is a major quality defect and therefore reduces the value of silver fir logs. The utilization of the less valuable wood and bark biomass of silver fir remains a major challenge but the extraction of valuable bioproducts such as nanocellulose and bioactive extracts from tree biomass continues to be intensively researched (Brennan et al. 2020b; Fernandez-Costas et al. 2017; Oleson and Schwartz 2016; Žepič et al. 2022).
Extracts from low-grade tissues of silver fir, i.e., bark and branch wood, have already been investigated for health-promoting properties, highlighting the antioxidant effect of the extracts (Drevenšek et al. 2016; Tavčar Benković et al. 2017). Silver fir samples were extracted using both conventional and advanced extraction methods, and extractives were analyzed using various in vitro antioxidant assays. Bark samples were extracted by maceration followed by purification by liquid-liquid extraction (Tavčar Benković et al. 2014), then using an accelerated extraction system (ASE) (Brennan et al. 2020b) or using standard procedures as demonstrated on Caucasian fir bark samples (Ozgenc et al. 2017). Hamada et al. (2018) used a Soxhlet apparatus for the extraction of sapwood and heartwood samples of silver fir. The wood of silver fir branches was also conventionally extracted at higher temperature and analyzed by Tavčar Benković et al. (2017) but, due to the patent process, detailed information on the extraction process is not available. Since conifer knotwood is generally known to be a potential source of bioactive polyphenols (Holmbom 2011), silver fir knotwood has also been studied most extensively, with ASE and Soxhlet often used for extraction (Kebbi-Benkeder et al. 2017; Willför et al. 2004). Stemwood and knotwood of Siberian fir (Abies sibirica) were extracted using ASE and the extracts were subsequently compared for their antioxidant properties; however, with respect to the sampling procedure, it is unclear whether sapwood or heartwood, or even a mixture of both, was extracted and analyzed (Ul’yanovskii et al. 2022). In terms of the solvents used for extraction, silver fir extractives have been obtained with water or various less and more polar organic solvents, e.g., hexane, toluene, aqueous acetone and aqueous ethanol (Hamada et al. 2018; Kebbi-Benkeder et al. 2015; Tavčar Benković et al. 2014; Willför et al. 2004).
The main objective of this study was to investigate the biorefinery potential of sapwood (SW), heartwood (HW), as well as bark (B) and knotwood (KW) extracts originating from lower value, wet-hearted silver fir logs, by comparing the contents of hydrophilic extractives (TEC) and total phenols (TPC) as well as the antioxidant capacity of the extracts. Two extraction methods were applied and compared: sonication (Son) and accelerated solvent extraction (ASE), in order to assess their potential for future use. An ethanol-water mixture was used as solvent for environmental and future application reasons. The chemical composition of the tissues was assessed and compared by measuring total extractive content (TEC), Folin-Ciocalteu total polyphenol content (TPC) and by the complex evaluation of the antioxidant capacity by FRAP (Ferric reducing antioxidant power), ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and DPPH (2,2-diphenyl-1-picrylhydrazyl) assays. The results contribute to a better understanding of the utilization of wet-hearted silver fir wood and to the elaboration of novel and cost-effective green extraction methods for the utilization of the extractives.
2 Materials and methods
2.1 Chemicals
Folin–Ciocalteu phenol reagent, gallic acid monohydrate, gallic acid (certified reference material), L-ascorbic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, an analogue of vitamin E), 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+ solution), 2,2-diphenyl-1-picrylhydrazyl (DPPH solution), 2,4,6-tripyridyl-S-triazine (TPTZ solution), potassium persulfate, iron(III)-chloride, acetic acid, sodium acetate, hydrochloric acid, sodium carbonate, potassium hydrogen phosphate and potassium dihydrogen phosphate were purchased from Merck (Sigma-Aldrich Chemie, Taufkirchen, Germany). Water, methanol and absolute ethanol were from J.T. Baker (Phillipsburg, NJ, USA). All chemicals used are commercially available, all were of pure/analytical grade and were used without further purification.
2.2 Material
The study was conducted on silver fir (Abies alba Mill.), i.e., a coniferous tree that plays an important role in the wood processing industry in Central Europe (Bianchi et al. 2014; Butnaru et al. 2022; Dobrowolska et al. 2017). Wood and bark samples from a number of trees were included in the investigation. Trees averaged 32.24 (SD, 2.48) meters in height and 125.83 (SD, 42.02) years old. Age was measured by counting the annual rings of the cross-section at the base of the silver fir (at about 0.2 m height). Since the study was conducted on mature silver firs, they were naturally characterized by the presence of the above-mentioned wet heart at the heartwood site. It was therefore not possible to compare the results with samples from intact and healthy trees, as all the firs sampled had a wet heart (Fig. 1). The presence of wet heart significantly reduces the value of silver fir logs. Nevertheless, an important goal of the research activity was to ensure the homogeneity of the silver fir wood and bark samples as much as possible. The samples were therefore cut from sample discs taken at different tree heights and homogenized. The silver firs were harvested from two locations: Kočevska Reka forest (45°34’31.5” N 14°46’27.8” E) and Kuželič forest, Grivac (45°28’11.7” N 14°50’43.8” E), Slovenia. The function of the tissues in a tree stem was considered in planning the study and selecting the sample groups, so wet heart, defined as defective or abnormal woody tissue, was not treated separately for the purposes of the present study. However, the precise identification of the different wood compartments of the wet heart of silver fir and the analysis of the chemical properties of these tissues with the investigation of their potential as a biorefinery raw material is an important goal of our future research activities. Samples of sapwood (SW), heartwood (HW), knotwood (KW) and bark (B) were thus sawn from each of the sampled discs in the manner previously described (Vek et al. 2021) (Fig. 1). SW and HW on the silver fir sample discs were distinguished with an iodine-starch test by applying iodine solution directly to the surface of the silver fir disc with a brush. In relation to the wood of knots, i.e., the base part of a branch imbedded in a tree stem, only the dark-colored central part of the knot was sampled, leaving out the adjacent light-colored tissues of the knot (Fig. 1). For our study, the KW samples were mechanically removed from the sample discs in the UL-BF workshop using a band saw and a chisel. A simple and efficient method for removing knotwood samples that is also suitable for higher Technology Readiness Levels (TRL) remains a major challenge that needs to be overcome. All collected silver fir samples were first oven-dried overnight at 50 °C, then ground using a cutting mill (Retsch SM 2000), and finally freeze-dried to a constant weight in a Telstar LyoQuest CC1930 freeze dryer at 0.045 mbar and − 85 °C. Finally, for the study of antioxidant properties, several homogenized samples of SW, HW, KW and B of silver fir were prepared by mixing the mass aliquots of each sample per tissue studied. The thus-prepared disintegrated samples of silver fir SW, HW, KW and B were stored in polypropylene bottles, which were air-tight sealed. The samples were kept in a cool, dark place until extraction.
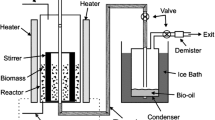
Schematic presentation of research activities. Sapwood (SW), heartwood (HW), knotwood (KW) and bark (B) of wet-hearted silver fir (Abies alba Mill.) were extracted by two extraction methods (sonication, ASE 350 system), and extracts were analyzed gravimetrically and colorimetrically for extractant content (soluble extractive content - TEC and total phenolic content - TPC) and antioxidant capacity (FRAP, ABTS, DPPH tests)
2.2.1 Extraction
The ground samples were first freeze-dried overnight. Extractions were performed in a Thermo Dionex system ASE 350 (Thermo Fisher Scientific Inc., Waltham, US) and in an Elma Transsonic T570 ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany) (Hofmann et al. 2015a; Keržič et al. 2019; Vek et al. 2021) (Fig. 1). The ASE extractions were performed under nitrogen, and the extracts were filtered through a cellulose filter, i.e., “on-line” during extraction. All wood and bark extracts obtained by ASE and sonication were prepared to achieve a sample to solvent ratio of 1:100 (w/v). The final volumes of the silver fir extracts produced were thus 100 ml for the ASE extracts and 30 ml for the extracts obtained by sonication (Table 1). As a solvent, 80% aqueous ethanol (v/v) was used. Further information on ASE and sonication of silver fir wood and bark samples can be found in Table 1.
Both extraction techniques can be also referred to as “green” due to the short extraction time and low solvent and energy consumption. Since water and ethanol are known to be environmentally friendly solvents because of their chemical properties and accessibility, e.g., ethanol can be produced by fermentation from renewable sources (Tekin et al. 2018), and to avoid the use of hazardous non-polar organic solvents, 80% aqueous ethanol (v/v) was chosen as the extraction solvent in the present study.
Before gravimetric analysis, all silver fir extracts were centrifuged twice at 10,000 rpm for 15 min in an Eppendorf 5810R centrifuge. The extracts were transferred to 2-mL polypropylene reaction tubes with safety caps and centrifuged again three times at 12,000 rpm for 10 min in an Eppendorf MiniSpin Mini centrifuge. Prior to the analyses, all reaction tubes containing the extracts were stored in a refrigerator at 4 °C.
2.2.2 Content of extractives and polyphenols in wood and bark samples
The total extractive content (TEC) and total phenol content (TPC) were measured according to the protocol described in our previous research reports (Vek et al. 2019, 2020). The TEC was determined by drying 10 mL of extract to constant weight in an oven at 105 °C, and results were expressed as percentage TEC per dry weight of sample (%, dw).
The TPC was measured by the Folin-Ciocalteu method (Albert et al. 2003; Singleton and Rossi 1965). Extracts were mixed with 10-fold diluted Folin-Ciocalteu phenol reagent (aq) and 0.7 M sodium carbonate solution (aq). The reaction mixtures were incubated for two hours and the absorbance was then measured at 765 nm in a Perkin-Elmer Lambda UV–VIS spectrophotometer. Gallic acid was used as a reference (Vek et al. 2019); the results in TPC are expressed as percentage of gallic acid equivalents (GAE) per dry weight of a silver fir sample (% GAE, dw).
2.2.3 Antioxidant properties
The antioxidant capacity of silver fir wood and bark extracts was measured according to the protocols described by Hofmann et al. (2015b). Colorimetric analyses of antioxidant capacities were performed using a Hitachi U-1500 spectrophotometer (Hitachi Ltd., Tokyo, Japan). Measurements of silver fir extracts were performed in two replicates. The final results are given as mean with standard deviation.
2.2.3.1 FRAP assay
The FRAP (ferric reducing antioxidant power) assay was run following the method of Benzie and Strain (1996) at 593 nm, applying 5 min reaction time and using ascorbic acid as standard. Results were expressed as mass equivalents of ascorbic acid per dry weight of silver fir sample (mg AAE/g dw) (Hofmann et al. 2015b).
2.2.3.2 ABTS assay
The ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) test was performed according to the protocol described by Stratil et al. (2007). After 10 min incubation at room temperature, the absorbance was determined at 734 nm. Results were expressed in milligrams of Trolox equivalents per gram of dry sample (mg TE / g dw) (Hofmann et al. 2015b).
2.2.3.3 DPPH assay
The DPPH radical neutralization assay was performed by measuring the radical scavenging activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) according to a modified protocol of Sharma and Bhat (2009). A methanolic solution of DPPH with a concentration of 80 µM/l was used for determination. DPPH reagent (2800 µl) was mixed with the sample (200 µl) and the mixture was incubated in the dark for 30 min at room temperature. The decrease in absorbance at 515 nm was measured. The DPPH radical scavenging capacity was expressed in milligrams of Trolox equivalents per gram of dry sample (mg TE / g dw) (Hofmann et al. 2015b).
2.3 Statistics and graphic material
Statistical significance was tested with basic statistical analysis using Statgraphics software. Before testing the differences between the means of the measurements using ANOVA, the results were tested for normal distribution. To determine which means were significantly different from the others, Fisher’s least significant difference (LSD) procedure was performed at a 95.0% confidence level. All structural formulas were generated using ChemDraw software from PerkinElmer.
3 Results and discussion
3.1 Total extractives content
According to the results, SW and HW contained significantly lower amounts of total extractives than B and KW (p < 0.001). On average, the two extractions of the silver fir samples yielded 2.22% of TEC and 0.55% TPC for SW, 3.80% of TEC and 1.07% TPC for HW, 24.65% of TEC and 9.89% TPC for KW and 11.82% of TEC and 3.22% TPC for B. Our results on extraction yields presented in Table 2 agree relatively well with previously published results on the chemistry of silver fir extracts, but there are some differences due to different sampling methods and extraction procedures (Brennan et al. 2020a; Hamada et al. 2018; Kebbi-Benkeder et al. 2017; Tavčar Benković et al. 2017; Willför et al. 2004). The following is a literature review of extractives obtained to date from various tissues of silver fir. It has been reported that the position of the bark on the silver fir can have a significant effect on extraction yield. Brennan et al. (2020a) demonstrated that ASE extraction of bark samples taken at different heights from a silver fir can yield TEC between 15% and even more than 30% (by weight). Treatment of Caucasian fir (Abies nordmanniana) bark with sodium hydroxide solution using the standard procedure (TAPPI; T 212 om-98) yielded 17.01% TEC (Ozgenc et al. 2017). In addition to the aforementioned longitudinal variation of water/ethanol soluble extractives in silver fir bark, radial variability in the content of total extractives was also observed. According to the results presented, Soxhlet extraction of sapwood and heartwood with a toluene/ethanol mixture yielded an average of 1.4% and 2.3% soluble extractives, respectively (Hamada et al. 2018). According to Willför et al. (2004) the total amount of hydrophilic extractives obtained with 95% acetone (v/v, aq) extraction was 0.7, 2.1 and 13–15% (dw) in SW, HW and KW, respectively. These amounts are lower than our measurements; however, their samples were washed with hexane (ASE) at a higher temperature and pressure prior to extraction with acetone, which probably also removed some of the nonstructural compounds that are soluble in acetone. Another possible explanation is that methanol and acetone are better suited for the extraction of polyphenols from fir bark samples. Secoisolariciresinol, lariciresinol and oligolignans have been reported to be abundant in silver fir KW samples (Willför et al. 2004). This was also confirmed in our preliminary study on the chemical composition of silver fir KW extracts (Vek et al. 2021). Significant longitudinal variation in extractives content has also been demonstrated for silver fir knotwood (Kebbi-Benkeder et al. 2017). In this case, the knotwood tissues were extracted sequentially in a Soxhlet apparatus using hexane and acetone as solvents. Soxhlet extraction of the samples from KW gave on average about 0.5% - < 2% lipophilic and 17 − 28% acetone soluble extractives. In addition to the dominant lignans, viz. secoisolariciresinol, hydroxymatairesinol and lariciresinol, the authors also found traces of matairesinol and pinoresinol in the KW extracts of silver fir (Kebbi-Benkeder et al. 2017). Similar results were also presented by Gérardin et al. (2023), whose KW samples contained 21.64% ethanol-soluble compounds. Ul’yanovskii et al. (2022) found that successive ASE extractions of KW of Siberian fir (Abies sibirica) with hexane and acetone (95%, aq) yielded 17.0% (dw) of TEC, and the amounts of TEC were significantly higher in the knotwood than in the stemwood (3.0%, dw). The TEC in KW of Siberian fir was significantly lower than the TEC in our KW samples (Table 2). In addition, the stemwood of Siberian fir contained comparable amounts of TEC to our stemwood samples (Table 2) but it is not clear whether either SW or HW or even a mixture of both was extracted in that case. In our study, the stemwood tissues were separated and analyzed separately. Butnaru et al. (2022) demonstrated that Soxhlet extraction of silver fir bark, needles and cones with an ethanol/toluene mixture yielded an average of 16.57–17.33%, 28.33–29.30% and 17.90–19.18% extractives, respectively. The bark was further described as an inexpensive and important source of phenols and flavonoids, containing about 95 mg GAE /g TPC and 130–175 mg QE /g flavonoids. Compared with the results of the present study (28.4–32.1 mg GAE /g TPC), the higher results for TPC in the B sample (Butnaru et al. 2022) could be explained by the composition of the solvent mixture and by the fact that the Folin-Ciocalteu reagent can also react with compounds that are soluble in less polar solvents than toluene, e.g., resin acids (Harju and Venäläinen 2006; Karppanen et al. 2007). Although the wood and bark of silver fir have been studied extensively for the chemical composition of extractives, this review of recent research reports clearly shows that only certain tissues of the fir trunk have been studied with the chosen extraction technique and solvent composition. The present report is the first comparative study to include all categories of silver fir stem tissues in one study, i.e., stemwood, knotwood and bark samples were extracted together and analyzed in the same manner.
The highest extractive contents were measured in KW samples. The extractives content in KW samples was more than 11 and 6 times higher than in SW and HW, respectively. Compared to SW and HW, much higher TEC and TPC were also measured in the B samples (Table 2). KW samples of silver fir were also richest in polyphenols, with ratios of TPC between KW and other samples even higher than the ratios of TEC (Table 2). Comparison of ASE and sonication showed that there was not much difference in the extraction yield of the studied samples. However, significantly more TEC and TPC were extracted from KW with ASE than with sonication (Table 2). ASE also proved to be more efficient than sonication in extracting hydrophilic compounds (TEC) from B samples. In the case of the B samples, the extraction technique had no significant effect on the TPC (Table 2). In terms of stem tissue, interestingly, higher TEC was obtained with sonication than with ASE from both SW and HW, but the significance here was not confirmed by statistical analysis (Table 2).
According to Table 3, ASE was more efficient for the extraction of polyphenols using aqueous ethanol solution compared to sonication. This difference between extraction methods in TPC/TEC (%, dw) was particularly evident for SW and HW samples. It has been described that the advantage of ASE extraction, commonly defined as subcritical application, is that organic solvents are heated above their boiling point during extraction but are maintained in a liquid state under high pressure. This increases the penetration of the solvent into the sample and enhances the desorption of the analyte, resulting in higher overall extraction performance with lower solvent consumption (Thurbide and Hughes 2000). This may explain the higher efficiency of ASE in extracting phenolic compounds from cell walls, especially from HW, compared with sonication (Table 3). For the extracts of KW and B, the difference between the TPC/TEC for ASE and the TPC/TEC for sonication was much smaller. KW samples, i.e., dark colored and impregnated wood tissue from branches embedded in the tree trunk, were confirmed to be rich in phenolic extractives. More than 40% of the dried ethanol extract KW was ascribed to polyphenols (Table 3). A lower percentage of TPC/TEC was measured in HW, SW and B samples, about one-third from TEC (Table 3). The results are in good agreement with the report of Tavčar Benković et al. (2017), who found that a dried aqueous extract of silver fir branchwood consists of 25% TPC (GAE).
3.2 Antioxidant capacity of silver fir wood and bark extracts
Plant polyphenols such as flavonoids and lignans are referred to as non-enzymatic antioxidants. The inactivation of oxidants by these reducing agents is described as a redox reaction in which one reactive species is reduced at the expense of the oxidation of another (Benzie and Strain 1996; Pietarinen et al. 2006; Rosales-Castro et al. 2012; Silva et al. 2014). The colorimetric methods commonly used to measure the antioxidant activity of plant extracts are based on oxidizing–reducing properties, in which phenolic compounds act as reducing agents and offer hydrogen radicals or electrons (Huang et al. 2005; Stratil et al. 2007). In general, these assays are known as rapid and reliable colorimetric methods for measuring antioxidant capacity. However, they all have some disadvantages, which have already been clearly summarized (Frankel and Meyer 2000; Hofmann et al. 2015b; Stratil et al. 2007) and which must be taken into account when interpreting the results. It is therefore advisable to perform at least 3 different methods and combine the evaluation of the results for each sample in order to obtain comprehensive results.
The antioxidant potential of the extracts was assessed using three different assays, all of which have different selectivity and reaction mechanisms (Prior et al. 2005; Shalaby and Shanab 2013; Sharma and Bhat 2009), so the results provide a complex insight into the comprehensive antioxidant power of the samples. The highest antioxidant capacities were measured with all three assays on KW extracts (FRAP: 159.75 mg AAE/g, ABTS: 316.15 mg TE /g, DPPH: 189.23 mg TE /g). In the case of Siberian fir, excellent antioxidant properties have also been found for KW samples (Ul’yanovskii et al. 2022. Lower antioxidant capacities were found for the B extracts (FRAP: 159.75 mg AAE/g, ABTS: 126.81 mg TE /g, DPPH: 74.52 mg TE /g). Butnaru et al. (2022) previously reported that ethanol/toluene extracts of silver fir bark were good DPPH radical scavengers but these results were only evaluated on the basis of a visible discoloration of the DPPH solutions. Water-soluble compounds of silver fir bark were found to be potent natural antioxidants tested by DPPH assay and a cell-based in vitro assay on primary human peripheral blood mononuclear cells (Tavčar Benković et al. 2014). According to the DPPH assay, silver fir bark extracts exhibited a significantly higher (91%) antioxidant activity than that of maritime pine bark extract (Tavčar Benković et al. 2014). The high application potential of silver fir bark extracts in nutrition, health and medicine is attributed to the at least 13 natural antioxidants, phenolic acids (gallic acid, homovanillic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic and p-coumaric acid), flavonoids (catechin, epicatechin and catechin tetramethyl ether) and lignans (taxiresinol, 7-(2-methyl-3,4-dihydroxytetrahydropyran-5-yloxy)-taxiresinol, secoisolariciresinol and lariciresinol) (Tavčar Benković et al. 2014).
Using FRAP and DPPH assay, the weakest antioxidant power was measured in SW (FRAP: 6.80 mg AAE/g, DPPH: 4.57 mg TE /g) and HW (FRAP: 12.35 mg AAE/g, DPPH: 10.19 mg TE /g). In the case of ABTS assay, a relatively high antioxidant capacity was measured for SW (13.80 mg TE /g) and HW (31.50 mg TE /g) extracts compared to B extracts. However, it has already been demonstrated that, in addition to knotwood and bark extracts, silver fir wood also possesses significant antioxidant activity. The latter has been demonstrated by both in vitro and in vivo tests (Tavčar Benković et al. 2017; Vek et al. 2021). A comprehensive study of antioxidant activities in vitro and in vivo was conducted by Tavčar Benković et al. (2017) using a water extract of silver fir branchwood. They found that the branchwood extract exhibited higher antioxidant activity in vitro than selected reference antioxidants, i.e., ascorbic acid, resveratrol and butylated hydroxytoluene (BHT), and similar antioxidant activity to epigallocatechin gallate. Branch wood extractives were found to be particularly effective OH radical scavengers. The in vivo antioxidant activity of silver fir extracts was also confirmed by intracellular assay. The in vitro and in vivo antioxidant activities of branchwood extracts were explained by the presence of lignans (Tavčar Benković et al. 2017). The effects of the chosen extraction technique on the antioxidant capacity of the silver fir samples studied are shown in Figs. 2, 3 and 4. It can be clearly seen that, for the SW and HW samples, the extraction technique had no significant effect on the antioxidant activity. Both extraction methods also had similar effects on the antioxidant capacity of the KW and B samples, but only when these extracts were analyzed with FRAP (Fig. 2). In contrast, the B extracts prepared by ASE extraction were found to be significantly better ABTS radical scavengers than the B extracts obtained by sonication (Fig. 3). In our study, the ASE extracts from KW proved to be the silver fir extracts with clearly the best DPPH radical scavenging activity (Fig. 4).
Ferric reducing antioxidant power (FRAP) for sapwood (SW), heartwood (HW), knotwood (KW) and bark extracts (B) of silver fir prepared with different extraction methods (sonication (Son) and accelerated solvent extraction (ASE)). Results are expressed in ascorbic acid mass equivalents per gram of dry sample (mg AAE/g dw). a-c, Different letters on bars indicate a significant difference at the p < 0.05 level
ABTS radical scavenging activity for extracts of sapwood (SW), heartwood (HW), knotwood (KW) and bark (B) of silver fir prepared with different extraction methods (sonication (Son) and accelerated solvent extraction (ASE)). Results are expressed in Trolox mass equivalents per gram of dry sample (mg TE/g dw). a-d, Different letters on the bars indicate a significant difference at the p < 0.05 level
DPPH radical scavenging activity for extracts of sapwood (SW), heartwood (HW), knotwood (KW) and bark (B) of silver fir prepared with different extraction methods (sonication (Son) and accelerated solvent extraction (ASE)). Results are expressed in Trolox mass equivalents per gram of dry sample (mg TE /g dw). a-d, Different letters on bars indicate a significant difference at the p < 0.05 level
The antioxidant capacity of wood and bark extracts has been explained by the presence of different classes of phenolic compounds (Miranda et al. 2021; Pietarinen et al. 2006; Rosales-Castro et al. 2012; Willför et al. 2003). Previous reports have indicated that hydrophilic extracts of silver fir bark consist of simple phenolic acids, flavonoids, lignans and condensed tannins, viz., prodelphinidins (Bianchi et al. 2014; Tavčar Benković et al. 2014; Vek et al. 2023), whereas the studied wood of silver fir contained mainly lignans, with a dominance of secoisolariciresinol (Kebbi-Benkeder et al. 2017; Vek et al. 2021; Willför et al. 2004). However, one of the objectives of the present study was to investigate the statistical relationships between the measured TEC and TPC of the extracts obtained by both ASE and sonication and the measured antioxidant capacities. The results of statistical analysis clearly showed a linear model to describe the significant relationships between the antioxidant capacities and the amount of extractives in the fir samples (Table 4). Since the p-values of the ANOVA are below 0.001 (Table 4), a significant relationship between the antioxidant capacities (measured by FRAP, ABTS and DPPH assay) and the content of silver fir polyphenols was confirmed at a confidence level of 95.0%. Extraction of the silver fir wood and bark samples, either with ASE or by sonication, revealed a strong correlation between the measured TEC and TPC (p ASE, sonication < 0.001, R ASE > 0.980, R sonication > 0.988). Our results show that the amount of TEC and TPC in the wood and bark extracts obtained by ASE extraction significantly described the antioxidant capacity (ANOVA, pASE < 0.001). These relationships were statistically confirmed for all antioxidant tests performed (Table 4). Similar results were also characteristic of the silver fir extracts prepared with sonication (Table 4). This could mean that the quality of the extracts obtained with ASE and sonication is similar. The extraction methods investigated here yielded similar phenolic contents and antioxidant activities. The chemical identity of the phenolic compounds contained in the silver fir extracts has already been reported (Kebbi-Benkeder et al. 2017; Tavčar Benković et al. 2014; Vek et al. 2021, 2023; Willför et al. 2004). To obtain more precise information on which isolates and phenolic compounds of silver fir are responsible for the antioxidant activity of the extracts, further research activities using appropriate sample-prep techniques for purification and the correct analytical tools for compound identification are needed. These samples should also be tested with other biological tests to determine the possible bioactivity of silver fir extractives.
4 Conclusion
This study investigated the antioxidant polyphenol content of wet-hearted silver fir stem tissues, including sapwood, heartwood, bark and knotwood, to find possible applications of the tissues of wet-hearted, thus lower grade wood logs. Accelerated solvent extraction and ultrasonic extraction techniques were compared using 80% aqueous ethanol (v/v) as a solvent. The highest contents of ethanol-soluble extractives were measured in knotwood and bark, which well supports the existing literature on extractives of silver fir. Although sonication proved to be an efficient, low-cost and fast extraction method, the present study revealed that, because of the higher temperature and pressure, the accelerated solvent extraction technique proved to be more efficient, resulting in significantly higher polyphenol contents. The results were also confirmed by the combined evaluation of multiple antioxidant capacity assays (FRAP, ABTS and DPPH). On the other hand, sonication operates at ambient temperature and pressure without a controlled atmosphere (unlike the nitrogen atmosphere in ASE), so the lower operating costs could also be understood as an advantage that could outweigh the higher yields of TEC and TPC extracted with ASE. The highest antioxidant concentrations were found in knotwood and bark, whereas sapwood and heartwood yielded significantly lower levels of polyphenols. The results show that the knotwood and bark of lower grade wet-hearted silver fir logs can be potential sources of natural antioxidants for future applications, whereas sapwood and heartwood are poor in these extractives, making them unlikely candidates for the extraction of natural antioxidants.
Data availability
No datasets were generated or analysed during the current study.
References
Albert L, Hofmann T, Németh ZI, Rétfalvi T, Koloszár J, Varga S, Csepregi I (2003) Radial variation of total phenol content in beech (Fagus sylvatica L.) wood with and without red heartwood. Holz Roh- Werkst 61:227–230. https://doi.org/10.1007/s00107-003-0381-x
Belt T, Hanninen T, Rautkari L (2017) Antioxidant activity of scots pine heartwood and knot extractives and implications for resistance to brown rot. Holzforschung 71:527–534. https://doi.org/10.1515/hf-2016-0232
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Bianchi S, Gloess AN, Kroslakova I, Mayer I, Pichelin F (2014) Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry Industrial. Crops Prod 61:430–437. https://doi.org/10.1016/j.indcrop.2014.07.038
Brennan M, Fritsch C, Cosgun S, Dumarcay S, Colin F, Gerardin P (2020a) Quantitative and qualitative composition of bark polyphenols changes longitudinally with bark maturity in Abies alba Mill. Ann Sci 77:14. https://doi.org/10.1007/s13595-019-0916-x
Brennan M, Fritsch C, Cosgun S, Dumarcay S, Colin F, Gerardin P (2020b) Yield and compositions of bark phenolic extractives from three commercially significant softwoods show intra- and inter-specific variation. Plant Physiol Biochem 155:346–356. https://doi.org/10.1016/j.plaphy.2020.07.033
Butnaru E, Pamfil D, Stoleru E, Brebu M (2022) Characterization of bark, needles and cones from silver fir (Abies alba Mill.) Towards valorization of biomass forestry residues. Biomass Bioenergy 159:106413. https://doi.org/10.1016/j.biombioe.2022.106413
Dobrowolska D, Boncina A, Klumpp R (2017) Ecology and silviculture of silver fir (Abies alba Mill.): a review. J for Res 22:326–335. https://doi.org/10.1080/13416979.2017.1386021
Drevenšek G, Lunder M, Benković ET, Štrukelj B, Kreft S (2016) Cardioprotective effects of silver fir (Abies alba) extract in ischemic-reperfused isolated rat hearts. Food Nutr Res 60:7. https://doi.org/10.3402/fnr.v60.29623
Dyderski MK, Paź S, Frelich LE, Jagodziński AM (2018) How much does climate change threaten European forest tree species distributions? Glob Change Biol 24:1150–1163. https://doi.org/10.1111/gcb.13925
Fernandez-Costas C, Palanti S, Charpentier JP, Sanroman MA, Moldes D (2017) A sustainable treatment for Wood Preservation: enzymatic grafting of Wood Extractives. ACS Sustain Chem Eng 5:7557–7567. https://doi.org/10.1021/acssuschemeng.7b00714
Frankel EN, Meyer AS (2000) The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Agric 80:1925–1941. https://doi.org/10.1002/1097-0010(200010)80:13%3C1925::AID-JSFA714%3E3.0.CO;2-4
Gérardin P, Hentges D, Gérardin P, Vinchelin P, Dumarçay S, Audoin C, Gérardin-Charbonnier C (2023) Knotwood and Branchwood Polyphenolic Extractives of Silver Fir, Spruce and Douglas Fir and Their Antioxidant, Antifungal and Antibacterial Properties. Molecules 28:13. https://doi.org/10.3390/molecules28176391
Gričar J, Prislan P, De Luis M, Gryc V, Hacurova J, Vavrčik H, Čufar K (2015) Plasticity in variation of xylem and phloem cell characteristics of Norway spruce under different local conditions. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.00730
Hamada J, Petrissans A, Ruelle J, Mothe F, Colin F, Petrissans M, Gerardin P (2018) Thermal stability of Abies alba wood according to its radial position and forest management. Eur J Wood Prod 76:1669–1676. https://doi.org/10.1007/s00107-018-1353-5
Hanewinkel M, Cullmann DA, Schelhaas M-J, Nabuurs G-J, Zimmermann NE (2013) Climate change may cause severe loss in the economic value of European forest land. Nat Clim Change 3:203–207. https://doi.org/10.1038/nclimate1687
Harju AM, Venäläinen M (2006) Measuring the decay resistance of scots pine heartwood indirectly by the Folin-Ciocalteu assay Canadian. J for Res 36:1797–1804
Hofmann T, Nebehaj E, Albert L (2015a) The high-performance liquid chromatography/multistage electrospray mass spectrometric investigation and extraction optimization of beech (Fagus sylvatica L.) bark polyphenols. J Chromatogr A 1393:96–105. https://doi.org/10.1016/j.chroma.2015.03.030
Hofmann T, Nebehaj E, Stefanovits-Bányai É, Albert L (2015b) Antioxidant capacity and total phenol content of beech (Fagus sylvatica L.) bark extracts. Industrial Crops Prod 77:375–381. https://doi.org/10.1016/j.indcrop.2015.09.008
Holmbom B (2011) Extraction and utilisation of non-structural wood and bark components. In: Alén R (ed) Biorefining of forest resources. vol book 20. Paper Engineers’ Association/Paperi ja Puu Oy, Helsinki, pp 178–224
Huang D, Ou B, Prior RL (2005) The Chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856. https://doi.org/10.1021/jf030723c
Humar M, Balzano A, Grbec S, Gričar J, Kržišnik D, Lesar B, Vek V (2021) Investigation of the material resistance and moisture performance of pubescent oak (Quercus Pubescens). Holzforschung 75:22–36. https://doi.org/10.1515/hf-2020-0045
Humar M, Vek V, Oven P et al (2022) Durability and moisture dynamics of douglas-fir wood from Slovenia. Front Plant Sci 13. https://doi.org/10.3389/fpls.2022.860734
Karppanen O, Venäläinen M, Harju AM, Willför S, Pietarinen S, Laakso T, Kainulainen P (2007) Knotwood as a window to the indirect measurement of the decay resistance of Scots pine heartwood. Holzforschung 61:600–604. https://doi.org/10.1515/hf2007.091
Kebbi-Benkeder Z, Colin F, Dumarcay S, Gerardin P (2015) Quantification and characterization of knotwood extractives of 12 European softwood and hardwood species. Ann Sci 72:277–284. https://doi.org/10.1007/s13595-014-0428-7
Kebbi-Benkeder Z, Manso R, Gérardin P, Dumarçay S, Chopard B, Colin F (2017) Knot extractives: a model for analysing the eco-physiological factors that control the within and between-tree variability. Trees 31:1619–1633. https://doi.org/10.1007/s00468-017-1573-z
Keržič E, Vek V, Poljanšek I, Oven P (2019) Optimization of accelerated solvent extraction (ASE) of silver fir wood (Abies alba Mill.): Optimizacija pospešene ekstrakcije s topili (ASE) na primeru lesa bele jelke (Abies alba Mill.). Les/Wood 68:69–77. https://doi.org/10.26614/les-wood.2019.v68n02a06
Lu JR, Venalainen M, Julkunen-Tiitto R, Harju AM (2016) Stilbene impregnation retards brown-rot decay of Scots pine sapwood. Holzforschung 70:261–266. https://doi.org/10.1515/hf-2014-0251
Miranda I, Ferreira J, Cardoso S, Pereira H (2021) Composition and antioxidant properties of extracts from Douglas fir bark. Holzforschung 75:677–687. https://doi.org/10.1515/hf-2020-0097
Oleson KR, Schwartz DT (2016) Extractives in Douglas-fir forestry residue and considerations for biofuel production. Phytochem Rev 15:985–1008. https://doi.org/10.1007/s11101-015-9444-y
Ozgenc O, Durmaz S, Kustas S (2017) Chemical Analysis of Tree Barks using ATR-FTIR spectroscopy and conventional techniques. BioResources 12:9143–9151. https://doi.org/10.15376/biores.12.4.9143-9151
Pietarinen S, Willför S, Ahotupa M, Hemming J, Holmbom B (2006) Knotwood and bark extracts: strong antioxidants from waste materials. J Wood Sci 52:436–444. https://doi.org/10.1007/s10086-005-0780-1
Prior RL, Wu XL, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302. https://doi.org/10.1021/jf0502698
Ragauskas AJ et al (2006) The path forward for biofuels. Biomaterials Sci 311:484–489. https://doi.org/10.1126/science.1114736
Rosales-Castro M, Gonzalez-Laredo RF, Rocha-Guzman NE, Gallegos-Infante JA, Rivas-Arreola MJ, Karchesy JJ (2012) Antioxidant activity of fractions from Quercus sideroxyla bark and identification of proanthocyanidins by HPLC-DAD and HPLC-MS. Holzforschung 66:577–584
Shalaby EA, Shanab SMM (2013) Antioxidant compounds, assays of determination and mode of action. African J Pharm Pharmacol 7:528–539. https://doi.org/10.5897/AJPP2013
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113:1202–1205. https://doi.org/10.1016/j.foodchem.2008.08.008
Shigo AL (1984) Development and characteristics of discoloured wood. IAWA Bull 5:99
Silva F, Figueiras A, Gallardo E, Nerin C, Domingues FC (2014) Strategies to improve the solubility and stability of stilbene antioxidants: a comparative study between cyclodextrins and bile acids. Food Chem 145:115–125. https://doi.org/10.1016/j.foodchem.2013.08.034
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticulture 16:144–158
Stratil P, Klejdus B, Kubáň V (2007) Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 71:1741–1751. https://doi.org/10.1016/j.talanta.2006.08.012
Tavčar Benković E, Grohar T, Žigon D, Švajger U, Janeš D, Kreft S, Štrukelj B (2014) Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Industrial Crops Prod 52:23–28. https://doi.org/10.1016/j.indcrop.2013.10.005
Tavčar Benković E, Žigon D, Mihailović V, Petelinc T, Jamnik P, Kreft S (2017) Identification, in vitro and in vivo antioxidant activity, and gastrointestinal stability of lignans from silver fir (Abies alba) wood extract. J Wood Chem Technol 1–11. https://doi.org/10.1080/02773813.2017.1340958
Tekin K, Hao N, Karagoz S, Ragauskas AJ (2018) Ethanol: a Promising Green Solvent for the deconstruction of. Lignocellulose ChemSusChem 11:3559–3575. https://doi.org/10.1002/cssc.201801291
Thurbide KB, Hughes DM (2000) A rapid method for determining the extractives content of wood pulp. Ind. Eng Chem Res 39:3112–3115. https://doi.org/10.1021/ie0003178
Torelli N (1990) Les&skorja (Wood&bark). Slovar Strokovnih Izrazov. Univerza v Ljubljani. Biotehniška fakulteta, Oddelek za lesarstvo, Ljubljana
Torelli N, Gorišek Ž, Oven P, Merela M (2005) Mokro srce pri jelki (Abies alba Mill.). Wetheart in silver fir (Abies alba Mill. Les wood 57:4–16
Torelli N, Trajkovic J, Sertic V (2006) Influence of phenolic compounds in heartwood of silver fir (Abies alba Mill.) On the equilibrium moisture content. Holz Roh- Werkst 64:341–342
Torelli N, Sinjur I, Gorišek Ž (2007) Biologija mokrega srca pri navadni jelki (Abies alba Mill.) In njegove lastnosti (Biology of wet heart in silver fir (Abies alba Mill.) And its propereties). Gozdarski Vestnik 65:443–460
Ul’yanovskii NV, Onuchina AA, Faleva AV, Gorbova NS, Kosyakov DS (2022) Comprehensive characterization of chemical composition and antioxidant activity of lignan-rich coniferous knotwood extractives. Antioxid 11:2338. https://doi.org/10.3390/antiox11122338
Valette N, Perrot T, Sormani R, Gelhaye E, Morel-Rouhier M (2017) Antifungal activities of wood extractives. Fungal Biology Reviews 31:113–123. https://doi.org/10.1016/j.fbr.2017.01.002
Välimaa A-L, Honkalampi-Hämäläinen U, Pietarinen S, Willför S, Holmbom B, von Wright A (2007) Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int J Food Microbiol 115:235–243. https://doi.org/10.1016/j.ijfoodmicro.2006.10.031
Vek V, Poljanšek I, Oven P (2019) Efficiency of three conventional methods for extraction of dihydrorobinetin and robinetin from wood of black locust. Eur J Wood Prod 77:891–901. https://doi.org/10.1007/s00107-019-01430-x
Vek V, Poljanšek I, Oven P (2020) Variability in content of hydrophilic extractives and individual phenolic compounds in black locust stem. Eur J Wood Prod 78:501–511. https://doi.org/10.1007/s00107-020-01523-y
Vek V, Keržič E, Poljanšek I, Eklund P, Humar M, Oven P (2021) Wood extractives of silver fir and their antioxidant and Antifungal properties. Molecules 26:6412. https://doi.org/10.3390/molecules26216412
Vek V, Šmidovnik T, Humar M, Poljanšek I, Oven P (2023) Comparison of the content of Extractives in the bark of the trunk and the bark of the branches of silver fir (Abies alba Mill.). Molecules 28:225. https://doi.org/10.3390/molecules28010225
Verkasalo E, Leppälä J, Muhonen T, Korpinen R, Möttönen V, Kurppa S (2019) Novel industrial ecosystems and value chains to utilize side-streams of wood products industries – Finnish approach. Pro Ligno J 15:157–165. http://www.proligno.ro/ro/articles/2019/4/VERKASALO.pdf
Vítková M, Müllerová J, Sádlo J, Pergl J, Pyšek P (2017) Black locust (Robinia pseudoacacia) beloved and despised: a story of an invasive tree in Central Europe. For Ecol Manage 384:287–302. https://doi.org/10.1016/j.foreco.2016.10.057
Willför SM et al (2003) Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J Agric Food Chem 51:7600–7606. https://doi.org/10.1021/jf030445h
Willför S, Nisula L, Hemming J, Reunanen M, Holmbom B (2004) Bioactive phenolic substances in industrially important tree species. Part 2: knots and stemwood of fir species. Holzforschung 58:650–659. https://doi.org/10.1515/HF.2004.119
Žepič V, Oven P, Čop M, Vek V, Janković B, Poljanšek I (2022) Physical, Rheological and Mechanical properties of Alkali activated Hydrogels based on Nanofibrillated Cellulose. J Nat Fibers 1–13. https://doi.org/10.1080/15440478.2022.2123879
Acknowledgements
The acknowledgements are listed in detail in the submitted manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, V.V., T.H. and P.O.; Methodology, V.V., T.H. and E.V.R.; Software and preparation of figures and tables, V.V. and U.O.; Validation and formal analysis, V.V., T.H., E.V.R.; Investigation, V.V., P.O., I.P., T.H., E.V.R., U.O.; Preparation of the original draft, V.V.; Resources, V.V. and P.O.; Writing, V.V., T.H., P.O., I.P., E.V.R., U.O. All authors have reviewed the manuscript. Revision and manuscript editing and preparation of the response to the reviewer reports, V.V., T.H., P.O., I.P., E.V.R., U.O.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vek, V., Hofmann, T., Rajczi, E.V. et al. Effect of accelerated extraction and sonication on the antioxidant capacity of wood and bark extracts of wet-hearted silver fir (Abies alba Mill.). Eur. J. Wood Prod. (2024). https://doi.org/10.1007/s00107-024-02102-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00107-024-02102-1