Abstract
Wood modification with thermosetting resins results in improved dimensional stability and durability. However, the treatment does not enhance fire resistance. To address this, Scots pine sapwood (Pinus sylvestris L.) was impregnated with thermosetting resins such as 1,3-dimethylol-4,5-dihydroxyethyleneurea, phenol-formaldehyde resin and melamine-formaldehyde resin, along with a phosphorus polyol as the flame retardant. Both weight percent gain and cell wall bulking were measured to investigate the deposition of resin and phosphorus polyol. Fire resistance was assessed through thermogravimetric analysis, Bunsen burner test and mass loss calorimeter. The inclusion of a phosphate polyol improved thermal stability, reduced flammability and heat release. Melamine-formaldehyde resin combined with phosphorus polyol demonstrated self-extinguishing capability with the heat release rate comparable to non-combustible materials inside 400 s. Moreover, the total heat release within 600 s shows an 84% reduction compared to untreated wood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As the world moves towards a carbon-neutral economy, the demand for biobased resources, such as wood, is increasing (Sathre and González-García 2014). However, wood is prone to biological degradation and moisture-induced dimensional changes, which limit its widespread application. Wood modification with thermosetting resins can improve the dimensional stability and biological resistance to fungal attack (Zelinka et al. 2022). However, the use of wood can be restricted by its flammability. Recently fire accidents, such as Notre Dame de Paris building (Praticò et al. 2020), underscore the burning hazard of wood materials. In addition, the restriction on using flammable wood for cladding after Grenfell disaster (Guillaume et al. 2020) has further emphasized the need for improving the reaction to fire of wood. One effective approach to achieve this is by incorporating flame retardants (FR), such as phosphate- and boron-based chemicals (Sauerbier et al. 2020). Therefore, developing a comprehensive treatment strategy that addresses these issues is crucial.
One such approach is to use a combined treatment that involves impregnating the wood with formaldehyde-based thermosetting resins, such as phenol formaldehyde (PF), melamine formaldehyde (MF) and dimethylol dihydroxyethyleneurea (DMDHEU), along with compatible fire retardants (FRs). Studies have shown that when used alone, wood modification with DMDHEU (Xie et al. 2014), PF and MF (Xie et al. 2016) cannot improve the reaction to fire significantly, but even further weakens flame retardancy.
In contrast, combining phosphate-nitrogen and boron-based FRs with DMDHEU (Jiang et al. 2014) and MF (Lin et al. 2020, 2021) resins for wood treatment has demonstrated to provide effective fire protection for wood. Impregnation of wood with boric-phenol-formaldehyde (BPF) resin also showed significant improvement in the reaction to fire (Yue et al. 2017, 2020). In recent years, there has been a growing preference for phosphate-based FRs due to their high effectiveness and low toxicity (Schartel 2010). Furthermore, our recent research findings have validated the effectiveness of a combined treatment utilizing PF resin and phosphoric acid esters in enhancing the reaction to fire (Wu et al. 2023). However, there is still a lack of research on using phosphate FRs alone with other formaldehyde-based thermosetting resins for wood modification. Both DMDHEU and MF are rich in nitrogen, however, it is still unknown if there could be an N-P synergism (Alongi et al. 2015) inside the wood matrix when combined with phosphate flame retardant. Further studies are needed to assess the efficacy and safety of these treatments in enhancing the fire resistance of wood.
The objective of this study was to assess the reaction to fire of Scots pine (Pinus sylvestris L.) sapwood using a phosphorus polyol (PP) in combination with DMDHEU, PF and MF resin, respectively. The effectiveness of these treatments was evaluated by measuring the thermal stability, flammability and heat release of the wood.
2 Experimental
2.1 Materials
Scots pine (Pinus sylvestris L.) sapwood was obtained from Lower Saxony state forest in Germany. The impregnation process involved the use of three thermosetting resins: DMDHEU resin (Fixapret CP liq (Archroma), ca. 67% solid content, density (20 °C) 1.36 g/cm³, pH (20 °C) 5–6); a low molecular weight PF resin (EXPK 195 (Metadynea), ca. 60% solid content, pH (20 °C) 9-10.5); MF resin (MW840 (Prefere Resins), ca. 68% solid content, density (20 °C) 1.26 g/cm³, pH (20 °C) 9–11). Furthermore, PP (Exolit OP 550 (Clariant), a polymeric ester of ethylene glycol and phosphoric acid with ethyl and hydroxyalkyl groups, ca. 70% solid content, pH (20 °C) 5) was added to the impregnation solution.
2.2 Treatment process
To determine the initial dry weight, all specimens were dried in an oven at 103 °C until a constant mass was achieved. The oven-dried specimens were then conditioned at 65% RH and 20 °C for two weeks. Aqueous solutions of thermosetting resins and a phosphorus polyol (PP) were prepared as described in Table 1. Sample preparation involved impregnation by vacuum (5 kPa, 1 h) followed by a pressure phase (1200 kPa, 2 h). After treatment, the specimens were air-dried for 1 week and then underwent a stepwise dry-curing process at 40 °C (24 h), 60 °C (24 h), 80 °C (24 h), 103 °C (24 h) and 120 °C (24 h) for sufficient curing without significant weight loss due to thermal degradation.
To calculate the weight percent gain (WPG, in %) and cell wall bulking (CWB, in %), wood specimens of 25 × 25 × 10 (ax.) mm³ were used (10 for each group). The WPG and CWB were determined based on the oven-dry weight (Sartorius CP 323 S, 0.001 g) and cross-sectional dimensions (Sylvac 100, 0.001mm) before (muntreated, Auntreated) and after treatment (Mtreated, Atreated), according to (1) and (2), respectively.
2.3 Experimental methods
2.3.1 Thermogravimetric analysis
The thermal stability of untreated and treated wood powder was assessed using thermogravimetric analysis (TGA). The test was conducted using Netzsch TG209 F1instrument IRIS (Selb, Germany). First, 10 mg of wood powder was ground to pass through a 0.5 mm mesh and then placed in an alumina crucible. The heating rate was set at 20 °C/min under a nitrogen atmosphere, ranging from 50 to 800 oC. Onset and end temperature of the mass loss step were recorded and the maximum of the first derivative of the TGA curve (DTG) was determined in the OriginPro2020 software.
2.3.2 Flammability test
For each treatment, five wood specimens measuring 13 × 4 × 125 mm3 were used for the flammability test, following the methodology of Pries and Mai (2013). Before conducting the fire resistance testing, the specimens were conditioned at 20 °C and 65% RH. The specimens were placed at an angle of 45° and positioned 8 cm away from the Bunsen burner flame. The tip of each specimen was then ignited with the Bunsen burner and exposed to the flame for 30 s. The test was continued until the specimen stopped burning. During the test, the weight of each wood specimen was recorded in 10-second intervals and the mass loss was calculated based on the weight of the specimens before (mmod, before burning) and after (mmod, after burning) the flammability test. Additionally, the time it took for the specimens to stop burning after the Bunsen burner flame was removed was measured. The mass loss was calculated according to (3) and the time to extinguish was determined using (4).
2.3.3 Mass loss calorimetry
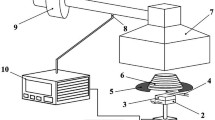
Mass loss calorimeter (Fire Testing Technology Ltd, UK) was used to evaluate the heat release of wood specimens according to the EN ISO 13927 (CEN, 2015). The thermopile system with the calibration by methane burner could produce similar results to cone calorimeter (Hasburgh et al. 2015) according to ISO 5660-1 (2002). The specimens were tested in a conditioned state (20 °C, 65% RH) under an external heat flux of 50 kW/m2. The baseline was collected with the empty sample holder measurement. Three replicates were tested and the mean value calculated for each group.
3 Results and discussion
3.1 Weight percent gain and cell wall bulking
Table 2 presents the average and standard deviation values of weight percent gain (WPG) and cell wall bulking (CWB) of the treated specimens. Wood modification using DMEHEU, PF and MF resins led to a WPG of over 30% for all specimens. The incorporation of phosphorus polyol resulted in similar WPG percentages of approx. 50% for DMDHEU-PP, PF-PP, and MF-PP, indicating successful penetration of the phosphorus polyol into the wood specimens regardless of the resin type used. The considerable deposition of phosphate flame retardant has been shown to contribute to the improvement of the reaction to fire (Wu et al. 2023).
The higher CWB indicate more chemicals inside the wood cell wall, which correlates with many properties such as biological resistance and dimensional stability (He and Riedl 2004). Also, the flame retardant could be more leaching-resistant in the cell wall than in the lumen due to the enveloping by water repellent agent (Yalinkilic et al. 1999). The inclusion of phosphorus polyol (PP) in the impregnation solutions containing DMDHEU and PF resins resulted in an increase in the cell wall bulking (CWB) value, indicating the deposition of the PP within the cell walls. However, these resins exhibit distinct modes of action in wood. DMDHEU can either react with the hydroxyl groups of wood polymers or undergo auto-condensation reactions, resulting in the formation of a resin that is unable to leach due to its size (Emmerich et al. 2019). In contrast, PF resin exclusively undergoes self-condensation within the wood, leading to the deposition of a long-chain polymer (Shams and Yano 2011). In contrast, MF-PP exhibited comparable CWB as MF, with the lowest CWB compared with DMDHEU and PF treated wood. This may be attributed to a higher molecular weight or polymerization of MF (Lin et al. 2021), which inhibits the penetration of the resin and flame retardant into the wood cell wall.
3.2 Thermal stability
The thermal stability of both untreated and treated wood was assessed using the thermogravimetric analysis (TGA) and the corresponding results are presented in Fig. 1. In the case of untreated Scots pine sapwood, the highest mass loss per unit time occurred at 383 oC, primarily attributed to cellulose dehydration and partially to lignin (Rowell 2012). The primary degradation temperature of DMDHEU is typically around 320 oC (Xie et al. 2014). Compared with untreated wood, the mass loss peak of DMDHEU treated wood shifted towards 371 oC. For MF-treated wood, the mass loss peak was observed at 364 oC, indicating the degradation of both MF resin and the wood itself (Girods et al. 2008). Similarly, the mass loss peak of PF-treated wood was found to shift to lower temperature (Xie et al. 2016), specifically 335 oC. Despite the minor change in the temperature corresponding to the maximum mass loss per unit time, treatment with any of the three thermosetting resins significantly increased the char residue. This increase can be attributed to the higher thermal stability of the thermosetting resin with chemically stable and cross-linked structure, when compared to untreated wood (Li et al. 2020; Wang et al. 2009; Dorieh et al. 2022). Notably, the PF resin treatment resulted in the highest char residue, reaching 31%, owing to the presence of thermally stable aromatic structures within the PF resin.
PF-PP and MF-PP treatments demonstrate notably higher final char residues, measuring 40% and 38% respectively, compared to DMDHEU-PP treatment which yielded a char residue of 34%. These findings were confirmed by higher thermal stability of PF and MF modified wood when compared to DMDHEU modified wood. Furthermore, all phosphorus polyol-treated wood samples exhibited lower onset degradation temperature (5% total weight loss) but significantly higher char residues than untreated wood, which only reached 18%. Additionally, in comparison to untreated wood, both the mass loss peak of DMDHEU-PP (287oC), PF-PP (304oC) and MF-PP (323oC) were observed to shift towards lower temperatures. This decrease in decomposition temperature can be attributed to the catalyzed dehydration of acids resulting from dephosphorylation occurring at lower temperatures. The catalytic action facilitates the formation of a thermally stable char, particularly at elevated temperatures (Green 1992).
3.3 Flammability
The Bunsen burner test is a quick and convenient method for assessing the flammability of wood. After conditioning for two weeks, the moisture of untreated wood reached approx. 9%, while resin treatment and resin combine PP treatment showed lower moisture content in a range from 6.24 to 7.83%. Variations in moisture content among different wood specimens can influence the vertical flame spread in larger wood specimens, while their impact on mass loss, flashover time, and heat release in a cone calorimeter may tend to be minor (Kraaijeveld and Log 2017). Figure 2 presents the mass loss and time to extinguish of each group. The mass loss of DMDHEU and PF treated wood was similar to the untreated wood, reaching approx. 70%. This indicates a high flammability and suggests that the resin treatment did not improve fire retardancy. The flammability test results for PF treatment contradict the positive TGA results. This discrepancy arises because the PF resin lacks oxidation resistance in high-temperature conditions (Gao et al. 1999). Moreover, treatment with these resins resulted in a longer time to extinguish compared to the untreated wood, which can be attributed to the increased density by resin treatment. In contrast, treatment with MF resin alone exhibited a significant improvement in fire resistance, with the mass loss of merely 20% and the ability to self-extinguish within 39 s. This is attributed to the release of nitrogen compounds (Ullah et al. 2014) and the formation of higher thermal stability cyameluric structure (Komatsu 2001). It is worth noting that even though the DMDHEU resin also contains nitrogen, it does not exhibit the same self-extinguishing effect. Further research is needed to fully understand the underlying mechanisms.
The incorporation of a phosphorus polyol into modified wood significantly enhanced the fire retardancy of the treated material. Among the tested combined treatments (resin + phosphorus polyol), the MF-PP collective displayed the most promising fire resistance, with the lowest mass loss at around 9% and a rapid self-extinguishing ability after the removal of the exterior flame. The flame retardancy mechanism of MF-PP can be partially attributed to its intumescent flame retardant system with N-P synergism, as suggested by Alongi et al. (2015). The MF resin, functioning as a nitrogen-based flame retardant, exhibits excellent compatibility with the phosphate flame retardant to synergistically improve the flame retardancy, as reported by Zhu and Xu (2020). The phosphorus polyol, containing polyol as a carbon source, acts as an acid source to facilitate the formation of a char layer. Simultaneously, the MF resin, functioning as a blowing agent, releases incombustible gas to prevent oxygen contact and dilute the combustible gas and heat. However, the combination of DMDEHU with a phosphate flame retardant did not exhibit the same N-P synergism in the thermogravimetric analysis (Holme and Patel 1982). Nevertheless, the char formation observed in the MF-PP system is not comparable to that of commercial intumescent systems, which generate a thick char layer. Instead, the formation of bubbles on the wood’s surface and within its structure acts as a barrier, providing effective flame retardancy.
3.4 Heat release
The fire performance of the treated wood was evaluated using a mass loss calorimeter. The key parameters used to assess the reaction to fire are the heat release rate (HRR) and the total heat release (THR), which could be the acceptance criteria used in codes and national regulations (Östman 2023). The output from the horizontal exposure could predict the fire performance for mid-scale fire test method such as single burning item (Hakkarainen and Kokkala 2001). Figure 3 illustrates the HRR and THR curves, displaying the average heat release for each group, while Table 3 provides a summary of the corresponding data. All wood specimens exhibit the characteristic combustion pattern of wood, characterized by two distinct peaks (Chung 2010). The first peak represents the rapid release of heat immediately after ignition, while the second peak (referred to as the peak heat release rate or PHRR) corresponds to complete combustion as the flame propagates to the bottom of the wood (Xu et al. 2015). Following the PHRR, the HRR of all wood specimens gradually decreased until the flame eventually extinguished.
Both DMDHEU- and PF-treated wood exhibited significantly higher heat release peaks (251.3 kW/m2 and 282.2 kW/m2, respectively) than the untreated wood (173.4 kW/m2). Furthermore, the time to reach PHRR was notably reduced in both cases, with DMDHEU-treated wood requiring 520 s and PF-treated wood requiring 472 s, compared to untreated wood (687 s). The higher and earlier heat release observed in these treatments increases the fire risk, which does not satisfy the regulatory requirements (Östman 2023). Nevertheless, it is noteworthy that MF-treated wood displayed a heat release pattern similar to that of untreated wood. This contrasts with the flammability test results, where MF-treated wood successfully demonstrated fire inhibition. The discrepancy can be attributed to the high constant heat flux in the cone calorimeter test, which surpasses the fire-retardant capabilities of the MF resin (Steen-Hansen and Kristoffersen 2007).
After incorporating phosphorus polyol, DMDHEU-PP and PF-PP showed significant improvements in flame retardancy compared to DMDHEU and PF. Their peak heat release rates (PHRR) occurred at later times (DMDHEU-PP: 148.6 kW/m², 567 s; PF-PP: 151.7 kW/m², 570 s) due to char formation inhibiting combustion. The similar fire performance of DMDHEU-PP and PF-PP with untreated wood is due to the introduction of combustible DMDHEU and PF resin. The increases in the fire loading reduce the effectiveness of phosphorus polyol. MF resin combined with phosphorus polyol resulted in quick self-extinguishing due to the formation of a protective char layer (Kurkowiak et al. 2023). The PHRR (161.7 kW/m², 795 s) of MF-PP is due to the prolonged extinguishing process before the HRR peak emerges. The THR of MF-PP was only 8.6 MJ/m² (approximately 16% of untreated wood) within 600 s.
4 Conclusion
In this study, we investigated the impregnation of Scots pine sapwood with aqueous solutions of three different thermosetting resins, both with and without a phosphorus polyol as a fire retardant. The WPG and CWB values confirmed a successful deposition of resin and phosphorus polyol in the wood structure. Our findings revealed that, when compared to untreated wood, none of the tested resins (DMDHEU, PF, and MF) resulted in a reduction of the heat release. However, incorporating phosphorus polyol with all three thermosetting resins exhibited several positive effects, including improved thermal stability, decreased flammability and inhibited combustion compared to the resin treated wood. Notably, the combination of MF resin and phosphorus polyol demonstrated significant suppression of combustion, with a total heat release within 600 s amounting to only 8.6 MJ/m², equivalent to approx. 16% of untreated wood. As the first screening, the addition of phosphorus polyol to thermosetting resins presents a promising strategy for enhancing the flame retardancy of the modified wood, thereby expanding its potential applications in public settings.
Data Availability
Not applicable, the raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Alongi J, Han Z, Bourbigot S (2015) Intumescence: tradition versus novelty. A comprehensive review. Prog Polym Sci 51:28–73. https://doi.org/10.1016/j.progpolymsci.2015.04.010
Chung Y-J (2010) Comparison of combustion properties of native wood species used for Fire pots in Korea. J Ind Eng Chem 16:15–19
Dorieh A, Pour MF, Movahed SG, Pizzi A, Selakjani PP, Kiamahalleh MV, Hatefnia H, Shahavi MH, Aghaei R (2022) A review of recent progress in melamine-formaldehyde resin based nanocomposites as coating materials. Prog Org Coat 165:106768
Emmerich L, Bollmus S, Militz H (2019) Wood modification with DMDHEU (1.3-dimethylol-4.5-dihydroxyethyleneurea) – state of the art, recent research activities and future perspectives. Wood Mater Sci Eng 14:3–18. https://doi.org/10.1080/17480272.2017.1417907
Gao J, Liu Y, Yang L (1999) Thermal stability of boron-containing phenol formaldehyde resin. Polym Degrad Stab 63:19–22. https://doi.org/10.1016/S0141-3910(98)00056-1
Girods P, Dufour A, Rogaume Y, Rogaume C, Zoulalian A (2008) Thermal removal of nitrogen species from wood waste containing urea formaldehyde and melamine formaldehyde resins. J Hazard Mater 159:210–221. https://doi.org/10.1016/j.jhazmat.2008.02.003
Green J (1992) A review of phosphorus-containing flame retardants. J Fire Sci 10:470–487
Guillaume E, Dréan V, Girardin B, Benameur F, Fateh T (2020) Reconstruction of Grenfell Tower Fire. Part 1: lessons from observations and determination of work hypotheses. Fire Mater 44:3–14. https://doi.org/10.1002/fam.2766
Hakkarainen T, Kokkala MA (2001) Application of a one-dimensional thermal flame spread model on predicting the rate of heat release in the SBI test. Fire Mater 25:61–70. https://doi.org/10.1002/fam.760
Hasburgh LE, White RH, Dietenberger MA, Boardman CR, Forest US, Forest S, Pinchot OG (2015) Comparison of the heat release rate from the mass loss calorimeter to the cone calorimeter for wood-based materials. Proceedings of the Fire and Materials
He G, Riedl B (2004) Curing kinetics of phenol formaldehyde resin and wood-resin interactions in the presence of wood substrates. Wood Sci Technol 38:69–81
Holme I, Patel SR (1982) 17—thermogravimetric analysis of n-methylolated resin finishes and diammonium hydrogen orthophosphate. J Text Inst 73:177–182. https://doi.org/10.1080/00405008208658935
Jiang T, Feng X, Wang Q, Xiao Z, Wang F, Xie Y (2014) Fire performance of oak wood modified with N-methylol resin and methylolated guanylurea phosphate/boric acid-based Fire retardant. Constr Build Mater 72:1–6. https://doi.org/10.1016/j.conbuildmat.2014.09.004
Komatsu T (2001) The first synthesis and characterization of Cyameluric High polymers. Macromol Chem Phys 202:19–25. https://doi.org/10.1002/1521-3935(20010101)202:1<19::AID-MACP19>3.0.CO;2-G
Kraaijeveld A, Log T (2017) Vertical Flame Spread in Wooden Corners as a Function of Fuel Moisture Content. In Proceedings of the 15th International Conference Fire and Materials, San Francisco, CA, USA; pp. 307–318
Kurkowiak K, Wu M, Emmerich L, Militz H (2023) Fire-retardant properties of wood modified with sorbitol, citric acid and a phosphorous-based system. Holzforschung 77:38–44. https://doi.org/10.1515/hf-2022-0114
Li W, Chen L, Li Y, Li X (2020) Bamboo modification with 1, 3-dimethylol-4, 5-dihydroxyethyleneurea (DMDHEU) catalyzed by maleic anhydride. J Wood Chem Technol 40:126–135
Lin C-F, Karlsson O, Mantanis GI, Sandberg D (2020) Fire performance and leach resistance of pine wood impregnated with guanyl-urea phosphate/boric acid and a melamine-formaldehyde resin. Eur J Wood Prod 78:107–111. https://doi.org/10.1007/s00107-019-01483-y
Lin C, Karlsson O, Martinka J, Rantuch P, Garskaite E, Mantanis GI, Jones D, Sandberg D (2021) Approaching highly leaching-resistant Fire-Retardant Wood by in situ polymerization with Melamine Formaldehyde Resin. ACS Omega 6:12733–12745. https://doi.org/10.1021/acsomega.1c01044
Östman B (2023) Acceptance criteria for products according to the cone calorimeter. Fire Mater 47:848–850. https://doi.org/10.1002/fam.3119
Praticò Y, Ochsendorf J, Holzer S, Flatt RJ (2020) Post-fire restoration of historic buildings and implications for Notre-Dame de Paris. Nat Mater 19:817–820. https://doi.org/10.1038/s41563-020-0748-y
Pries M, Mai C (2013) Fire resistance of wood treated with a cationic silica sol. Eur J Wood Prod 71:237–244. https://doi.org/10.1007/s00107-013-0674-7
Rowell RM (ed) (2012) - Thermal properties, Combustion, and Fire Retardancy of Wood. Handbook of Wood Chemistry and Wood composites. CRC Press, pp 144–167. https://doi.org/10.1201/b12487-11
Sathre R, González-García S (2014) Life cycle assessment (LCA) of wood-based building materials. Eco-efficient Construction and Building materials. Elsevier, pp 311–337. https://doi.org/10.1533/9780857097729.2.311
Sauerbier P, Mayer AK, Emmerich L, Militz H (2020) Fire Retardant Treatment of Wood – State of the art and future perspectives. In: Makovicka Osvaldova L, Markert F, Zelinka SL (eds) Wood & Fire Safety. Springer International Publishing, Cham, pp 97–102. https://doi.org/10.1007/978-3-030-41235-7_14
Schartel B (2010) Phosphorus-based Flame Retardancy mechanisms—old hat or a starting point for Future Development? Materials 3:4710–4745. https://doi.org/10.3390/ma3104710
Shams MI, Yano H (2011) Compressive deformation of phenol formaldehyde (PF) resin-impregnated wood related to the molecular weight of resin. Wood Sci Technol 45:73–81. https://doi.org/10.1007/s00226-010-0310-1
Steen-Hansen A, Kristoffersen B (2007) Prediction of Fire classification for wood based products. A multivariate statistical approach based on the cone calorimeter. Fire Mater 31:207–223. https://doi.org/10.1002/fam.934
Ullah S, Bustam MA, Nadeem M, Naz MY, Tan WL, Shariff AM (2014) Synthesis and thermal degradation studies of Melamine Formaldehyde resins. Sci World J 2014:1–6. https://doi.org/10.1155/2014/940502
Wang J, Jiang H, Jiang N (2009) Study on the pyrolysis of phenol-formaldehyde (PF) resin and modified PF resin. Thermochimica Acta 496:136–142. https://doi.org/10.1016/j.tca.2009.07.012
Wu M, Emmerich L, Kurkowiak K, Militz H (2023) Fire resistance of pine wood treated with phenol-formaldehyde resin and phosphate-based flame retardant. Wood Mater Sci Eng 1–7. https://doi.org/10.1080/17480272.2023.2205379
Xie Y, Liu N, Wang Q, Xiao Z, Wang F, Zhang Y, Militz H (2014) Combustion behavior of oak wood (Quercus mongolica L.) modified by 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU). Holzforschung 68:881–887. https://doi.org/10.1515/hf-2013-0224
Xie Y, Xu J, Militz H, Wang F, Wang Q, Mai C, Xiao Z (2016) Thermo-oxidative decomposition and combustion behavior of scots pine (Pinus sylvestris L.) sapwood modified with phenol- and melamine-formaldehyde resins. Wood Sci Technol 50:1125–1143. https://doi.org/10.1007/s00226-016-0857-6
Xu Q, Chen L, Harries KA, Zhang F, Liu Q, Feng J (2015) Combustion and charring properties of five common constructional wood species from cone calorimeter tests. Constr Build Mater 96:416–427. https://doi.org/10.1016/j.conbuildmat.2015.08.062
Yalinkilic MK, Imamura Y, Takahashi M, Yalinkilic AC (1999) In situ polymerization of vinyl monomers during compressive deformation of wood treated with boric acid to delay boron leaching. For Prod J 49:43
Yue K, Chen Z, Lu W, Liu W, Li M, Shao Y, Tang L, Wan L (2017) Evaluating the mechanical and fire-resistance properties of modified fast-growing Chinese fir timber with boric-phenol-formaldehyde resin. Constr Build Mater 154:956–962. https://doi.org/10.1016/j.conbuildmat.2017.08.035
Yue K, Wu J, Xu L, Tang Z, Chen Z, Liu W, Wang L (2020) Use impregnation and densification to improve mechanical properties and combustion performance of Chinese fir. Constr Build Mater 241:118101. https://doi.org/10.1016/j.conbuildmat.2020.118101
Zelinka SL, Altgen M, Emmerich L, Guigo N, Keplinger T, Kymäläinen M, Thybring EE, Thygesen LG (2022) Rev Wood Modif Wood Functionalization Technol Forests 13:1004. https://doi.org/10.3390/f13071004
Zhu H, Xu S (2020) Preparation of flame-retardant rigid polyurethane foams by combining modified melamine–formaldehyde resin and phosphorus flame retardants. ACS Omega 5:9658–9667
Funding
Open Access funding enabled and organized by Projekt DEAL. The research was funded by China Scholarship Council, 202003270024.
Author information
Authors and Affiliations
Contributions
M.W., L.E., K.K., H.M., conceived and designed the experiments; M.W., carried out the experiments and data collection; M.W., writing—original draft preparation; M.W., L.E., K.K., H.M., writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, M., Emmerich, L., Kurkowiak, K. et al. Combined treatment of wood with thermosetting resins and phosphorous flame retardants. Eur. J. Wood Prod. 82, 167–174 (2024). https://doi.org/10.1007/s00107-023-02012-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-023-02012-8