Abstract
Introduction
Surgically managed appendicitis exhibits great heterogeneity in techniques for mesoappendix transection and appendix amputation from its base. It is unclear whether a particular surgical technique provides outcome benefit or reduces complications.
Material and methods
We undertook a pre-specified subgroup analysis of all patients who underwent laparoscopic appendectomy at index admission during SnapAppy (ClinicalTrials.gov Registration: NCT04365491). We collected routine, anonymized observational data regarding surgical technique, patient demographics and indices of disease severity, without change to clinical care pathway or usual surgeon preference. Outcome measures of interest were the incidence of complications, unplanned reoperation, readmission, admission to the ICU, death, hospital length of stay, and procedure duration. We used Poisson regression models with robust standard errors to calculate incident rate ratios (IRRs) and 95% confidence intervals (CIs).
Results
Three-thousand seven hundred sixty-eight consecutive adult patients, included from 71 centers in 14 countries, were followed up from date of admission for 90 days. The mesoappendix was divided hemostatically using electrocautery in 1564(69.4%) and an energy device in 688(30.5%). The appendix was amputated by division of its base between looped ligatures in 1379(37.0%), with a stapler in 1421(38.1%) and between clips in 929(24.9%). The technique for securely dividing the appendix at its base in acutely inflamed (AAST Grade 1) appendicitis was equally divided between division between looped ligatures, clips and stapled transection. However, the technique used differed in complicated appendicitis (AAST Grade 2 +) compared with uncomplicated (Grade 1), with a shift toward transection of the appendix base by stapler (58% vs. 38%; p < 0.001). While no statistical difference in outcomes could be detected between different techniques for division of appendix base, decreased risk of any [adjusted IRR (95% CI): 0.58 (0.41–0.82), p = 0.002] and severe [adjusted IRR (95% CI): 0.33 (0.11–0.96), p = 0.045] complications could be detected when using energy devices.
Conclusions
Safe mesoappendix transection and appendix resection are accomplished using heterogeneous techniques. Technique selection for both mesoappendix transection and appendix resection correlates with AAST grade. Higher grade led to more ultrasonic tissue transection and stapled appendix resection. Higher AAST appendicitis grade also correlated with infection-related complication occurrence. Despite the overall well-tolerated heterogeneity of approaches to acute appendicitis, increasing disease acuity or complexity appears to encourage homogeneity of intraoperative surgical technique toward advanced adjuncts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute appendicitis is one of the most common abdominal emergencies requiring acute surgery. The incidence of appendicitis is estimated at 100, 105, and 151 per 100,000 person years in North America, Eastern Europe, and Western Europe, respectively [1]. Since its description by Fitz in 1886, clinical investigations underscore the need for prompt therapy of the non-perforated appendix [2]. The 1894 publication describing the muscle splitting incision standardized the operative exposure for appendectomy. Since then, mortality associated with acute appendicitis has been reduced to nearly 0.1% due to further improvements in medical and surgical management [3]. The surgical approach has evolved over the decades from Fitz’s laparotomy and McArthur’s ‘gridiron incision’ at McBurney’s point for targeted appendectomy to the minimally invasive procedures that sprang from Semm’s innovative ‘pelvikoscopie’ in 1982 [4, 5]. However, there remains some opacity regarding the most efficacious surgical technique for mesoappendix transection, and for management of the appendix base. Each of these is key operative elements of appendectomy regardless of open or minimally invasive approach.
Safe mesoappendix division may be achieved by mechanical means (clips, intracorporeal suture), electrocautery, of tissue sealing devices using ultrasonic or tissue sealing energy approaches. To date, no randomized control trial or large prospective cohort study has compared the bleeding complication profile between techniques. Similarly, appendix resection at its base may be achieved by sharp division between suture ligatures or clips, or linearly arrayed staples. Despite the length of time that these approaches have been used, it remains unclear whether one technique is superior regarding post-appendectomy infection including appendiceal stump leak and cecal fistula formation. These questions are more difficult to answer using randomized controlled trials as entry criteria often exclude patients in whom the techniques would be deployed during routine clinical care. Instead, a prospective non-randomized observational study of strictly controlled patient cohorts using time-bound patient accrual and multicenter assessment may be better suited to answer current care questions. Such studies are known as snapshot audits, and this exploration parses data from the SnapAppy cohort study to answer questions regarding mesoappendix and appendix management as a preplanned evaluation of the specifics of appendicitis management using laparoscopic techniques [6,7,8].
Methods
Protocol
We conducted a prospective, observational, non-randomized multicenter cohort study, using standardized published methodology [9], in line with a pre-specified protocol which was registered with ClinicalTrials.gov (Trial # NCT04365491). The study enrolled all consecutive patients admitted with acute appendicitis in a 90-day window between November 1, 2020, and May 28, 2021, and followed those patients for 90 days post-admission (up to August 31, 2021). The study complied with both the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and the Declaration of Helsinki.
Center eligibility
Any unit undertaking adult acute care surgery was eligible to register to enter patients into the study. No minimum case volume, or center-specific limitations were applied. The study protocol was disseminated to registered members of the European Society of Trauma and Emergency Surgery (ESTES) and through national surgical societies.
Patient eligibility
All adult patients (over 15 years of age) admitted for acute appendicitis who underwent laparoscopic appendectomy during index admission were included in the current study. Appendicitis was graded using the AAST Anatomic Disease Severity grading system for emergency general surgery that provides a uniform method to assess disease severity for a variety of conditions, including acute appendicitis [10,11,12]. The grading system uses clinical, radiographic, operative, and pathologic criteria to assign an incrementing ordinal severity score of 1 (mild disease limited to the organ) to 5 (widespread severe disease).
Data capture
Data were recorded contemporaneously and stored on a secure, user-encrypted online platform (SMARTTrial®) without patient-identifiable information. Centers were asked to validate that all eligible patients during the study period had been entered, and to attain > 95% completeness of data field entry prior to final submission. The database was closed for analysis on October 1, 2021. Quality assurance guidance to ensure data fidelity was provided by at least one consultant/attending-level surgeon at each site.
Outcome measures
The primary outcome measure was any postoperative complication within 30 days. Secondary outcomes were severe complications within 30 days defined as Clavien–Dindo classification grade 3 to 5 (reoperation, reintervention, unplanned admission to intensive care unit, organ support requirement, or death), length of stay (LOS), and procedure duration (PD) in minutes.
Statistical analysis
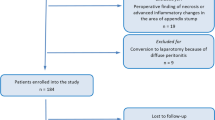
Patients who underwent a laparoscopic appendectomy were included for analysis. Patients who were missing data related to the technique used for mesoappendix transection or appendix resection were excluded from the associated analyses (study flow diagram; Fig. 1).
Patients were grouped based on the technique used for transection of the mesoappendix (electrocautery or energy device) or resection of the appendix (loop ligature, stapled, clipped). Descriptive results are presented as means and standard deviations (SDs) for continuous, normally distributed variables, medians and interquartile ranges (IQRs) for non-normally distributed continuous variables, as well as counts and percentages for categorical variables. Continuous, normally distributed variables were compared using a Student’s t-test, while non-normally distributed variables were compared using the Mann–Whitney U-test. A Chi-square test or Fisher’s exact test was used for categorical variables, as appropriate.
The relationship between the technique used and complications was determined using Poisson regression models with robust standard errors. The dependent variable was either any complication or severe complications, while the independent variables were the surgical technique as well as the patient’s age, sex, American Society of Anesthesiologists (ASA) classification, a history of previous abdominal surgery, ischemic heart disease, insulin-dependent diabetes, congestive heart failure, chronic renal disease, current smoking status, immunosuppression, the American Association for the Surgery of Trauma (AAST) appendicitis grade, time to surgery from admission, white blood cell count on admission, neutrophil percent on admission, C-reactive protein level on admission, as well as the country where the surgery was performed. Results of the statistical analyses are presented as incident rate ratios (IRRs) and 95% confidence intervals (CIs).
The association between the technique used and PD as well as LOS was evaluated using a quantile regression model. The independent variables were the same as previously utilized to assess the relationship between technique and complications. Results are presented as the median change in PD or LOS along with 95% CIs. Separate analyses were performed for each outcome as well as the stage of the appendectomy being investigated.
Multiple imputation by chained equations (MICE) was used to treat missing data [13]. In all analyses, a two-tailed p-value of less than 0.05 was considered statistically significant. Analyses were conducted with the statistical software R 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) using the tidyverse, mice, lubridate, readxl, writexl, robustbase, and quantreg packages [14].
Ethical considerations
All participating centers had Institutional Review Board approval or equivalent. No patient consent was sought since the current study was purely observational and did not impact patient care. All data were de-identified when uploaded to the secure study database.
Results
Participating centers
Following an open call for participation in May 2020, 71 centers across 14 countries (Bahrain, Estonia, Finland, Iran, Ireland, Israel, Italy, Portugal, Romania, Spain, Sweden, Switzerland, UK, and USA) completed the local ethics approval process and proceeded to prospectively enroll patients.
Comparison of surgical techniques for management of the appendix base
A total of 3729 patients were included in the analyses of the technique used for dividing the appendix base (Fig. 1). Histopathology revealed acute inflammation of the appendix in 90.4%, a normal appendix in 3.5% and neoplasm in 1.5%. The appendix was reportedly not sent for histopathologic evaluation in 4.5%, and data were missing or incomplete in 0.1%. Compared to patients whose appendix base was looped, those who had their appendix base divided using staples or clips were slightly older (33 vs. 38 and 35 years, p < 0.001), had a marginally higher BMI (25.8 vs. 27.2 and 26.8, p = 0.036), and were less fit for surgery according to their ASA classification (ASA ≥ 3: 5.1% vs. 10.6% and 7.7%, p < 0.001). These patients were also more likely to have had their diagnosis confirmed using a computerized tomography (CT) scan (48.7% vs 66.2% and 55.1%, p < 0.001). Compared to patients whose appendix base was looped or clipped, those who had their appendix base divided using staples were also more likely to have a perforated appendix (AAST grade ≥ 3: 15% vs 6.9% and 6.5%, p < 0.001) (Table 1).
Temperature is measured in degrees Celsius. Length of stay is measured in days. Procedure duration is measured in days. A severe complication is defined as a Clavien–Dindo classification ≥ 3a
The appendix was amputated by division from its base using looped ligatures in 1379 (37.0%), staples in 1421 (38.1%) and clips in 929 (24.9%). The technique for transection of the appendix at its base in acutely inflamed (AAST Grade 1) appendicitis was relatively equally divided between division between looped ligatures (37%), clips (25%) and stapler (38%). However, the technique used differed in complicated appendicitis (AAST Grade 2 +) migrated toward transection of the appendix base by stapler (58%) vs. loops (24%) or clips (18%) (p < 0.001) (Table 1).
Compared to patients whose appendix base was looped, overall procedural duration was greater for those who had their appendix base divided using staples (65 min vs 60 min, p < 0.001), while it was shorter for those who had their appendix base divided using clips (51 min vs 60 min, p < 0.001) (Fig. 2). The overall crude rate of any complication within 30 days was also marginally higher for those who had their appendix base divided using staples (14.5% vs 13.3%, p < 0.001) and lower for those who had their appendix base divided using clips (8.7% vs 13.3%, p < 0.001), compared to those who had their appendix base looped. However, patients who received staples or clips both had a higher crude rate of severe complications (3.7% and 1.9% vs 1.2%, p < 0.001). There was no statistically significant difference in the crude rate of reoperations within the first 90 postoperative days (Table 1). After adjusting for potential confounders, there was no statistically significant difference in the risk of any [IRR (95% CI) 1.20 (0.94–1.53) and IRR (95% CI) 0.79 (0.57–1.08)] or severe complications [IRR (95% CI) 1.79 (0.97–3.31) and 1.09 (0.49–2.42)], when comparing the use of staples and clips with having the appendix base looped (Table 2). Similarly, while the median LOS was longer for patients who received staples [median LOS (95% CI): 0.26 days (0.14–0.37), p < 0.001] and shorter for patients who received clips [median LOS (95% CI): − 0.16 days (− 0.08 to 0.25), p < 0.001], compared to patients who had their appendix base looped, this difference was not clinically significant (Table 3). The median PD in patients who had their appendix base divided using stapler device was statistically significantly longer PD [median PD (95% CI): 5.75 min (3.35–8.15), p < 0.001] compared to patients who had their appendix base looped or clipped (Table 4); however, this difference in PD holds no clinical significance.
Comparison of surgical techniques for management of the mesoappendix
A total of 2252 patients were included in the analyses of the technique used for dividing the mesoappendix (Fig. 1). The mesoappendix was divided using electrocautery in 1564 (69.4%) patients and an ultrasonic energy device in 688 (30.5%) and by other means (e.g., stapled or between clips or ligatures) in 1371(31.3%). Compared to patients who had their appendix divided using electrocautery, patients where an energy device was used instead were marginally older (37 vs 35, p = 0.047) and less fit for surgery according to their ASA classification (ASA ≥ 3: 10.3% vs 7.4%, p = 0.018). Energy device usage was also more prevalent in patients with perforation (AAST grade ≥ 3: 12.9% vs 9%, p = 0.038) (Table 5).
Patients who had their mesoappendix divided using an energy device both had an overall longer PD (65 min vs 57 min, p < 0.001) and LOS (2.1 days vs 1.8 days, p < 0.001). There were no statistically significant differences in the overall crude rate of any complications, severe complications, or reoperations (Table 5, Fig. 3). However, after adjusting for confounders in the regression analyses, energy devices were associated with a lower rate of any [adjusted IRR (95% CI): 0.58 (0.41–0.82), p = 0.002] and severe [adjusted IRR (95% CI): 0.33 (0.11–0.96), p = 0.045] complications (Table 2). The differences present in the crude PD and LOS were not statistically significant after adjusting for confounders in the regression analyses (Tables 3 and 4).
Procedure duration (a surrogate for case complexity) and the utilization of advanced surgical instruments to divide the mesoappendix, increased with AAST anatomic severity grade in patients undergoing laparoscopic appendectomy. Electrocautery devices include hook and Maryland dissectors connected to a current generator and can provide cutting or coagulation at the instrument tip. Ultrasonic or tissue sealing energy devices (including Ligasure and Harmonic Scalpel)
Specific complications
Postoperative complications within 30 days were reported in 12.6% of patients in the appendix base management cohort. The data-entry completion rate was high, with just 5 patients (0.1%) where data were unavailable. Infection-related complications accounted for 35% of all complications—surgical site infection was present in 46 patients (1.2%), postoperative organ-space infection was seen as pelvic abscess in 114 patients (3.1%), or subphrenic abscess in 5 patients (0.1%). Percutaneous interventional radiologic drainage of a postoperative intraperitoneal abscess was performed in 38 patients (1%). Postoperative ileus was seen in 82 patients (2.1%) and postoperative hemorrhage in 10 patients (0.2%). Other unspecified complications were recorded in 249 patients (6.7%). Forty-seven patients (1.3%) underwent re-operation during index admission (Fig. 4).
Postoperative complications within 30 days were reported in 315 (13.9%) of patients in the mesoappendix management cohort. Hemorrhagic complications were noted in 5 (0.2%) patients operated using electrocautery, versus 0 (0.0%) patients operated using an ultrasonic device (p = 0.318). Infection-related complications were noted in 84 (5.4%) cases using electrocautery, versus 34 (4.9%) using an ultrasonic device (p = 0.750). Severe complications (Clavien–Dindo 3 or above) within 30 days of operation were developed in 47 (3.0%) patients managed using electrocautery versus 14 (2.0%) patients managed using an ultrasonic device; (p = 0.231).
Discussion
Performance improvement in acute care surgery requires continuous re-evaluation of diagnostics, operative decision-making, and perioperative care. Nonetheless, contemporary appendicitis management exhibits great heterogeneity despite the frequency with which appendicitis is managed either operatively, or non-operatively. Indeed, several recent prospective randomized control trials both in Europe and the USA have demonstrated non-inferiority of antimicrobial-based pharmacologic management of early-stage acute appendicitis compared with appendectomy [15,16,17,18,19]. While these results have been met with great interest, and have provided therapeutic alternatives in the appropriate patient, the incidence of recurrent acute appendicitis, principally in patients with an appendicolith, ranging from 24 to 31%, is seen by some as a failure of non-operative management [20, 21]. Thus, outcomes related to surgical removal of the appendix remain of great importance for surgeons, Emergency Medicine clinicians, Primary Care physicians, as well as patients and their families.
Since outcomes are often related to condition stage or severity, a uniform manner by which to assess a process allows comparison across sites. The AAST disease severity grading system provides a mechanism to account for disease burden when performing comparative effectiveness research in emergency general surgery (EGS), including appendicitis [22, 23]. Increasing AAST grade for acute appendicitis is associated with increasing cost, complication rate, operative duration, length of stay, and need for open surgical technique in a variety of populations [22,23,24,25]. These data suggest that intraoperative assessment of appendicitis severity appears to impact surgical technique by influencing instrumentation selection. Energy device-driven mesoappendix transection and stapler-based appendix resection predominated as AAST appendicitis grade increased in comparison with all other approaches. It is intuitively attractive to link these two approaches with the notion of improved performance compared to electrocautery, clips, or loop ligatures. Nonetheless, other high-grade Clavien–Dindo classification complications were more common in those who underwent stapled appendix resection, before adjusting for confounders, reinforcing the notion that intraoperative tissue characteristics, anesthesia tolerance, or pre-operative assessment of comorbidities influenced instrument selection.
Instrument cost for frequent procedures such as laparoscopic appendectomy is important consideration in the OR and hospital budget assessments. Quantifying the total direct and indirect costs associated with an individual patient’s operation and hospital care is complex and multifactorial. A variety of factors impact the total assessment including but not limited to OR occupancy time, surgeon and anesthesiologist professional fees, pharmaceutical costs, equipment and supplies and personnel for room cleaning, reusable medical equipment acquisition and reprocessing/resterilization costs and single-use instrument acquisition costs (e.g., energy and stapling devices, loop ligatures, clips), acute care facility room costs, nursing costs, laboratory fees, and radiology tests as well as professional interpretations fees. Accordingly, instrumentation is only a small part of the total cost of operation related hospital care. Given the global heterogeneity in costs as well as patient charges, we did not undertake a cost analysis that would likely have been less fruitful than desired. Unfortunately, while surgeons are aware of their professional fees, many are less well informed regarding the costs of care such as those related to laboratory studies, general floor bed as opposed to ICU bed fees, or the cost of routine equipment or supplies. On the other hand, surgeons are acutely aware of postoperative complications and their impact on patient outcomes [26, 27].
In some healthcare systems, surgeon outcomes are publicly reportable. Importantly, many of the comparative assessment metrics are not acuity adjusted, nor coupled with other metrics of the acute care facility such as the case mix index. Additionally, completion of voluntary surveys may be freighted with subjectivity, as well as potentially overrepresenting those dissatisfied with care as they may be more likely to devote the required time. Therefore, postoperative complications may drive a skewed assessment of the surgeon when those assessments exist at a remove from an explanatory context.
Relatedly, the incidence of postoperative complications is a key quality metric for evaluating surgeon and hospital performance [28,29,30,31,32,33,34,35]. In over 4000 consecutive patients with acute appendicitis, the overall 90-day incidence of postoperative complications following appendectomy was 12.6%. Placing this in the context of existing literature (comprising retrospective administrative data and prospective randomized and non-randomized efficacy studies) [24,25,26,27,28,29,30,31] demonstrated divergent reporting practices as well as definitions used to identify complications. Many relevant retrospective contemporary studies reported an overall complication rate, with few defining complications in their methodology, or reporting the incidence of specific complications [24,25,26,27,28,29,30,31]. Total serious morbidity-modified National Surgical Quality Improvement Program (NSQIP) events were documented in 3.5% of patients randomized to surgical intervention in the 2020 Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) Trial. In 2015, the overall (not just serious) complication rate was 20.5% in the Finnish Antibiotic Therapy vs Appendectomy for Treatment of Uncomplicated Acute Appendicitis (APPAC) study [15, 20, 36]. An aggregated incidence of postoperative complications in a mixed population including uncomplicated and complicated appendicitis of 18.4% was derived from a meta-analysis of eleven trials assessing 1288 patients [37]. Therefore, our aggregate data align well with other studies—as does our specific major complication data—supporting the assertion that the captured audit data appropriately reflect outcomes in an unselected patient population that received contemporary acute appendicitis management.
Whether the untoward outcomes identified in our audit were specifically related to patient comorbidities, stage of presentation, surgical technique, instrumentation, or a combination of all four elements is unable to be parsed from the data. However, that geography did not directly link to a specific complication, nor a set of complications, argue that existing influencers were operative across all study sites, surgeons, and patients. Furthermore, the notion that emergency general surgery (EGS) patient outcomes are distinctly identifiable from those of elective patients regarding complication incidence and impact is a key point. Nascent efforts to establish an EGS database and morbidity and mortality review process metrics, morbidity and mortality calculators that assess comorbidity interaction rather than simple presence, as well as the growth of the AAST’s Acute Care Surgery fellowship programs underscores the differences that separate elective and emergency surgery patients. Rigorous evaluation of complications using a single data dictionary is likely to increase complication recognition and reporting in this unique patient group. It will be essential to frame the increased reporting in the context of condition severity, comorbidity, and acuity of intervention instead of simple presentation as an event frequency.
Study limitations
Our data are supported by the strength of a time-bound prospective observational approach to a common condition managed across 14 countries, but nonetheless demonstrates important limitations. First, we did not secure long-term outcome data. In evaluating causality, or the impact of a trialed intervention compared to a control measure, long-term outcome data are key. This study was instead targeted at capturing an environmental scan of current practice and immediate outcomes to inform hypothesis generation. Moreover, the patients in this audit are all part of the “control” arm as they reflected usual care at each institution. Second, we did not assess the impact of ERAS protocols (if any were used), the use of nasogastric drainage, time to oral intake, the duration of postoperative antibiotic therapy, or the occurrence of multi-drug resistant organisms in those with infection-related complications. These are questions that have been assessed by other studies and would have expanded the collected data set without enhancing evaluation of the specifically targeted outcomes. Third, neither insurance status, socioeconomic group, nor ethnicity was assessed due to the wide variability across the 14 countries that was unified only by focusing on those undergoing laparoscopic appendectomy and their immediate outcomes. Assessments of community care, return to gainful employment, capability of completing activities of daily living and related elements was beyond the scope of this specific audit. While we present a large contemporary dataset, our analyses, and the conclusions drawn from them, are limited by the completeness of the data available (Fig. 1). We did not capture instances where surgeons chose to use a combination of approaches, nor did we capture the complex considerations around laparoscopic port size, design, and placement. We acknowledge this potential shortcoming in our data collection instrument design. The insights provided from these data would provide an interesting study of surgical ergonomics, as well as providing information on the potential impact of these equipment choices on procedure duration and rates of complication. The anticipated heterogeneity in port sizes, blunt versus sharp trocar design, reusable versus single-use, medical device manufacturer, modes of initial entry and insufflation, laparoscopic cameras, etc., used across 71 centers in 14 countries would greatly reduce power and strongly hamper any assumptions of association. This inquiry into efficacy would perhaps be best achieved by a different methodology, perhaps a well-designed tightly controlled prospective randomized control trial.
Conclusions
Heterogeneity exists in the surgical techniques used to safely transect the mesoappendix and resect the appendix. Despite different instrumentation, operative time was remarkably similar. Instrument selection (energy devices and stapler) became more homogeneous with increasing AAST severity grade. This study’s findings may inform questions to be assessed that explore the specifics of intraoperative decision-making regarding instrumentation, as well as specific interventions to reduce the frequency of identified postoperative complications unrelated to the technical aspects of mesoappendix transection or appendix resection.
Data availability
The ESTES SnapAppy Group welcomes the use of these de-identified pooled data for further research that benefits patients. Requests can be submitted to the ESTES Research Committee. Release is subject to their approval and the appropriate safeguarding as determined by applicable legislation (GDPR and HIPAA).
References
Ferris M, Quan S, Kaplan BS, Molodecky N, Ball CG, Chernoff GW, et al. The global incidence of appendicitis: a systematic review of population-based studies. Ann Surg. 2017;266:237–41.
Fitz R. On perforating inflammation of the vermiform appendix with special reference to its early diagnosis and treatment. New Engl J Med. 1935;213:245–8.
Worni M, Østbye T, Gandhi M, Rajgor D, Shah J, Shah A, et al. Laparoscopic appendectomy outcomes on the weekend and during the week are no different: a national study of 151,774 patients. World J Surg. 2012;36:1527–33.
McBurney C. The incision made in the abdominal wall in cases of appendicitis, with a description of a new method of operating. Ann Surg. 1894;20:38–43.
Thomas CG. Experiences with early operative interference in cases of disease of the vermiform appendix by Charles McBurney, M.D., visiting surgeon to the Roosevelt Hospital, New York City. Rev Surg. 1969;26:153–66.
Bass GA, Gillis A, Cao Y, Mohseni S. Patterns of prevalence and contemporary clinical management strategies in complicated acute biliary calculous disease: an ESTES “snapshot audit” of practice. Eur J Trauma Emerg Surg. 2022;48:23–35.
Bass GA, Gillis AE, Cao Y, Mohseni S, For TES, Studies GESC. Self-reported and actual adherence to the Tokyo guidelines in the European snapshot audit of complicated calculous biliary disease. Bjs Open. 2020;4:622–9. https://doi.org/10.1002/bjs5.50294.
Bass GA, Gillis AE, Cao Y, Mohseni S, Shamiyeh A, Rosetti L, et al. Patients over 65 years with acute complicated calculous biliary disease are treated differently: results and insights from the ESTES snapshot Audit. World J Surg. 2021;45:2046–55. https://doi.org/10.1007/s00268-021-06052-0.
Bass GA, Kaplan LJ, Ryan ÉJ, Cao Y, Lane-Fall M, Duffy CC, et al. The snapshot audit methodology: design, implementation and analysis of prospective observational cohort studies in surgery. Eur J Trauma Emerg Surg. 2022. https://doi.org/10.1007/s00068-022-02045-3.
Crandall ML, Agarwal S, Muskat P, Ross S, Savage S, Schuster K, et al. Application of a uniform anatomic grading system to measure disease severity in eight emergency general surgical illnesses. J Trauma Acute Care. 2014;77:705–8.
Shafi S, Aboutanos M, Brown CV, Ciesla D, Cohen MJ, Crandall ML, et al. Measuring anatomic severity of disease in emergency general surgery. J Trauma Acute Care. 2014;76:884–7.
Vasileiou G, Ray-Zack M, Zielinski M, Qian S, Yeh DD, Crandall M. Validation of the American Association for the Surgery of Trauma emergency general surgery score for acute appendicitis-an EAST multicenter study. J Trauma Acute Care. 2019;87:134–9.
Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Medicine. 2016;4:30.
Weinberg SL, Harel D, Abramowitz SK. Statistics using R. Cambridge: Cambridge University Press; 2020. p. 268–86.
Flum DR, Davidson GH, Monsell SE, Shapiro NI, Odom SR, Sanchez SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. New Engl J Med. 2020;383:1907–19.
Saverio SD, Sibilio A, Giorgini E, Biscardi A, Villani S, Coccolini F, et al. The NOTA Study (Non Operative Treatment for Acute Appendicitis): prospective study on the efficacy and safety of antibiotics (amoxicillin and clavulanic acid) for treating patients with right lower quadrant abdominal pain and long-term follow-up of conservatively treated suspected appendicitis. Ann Surg. 2014;260:109–17.
Salminen P, Paajanen H, Rautio T, Nordström P, Aarnio M, Rantanen T, et al. Antibiotic therapy vs appendectomy for treatment of uncomplicated acute appendicitis: the APPAC randomized clinical trial. JAMA. 2015;313:2340–8.
Prechal D, Post S, Pechlivanidou I, Ronellenfitsch U. Feasibility, acceptance, safety, and effectiveness of antibiotic therapy as alternative treatment approach to appendectomy in uncomplicated acute appendicitis. Int J Colorectal Dis. 2019;34:1839–47.
O’Leary DP, Walsh SM, Bolger J, Baban C, Humphreys H, O’Grady S, et al. A randomized clinical trial evaluating the efficacy and quality of life of antibiotic-only treatment of acute uncomplicated appendicitis: results of the COMMA trial. Ann Surg. 2021;274:240–7.
Emile SH, Sakr A, Shalaby M, Elfeki H. Efficacy and safety of non-operative management of uncomplicated acute appendicitis compared to appendectomy: an umbrella review of systematic reviews and meta-analyses. World J Surg. 2022;46(5):1022–38.
Luo WY, Halabi R, Dodo-Williams T, Lobova VA, Matson J, Cui CL, et al. Doubts about cutting it out: systematic review and meta-analysis of patient-centered outcomes in nonoperative management vs surgery in acute uncomplicated appendicitis in adults. J Am Coll Surg. 2021;233:S91.
Mouch CA, Cain-Nielsen AH, Hoppe BL, Giudici MP, Montgomery JR, Scott JW, et al. Validation of the American Association for the Surgery of Trauma grading system for acute appendicitis severity. J Trauma Acute Care Surg. 2020;88:839–46.
Hernandez MC, Aho JM, Habermann EB, Choudhry AJ, Morris DS, Zielinski MD. Increased anatomic severity predicts outcomes: validation of the American Association for the Surgery of Trauma’s Emergency General Surgery score in appendicitis. J Trauma Acute Care. 2017;82:73–9.
Finnesgard EJ, Hernandez MC, Aho JM, Zielinski MD. The American Association for the Surgery of Trauma Emergency General Surgery Anatomic Severity Scoring System as a predictor of cost in appendicitis. Surg Endosc. 2018;32:4798–804.
Collins CM, Davenport DL, Talley CL, Bernard AC. Appendicitis grade, operative duration, and hospital cost. J Am Coll Surg. 2018;226:578–83.
Lai CH, Shapiro LM, Amanatullah DF, Chou LB, Gardner MJ, Hu SS, et al. A framework to make PROMs relevant to patients: qualitative study of communication preferences of PROMs. Qual Life Res. 2022;31:1093–103.
Saunders DI, Sinclair RCF, Griffiths B, Pugh E, Harji D, Salas B, et al. Emergency Laparotomy Follow-Up Study (ELFUS): prospective feasibility investigation into postoperative complications and quality of life using patient-reported outcome measures up to a year after emergency laparotomy. Perioper Med. 2021;10:22.
Zorzetti N, Lauro A, Vaccari S, Ussia A, Brighi M, D’andrea V, et al. A systematic review on the cost evaluation of two different laparoscopic surgical techniques among 996 appendectomies from a single center. Updates Surg. 2020;72:1167–74.
Ortega AE, Hunter JG, Peters JH, Swanstrom LL, Schirmer B, Group LAS. A prospective, randomized comparison of laparoscopic appendectomy with open appendectomy. Am J Surg. 1995;169:208–13.
Group SA of L and TSS, Beldi G, Vorburger SA, Bruegger LE, Kocher T, Inderbitzin D, et al. Analysis of stapling versus endoloops in appendiceal stump closure. Br J Surg. 2006;93:1390–3.
Sadat-Safavi SA, Nasiri S, Shojaiefard A, Jafari M, Abdehgah AG, Notash AY, et al. Comparison the effect of stump closure by endoclips versus endoloop on the duration of surgery and complications in patients under laparoscopic appendectomy: a randomized clinical trial. J Res Med Sci Off J Isfahan Univ Med Sci. 2016;21:87.
Sahm M, Kube R, Schmidt S, Ritter C, Pross M, Lippert H. Current analysis of endoloops in appendiceal stump closure. Surg Endosc. 2011;25:124–9.
Group O behalf of the snapshot appendicitis collaborative study, van Rossem CC, van Geloven AAW, Schreinemacher MHF, Bemelman WA. Endoloops or endostapler use in laparoscopic appendectomy for acute uncomplicated and complicated appendicitis. Surg Endosc. 2017;31:178–84.
Swank HA, van Rossem CC, van Geloven AAW, Hof KH, Kazemier G, Meijerink WJHJ, et al. Endostapler or endoloops for securing the appendiceal stump in laparoscopic appendectomy: a retrospective cohort study. Surg Endosc. 2014;28:576–83.
Mehdorn M, Schürmann O, Mehdorn HM, Gockel I. Intended cost reduction in laparoscopic appendectomy by introducing the endoloop: a single center experience. Bmc Surg. 2017;17:80.
Monsell SE, Voldal EC, Davidson GH, Fischkoff K, Coleman N, Bizzell B, et al. Patient factors associated with appendectomy within 30 days of initiating antibiotic treatment for appendicitis. Jama Surg. 2022;157: e216900.
Yang Z, Sun F, Ai S, Wang J, Guan W, Liu S. Meta-analysis of studies comparing conservative treatment with antibiotics and appendectomy for acute appendicitis in the adult. Bmc Surg. 2019;19:110.
Acknowledgements
ESTES SnapAppy Group: Gary Alan Bass, Division of Traumatology, Surgical Critical Care & Emergency Surgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA; Division of Trauma & Emergency Surgery, Orebro University Hospital and School of Medical Sciences, Orebro University, Sweden; Center for Perioperative Outcomes Research and Transformation (CPORT), University of Pennsylvania, Philadelphia, USA; Leonard Davis Institute of Health Economics (LDI), University of Pennsylvania, Philadelphia, USA. Shahin Mohseni, Division of Trauma & Emergency Surgery, Orebro University Hospital and School of Medical Sciences, Orebro University, Sweden. Lewis J. Kaplan, Division of Traumatology, Surgical Critical Care & Emergency Surgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA; Corporal Michael Crescenz Veterans Affairs Medical Center, Philadelphia, USA. Rebecka Ahl Hulme, Division of Trauma and Emergency Surgery, Department of Surgery, Karolinska University Hospital, and Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden. Alan Biloslavo, Department of General Surgery, Trieste University Hospital, Trieste, Italy. Yang Cao, Clinical Epidemiology and Biostatistics, School of Medical Sciences, Orebro University, Sweden. , Maximilian P. Forssten, Division of Trauma & Emergency Surgery, Orebro University Hospital and School of Medical Sciences, Orebro University, Sweden. Hayato Kurihara, Humanitas Clinical and Research Center, IRCCS, Milano, Italy. Isidro Martinez-Casas, Servicio de Cirugía General. Unidad de Cirugía de Urgencias. Hospital Universitario Virgen del Rocío, Sevilla Spain. Jorge Pereira, Centro Hospitalar Tondela, Viseu, Portugal. Arvid Pourlotfi, Division of Trauma & Emergency Surgery, Orebro University Hospital and School of Medical Sciences, Orebro University, Sweden. Éanna J. Ryan, Division of Trauma & Emergency Surgery, Orebro University Hospital and School of Medical Sciences, Orebro University, Sweden; Tallaght University Hospital, Dublin, Ireland. Matti Tolonen, Helsinki University Hospital HUS Meilahden Tornisairaala, Helsinki, Finland.
Bahrain: Bahrain Defence Force-Royal Medical Services: Nayef Louri (ORCID 0000-0001-9280-875X); Fatema Nedham (ORCID 0000-0002-5511-684X); Thomas Noel Walsh (ORCID 0000-0002-1600-8029); Jamal Hashem (ORCID 0000-0003-1544-8177). King Hamad University Hospital: Martin Corbally (ORCID 0000-0003-2599-6134); Abeer Farhan (ORCID 0000-0002-5121-6528); Hamad Al Hamad (ORCID 0000-0003-0568-3982); Rawan Elhennawy (ORCID 0000-0001-7076-6886). Salmaniya Medical Complex: Mariam AlKooheji (ORCID 0000-0002-3125-7939); Manar AlYusuf (ORCID 0000-0002-7206-8448); Wissal Aknouche (ORCID 0000-0001-9479-112X); Anas A. Zeidan (ORCID 0000-0002-9560-8187); Yusuf S. Alsaffar (ORCID 0000-0001-7492-4773). Estonia: North Estonia Medical Center: Edgar Lipping (ORCID 0000-0002-9593-5460); Peep Talving (ORCID 0000-0002-9741-2073); Sten Saar (ORCID 0000-0002-8958-5169); Katrina Graumann (ORCID 0000-0003-1429-4874); Liis Kibuspuu (ORCID 0000-0001-7321-3953); Eduard Harkov (ORCID 0000-0003-1194-2864). Finland: HUS Meilahden Tornisairaala: Gisele Aaltonen (ORCID 0000-0002-8265-6681); Iines S. Sillman (ORCID 0000-0001-7779-0432); Sami Haapanen (ORCID 0000-0001-8983-6733). HUS Jorvin sairaala: Hanna Lampela (ORCID 0000-0001-9619-1099). Henna Sammalkorpi (ORCID 0000-0001-8848-9346). Sofia Eskola (ORCID 0000-0001-8092-4550); Altti Laakso (ORCID 0000-0003-0417-9274). Hyvinkää Hospital Area: Johan Back (ORCID 0000-0002-9646-1914); Ulla Kettunen (ORCID 0000-0002-4107-8627); Antti M. Nummi (ORCID 0000-0002-4461-3373); Anika Szwedyc (0000-0002-4399-1639); Taina Nykänen (ORCID 0000-0003-3160-2816); Rolle Rantala (ORCID 0000-0002-7689-1685). Oulun Yliopistollinen Sairaala: Elisa J. Mäkäräinen-Uhlbäck (ORCID 0000-0003-4408-2514); Sanna A. Meriläinen (ORCID 0000-0002-0789-9042); Heikki I. Huhta (ORCID 0000-0002-6273-198X); Jukka M.J. Rintala (ORCID 0000-0003-1865-8444); Kirsi E. M. Laitakari (ORCID 0000-0001-7837-7025). Turku University Hospital: Elina Lietzen (ORCID 0000-0002-7514-7176); Paulina Salminen (ORCID 0000-0001-6435-9264); Risto K.A. Rapola (ORCID 0000-0002-0204-7027). Iran: Namazi Hospital, Shiraz University of Medical Sciences: Vahid Zangouri, Mohammad Y. Karami, Sedigheh Tahmasebi, Majid Akrami, Alireza Golchini, Faranak Bahrami. Ireland: Tullamore General Hospital: Sean M. Johnston (ORCID 0000-0001-9445-9645); Sean T. Lim (ORCID 0000-0002-8448-7121); Irele Ifijeh Ahonkhai (ORCID 0000-0002-9572-1235); Eltahir Eltagani (ORCID 0000-0001-6627-8804); Odhran K. Ryan (ORCID 0000-0003-2750-4607). St Vincent's University Hospital: Ailbhe O’Driscoll-Collins (ORCID 0000-0002-8447-0944); Aine O'Neill (ORCID 0000-0001-9190-6008); Zakiya Penny (ORCID 0000-0002-0235-6671); Orlaith Kelly (ORCID 0000-0002-9123-7024); Carolyn Cullinane (ORCID 0000-0002-9320-1586); Ian Reynolds (ORCID 0000-0002-8790-2538); Helen Heneghan (ORCID 0000-0002-2009-3406); Sean Martin (ORCID 0000-0002-6351-8857); Des Winter. Galway University Hospitals: Matthew Davey; Maha Alkhattab; Aoife J. Lowery; Michael J. Kerin; Aisling M. Hogan; Martin S. Davey; Ke En Oh. Letterkenny University Hospital: Syed Mohammad Umar Kabir (ORCID 0000-0003-4801-042X); Huilun Huan (ORCID 0000-0002-3007-2342); Charlotte Aziz (ORCID 0000-0003-2384-0718); Michael Sugrue (ORCID 0000-0002-9337-8939). University Hospital Waterford: Jessica M. Ryan (ORCID 0000-0001-6161-9630), Tara M. Connelly (ORCID 0000-0002-3178-7091), Mohammad Alhazmi (ORCID 0000-0002-8226-328X), Youssef Al-Mukhaizeem (ORCID 0000-0003-3935-8619), Fiachra Cooke (ORCID 0000-0001-9289-4221), Peter M. Neary (ORCID 0000-0002-9319-286X). Beaumont Hospital: Arnold D. K. Hill (ORCID 0000-0001-9617-7983); Michael R. Boland (ORCID 0000-0001-9024-8189); Angus J. Lloyd (ORCID 0000-0002-3043-6900); Frances Fallon (ORCID 0000-0003-4481-0796); Eoin F. Cleere (ORCID 0000-0003-2750-3057); James Toale (ORCID 0000-0002-2026-7584). Mayo University Hospital: Patrick A. Boland; Michael Devine; Conor Keady; Sarah Hunter; M. Kevin Barry. Tallaght University Hospital: Michael E. Kelly (ORCID 0000-0002-0757-6411); Aidan T. O’Dowling (ORCID 0000-0003-0534-0568); Ben Creavin (ORCID 0000-0001-8209-4810); Dara O. Kavanagh (ORCID 0000-0001-9535-0844); Paul Neary (ORCID 0000-0002-7464-1287); Paul F. Ridgway (ORCID 0000-0002-8500-8532); Cathleen A. McCarrick (ORCID 0000-0002-9713-9038). St James' University Hospital: Jarlath Bolger; Barry Maguire; Cian Keogh; Surbhi Chawla. Mater Misericordiae University Hospital: John Conneely (ORCID 0000-0001-6352-9444); Emilie McCormack (ORCID 0000-0001-5217-1977);Ben Shanahan (ORCID 0000-0001-6890-5007);Nicola Raftery (ORCID 0000-0002-4684-5573);Darragh Rice (ORCID 0000-0001-7857-8273);Niall McInerney (ORCID 0000-0003-4051-3882);Aine Stakelum (ORCID 0000-0002-1593-758X);Jan Mares (ORCID 0000-0001-8217-647X);Jonavan Tan (ORCID 0000-0002-4202-804X);Mark Hanna (ORCID 0000-0002-2878-392X);Ishwarya Balasubramanian (ORCID 0000-0002-3864-5651);Christina Fleming (ORCID 0000-0003-2326-2655). Israel: Soroka University Medical Center: Guy Barsky (ORCID 0000-0001-6290-0619); Gad Shaked (ORCID 0000-0002-4992-252X). Italy: Emergency Surgery and Trauma Section, Humanitas Research Hospital, Rozzano: Simone Giudici (ORCID 0000-0002-5721-2229); Martina Ceolin (ORCID 0000-0002-4689-480X); Simona Mei (ORCID 0000-0001-7952-0014); Francesca Mazzarella (ORCID 0000-0002-7132-3945). Trieste University Hospital: Annalisa Zucca (ORCID 0000-0002-7179-1191); Susanna Terranova (ORCID 0000-0003-1417-1903); Nicolo de Manzini (ORCID 0000-0002-8362-9044). Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino: Diego Visconti (ORCID 0000-0003-4041-1691); Emanuele Doria (ORCID 0000-0002-7941-1886); Mauro Santarelli (ORCID 0000-0017-9842-2240). San Maurizio Hospital, Bolzano: Giovanni Scotton (ORCID 0000-0003-2222-3822); Francesca Notte (ORCID 0000-0002-3559-1782); Giacomo Bertelli (ORCID 0000-0001-7681-1211); Anna Malpaga (ORCID 0000-0003-1171-6197); Giulia Armatura (ORCID 0000-0002-8536-7551); Antonio Frena (ORCID 0000-0001-9461-5345). Cisanello Hospital, University of Pisa: Dario Tartaglia (ORCID 0000-0003-1615-3370); Federico Coccolini (ORCID 0000-0001-6364-4186); Camilla Cremonini (ORCID 0000-0003-1503-7087); Enrico Cicuttin (ORCID 0000-0003-4584-9940); Alessio Mazzoni (ORCID 0000-0003-3325-2063); Massimo Chiarugi (ORCID 0000-0001-6905-2499). Portugal: Centro Hospitalar Universitário da Cova da Beira: Constança M. Azevedo (ORCID 0000-0002-9808-4083); Filipa D. Mendes (ORCID 0000-0002-7864-467X); Luis Q. Faria (ORCID 0000-0002-8089-9815); Carlos Nazario (ORCID 0000-0002-5751-5326); Daniela Machado (ORCID 0000-0002-3124-8952); Miguel Semiao (ORCID 0000-0002-1342-6081). Centro Hospitalar Tondela-Viseu: Jorge Pereira; Carlos Casimiro; Jose Pinto; Tiago Pavão; Raquel Pereira; Bruno Barbosa. Centro Hospitalar de Trás-os-Montes e Alto Douro: Nadia Tenreiro (ORCID 0000-0002-6869-0795); Catia Ferreira (ORCID 0000-0002-5651-3791); Goncalo Guidi (ORCID 0000-0003-3564-1468); Daniela C Martins (ORCID 0000-0003-3189-4599); Clara Leal (ORCID 0000-0003-2387-5144); Bruno B. Vieira (ORCID 0000-0001-9705-6489). North Lisbon University Hospital Centre: Luís S. Castro (ORCID 0000-0002-4214-2539); Aldara Faria (ORCID 0000-0002-8621-7846); Alberto Figueira (ORCID 0000-0003-0737-4200); Mauro Sousa (ORCID 0000-0002-8872-1093); Pedro Rodrigues (ORCID 0000-0001-7536-5635); Rodrigo Roquette (ORCID 0000-0002-7885-3076). Centro Hospitalar Universitário do Algarve - Hospital de Faro: Ricardo Ribeiro (ORCID 0000-0001-7411-0577); Paulo Cardoso (ORCID 0000-0002-7204-0402); Joana Domingues (ORCID 0000-0001-8231-3040); Maria Isabel Manso (ORCID 0000-0003-4796-5857); Rute Pereira (ORCID 0000-0003-3989-565X); Tatiana Revez (ORCID 0000-0002-8751-2097). Romania: Ponderas Academic Hospital, Bucharest: Bogdan D. Dumbrava (ORCID 0000-0003-4587-8792); Florin Turcu (N/A); Ionut Hutopila (N/A); Bogdana Banescu (N/A); Gerald Filip (N/A), Catalin Copaescu (ORCID 0000-0002-7270-0706). Spain: Hospital Universitario Juan Ramón Jiménez: Marcos Alba Valmorisco (ORCID 0000-0002-4449-7042); Isabel Manzano Martín (ORCID 0000-0003-4952-1934); Rocio Martín García de Arboleya; José Ortega Seda (ORCID 0000-0002-8759-8206); Pablo Rodríguez González (ORCID 0000-0001-6887-6074); Jose Antonio Becerra Toro (ORCID 0000-0003-4644-8903); Enrique Rodríguez Lara (ORCID 0000-0002-5121-9657); Jose Antonio González Minchón (ORCID 0000-0002-4059-6246). Hospital Universitario Son Espases: Juan José Segura-Sampedro (ORCID 0000-0003-0565-3791); Sebastián Jerí-McFarlane (ORCID 0000-0003-0319-2872); Alejandro Gil-Catalán (ORCID 0000-0001-8642-3925); Andrea Craus-Miguel (ORCID 0000-0002-2506-8621); Laura Fernández-Vega (ORCID 0000-0002-1998-5976); Xavier González-Argenté (ORCID 0000-0003-0003-9055). Hospital General Universitario de Ciudad Real: Mercedes Estaire-Gómez (ORCID 0000-0001-9153-9201); Borja Camacho Fernández-Pacheco (ORCID 0000-0003-3242-2892); Rebeca Vitón-Herrero (ORCID 0000-0003-4046-2691); Elisa Jimenez-Higuera (ORCID 0000-0003-2547-5814); Alejandro Barbero (ORCID 0000-0003-0818-0870); José M. Valverde (ORCID 0000-0001-6867-7710). Hospital Universitario Son Llàtzer: Enrique Colás-Ruiz (ORCID 0000-0001-8821-2531); Maria del Mar Escales-Oliver (ORCID 0000-0002-3991-071X); Olga Claramonte-Bellmunt (ORCID 0000-0002-0805-0710); Marta Castro-Suárez (ORCID 0000-0003-1837-8362); Naila Pagés-Valle (ORCID 0000-0003-0898-9303); José Andrés Cifuentes-Ródenas (ORCID 0000-0003-4886-0176). Hospital Universitario Central de Asturias: Marta Merayo Alvarez; Jose Luis Michi Campos; Luis Alejandro García González; Beatriz Carrasco Aguilera; Jaime Iturbe Menéndez; Jose Luis Rodicio Miravalles. Infanta Sofía University Hospital: Carmen Rodríguez Haro (ORCID 0000-0002-2086-694X); Sara Núñez O'Sullivan (ORCID 0000-0001-8418-6145); Mariana García Virosta (ORCID 0000-0003-2021-4623); María Hernández O'Reilly (ORCID 0000-0003-0913-4446). Hospital Universitario de La Ribera: Izaskun Balciscueta-Coltell (ORCID 0000-0002-5787-5647); Javier Lorenzo-Perez (ORCID 0000-0003-0036-144X); Sonia Martinez-Alcaide (ORCID 0000-0002-8213-5616); Susana Martinez-Ramos (ORCID 0000-0001-5651-1246); Maria Sebastian-Fuertes (ORCID 0000-0003-2729-657X); Laura Gomez-Romer (ORCID 0000-0002-6061-8174). Hospital Universitario de Gran Canaria Dr Negrín: Maria M. Pelloni (ORCID 0000-0002-5950-820X); Aida Cristina Rahy-Martín (ORCID 0000-0002-2791-529X); Andrés Felipe Yepes-Cano (ORCID 0000-0001-5827-8951). Hospital Universitario Virgen Macarena: Julio Reguera-Rosal (ORCID 0000-0003-4364-0908); Jose A. Lopez-Ruiz (ORCID 0000-0002-5905-7007); Beatriz Marenco (ORCID 0000-0002-0372-5707); Marina Retamar-Gentil (ORCID 0000-0002-0209-2047); Estela Romero-Vargas (ORCID 0000-0002-9012-6090); Angeles Gil-Olarte (ORCID 0000-0002-1324-9660). Urduliz Alfredo Espinosa Hospital: Aitor Landaluce-Olavarria (ORCID 0000-0002-8631-0151); Begoña Estraviz-Mateos (ORCID 0000-0002-5687-7667); Jose-Mario De Francisco-Rios (ORCID 0000-0001-6589-4250); Aitor Sainz-Lete (ORCID 0000-0003-2215-0870); Ane Emaldi-Abasolo (ORCID 0000-0001-5669-4441); Manolo Leon-Valarezo (ORCID 0000-0002-1755-5200). Donostia University Hospital: Claudia C. Lopes Moreira (ORCID 0000-0003-0404-736X); Aintzane Lizarazu Perez (ORCID 0000-0001-9091-4727); Araceli Rodriguez Gonzalez (ORCID 0000-0001-5249-6368); Iñigo Augusto Ponce (ORCID 0000-0003-0639-1834); Ignacio Maria Goena Iglesias (ORCID 0000-0002-7753-1606). Hospital Universitario de Burgos: Cristina González-Prado (ORCID 0000-0002-9548-505X); Guillermo Cabriada (ORCID 0000-0002-7161-7628); Beatriz López (ORCID 0000-0002-1541-8691); Michelle C. Otero (ORCID 0000-0002-3031-0953); Nerea Muñoz-Plaza (ORCID 0000-0001-8612-9849); Alberto Palomo (ORCID 0000-0002-7660-2134). Hospital Universitario Príncipe de Asturias: Fernando Mendoza-Moreno (ORCID 0000-0002-1046-6344); Manuel Díez-Alonso (ORCID 0000-0002-0993-8498); Francisca García-Moreno-Nisa (ORCID 0000-0001-5360-0577); Belén Matías-García (ORCID 0000-0001-9209-7724); Enrique Ovejero-Merino (ORCID 0000-0002-4254-3594); Ana Quiroga-Valcárcel (ORCID 0000-0001-5921-6920). Elche University General Hospital, Alicante: Luis Sánchez-Guillén (ORCID 0000-0003-0623-9074); Inmaculada Oller-Navarro (ORCID 0000-0003-0417-3489); Álvaro Soler-Silva (ORCID 0000-0002-5228-2746); Antonio Francisco Sanchís-López (ORCID 0000-0002-3421-1389). Complejo Asistencial Universitario de Salamanca: Francisco Blanco-Antona (ORCID 0000-0001-5946-9944); Luis Muñoz-Bellvis (ORCID 0000-0002-7709-5201); Jaime López-Sánchez (ORCID 0000-0002-4506-4951); Sonsoles Garrosa-Muñoz (ORCID 0000-0002-3496-5068); Beatriz Barón-Salvador (ORCID 0000-0002-8591-0278); Juan Manuel Nieto-Arranz (ORCID 0000-0002-6545-9790). Hospital Universitari Parc Taulí: Andrea Campos-Serra (ORCID 0000-0001-6970-7141); Raquel Gràcia-Roman (ORCID 0000-0003-2861-9270); Anna Muñoz-Campaña (ORCID 0000-0003-4136-3046); Carla Zerpa-Martin (ORCID 0000-0002-5731-409X); Andrea Torrecilla-Portoles (ORCID 0000-0002-6585-1944); Tessa Landa (ORCID 0000-0001-5611-4486). Virgen del Rocío University Hospital: Virginia Durán Muñoz-Cruzado (ORCID 0000-0003-4499-0483); Felipe Pareja-Ciuró (ORCID 0000-0001-9192-3465); Daniel Aparicio-Sánchez (ORCID 0000-0001-7061-345X); Eduardo Perea del Pozo (ORCID 0000-0003-3219-2601); Sandra Dios-Barbeito (ORCID 0000-0002-5944-1826); Carlos García-Sánchez (ORCID 0000-0001-7573-295X); Antonio Jesús García-Moriana (ORCID 0000-0002-4866-3511). Hospital Clinic Barcelona: Victor Turrado-Rodriguez (ORCID 0000-0002-0573-7373); Roser Termes-Serra (ORCID 0000-0002-6982-3860); Paula Gonzalez-Atienza (ORCID 0000-0003-1957-6774); Xavier Morales-Sevillano (ORCID 0000-0002-4514-007X); Alba Torroella (ORCID 0000-0002-1911-2446); César Ginestà (ORCID 0000-0002-5867-7503). Hospital Universitario Arnau de Vilanova: Alfredo Escartín (ORCID 0000-0002-5234-2366); Ferney Gomez (ORCID 0000-0002-0022-1327); Ana Pinillos (ORCID 0000-0003-2507-1318); Jaume Ortega (ORCID 0000-0001-9196-4689); Guillermo Lopez (ORCID 0000-0002-6368-5591); Eric Gutierrez (ORCID 0000-0001-9005-6523). Hospital Del Mar de Barcelona: Estela Membrilla-Fernandez; Francisco Ocho-Segarra; Ana María González-Castillo; Amalia Pelegrina-Manzano; Juan Guzmán-Ahumada; Juan Jose Sancho-Insenser. Complejo Hospitalario Universitario de A Coruña: María Lourdes García-Jiménez; Laura Castro-Diez; Manuel González-Bermúdez; Mónica Torres-Díaz; Carla Madarro Pena; Angélica Blanco Rodríguez. Sweden: Örebro University Hospital: Dhanisha Trivedi (ORCID 0000-0003-3875-5831); Souheil Reda (ORCID 0000-0001-6145-1233). Capio S:t Göran Hospital: Hans Edvardsson (ORCID 0000-0001-9511-9730); Lovisa Strömmer (ORCID 0000-0001-5424-7111). Sahlgrenska University Hospital: Eva-Corina Caragounis (ORCID 0000-0001-5637-2637); Karin Sillén (ORCID 0000-0003-2942-0810); Sofia Warfvinge. Sahlgrenska University Östra Hospital: Fredrik Bergstedt; Philip Enström; Harald Olsson; Anders Rosemar. Karolinska University Hospital: Nathalie Young (ORCID 0000-0002-4675-7581); Agnieszka Popowicz (ORCID 0000-0001-7960-0962); Johanna Lerström; Johanna Jäderbo (ORCID 0000-0002-0115-4242); Folke Hammarqvist (ORCID 0000-0001-9175-4534). Danderyds Hospital: Hanna Zacharias (ORCID 0000-0003-1180-0970). Karlstad Hospital: Maria B. Wikström (ORCID 0000-0001-8864-7068); Anna Stene Hurtsén (ORCID 0000-0003-0646-2508). Östersund County Hospital: Haytham Bayadsi (ORCID 0000-0002-4877-5150); Emma Jansson (ORCID 0000-0002-9944-5733); Nils Brunstrom (ORCID 0000-0002-0398-7328); Ellen B. Malers (ORCID 0000-0002-7148-3252). Linköping University Hospital: Per I. Loftås (ORCID 0000-0002-8289-3054); Anders Möller (ORCID 0000-0001-9608-3864); Elena Atanasova. Switzerland: Bern University Hospital, University of Bern: Simone N. Zwicky (ORCID 0000-0003-4465-6813); Beat Schnüriger (ORCID 0000-0002-1672-2775). United Kingdom: Aintree University Hospital: Olga Rutka (ORCID 0000-0002-8791-1987); Arjun T. Kattakayam (ORCID 0000-0002-7664-609X); Mushfique Alam (ORCID 0000-0001-9924-8791); John V. Taylor (ORCID 0000-0003-3059-9766). Tameside and Glossop Integrated Care NHSFT: Andrei Mihailescu (ORCID 0000-0002-4364-6042); Eszter T. Karip (ORCID 0000-0002-7610-1604); Ehtisham Zeb (ORCID 0000-0002-2947-2082); Adam O’Connor (ORCID 0000-0001-5267-2819); Goran Pokusevski (ORCID 0000-0003-3585-1860). Brighton and Sussex University Hospitals, Brighton: Mansoor Khan (ORCID 0000-0002-0496-7652); Charlotte Florance; Christie Swaminathan; Shameen Jaunoo; Mohammed Sajid. United States of America: University of Pennsylvania Hospital System, Philadelphia: Caoimhe C. Duffy (ORCID 0000-0001-6302-1683); John Rees (ORCID 0000-0003-4343-395X); Mark J. Seamon (ORCID 0000-0001-7536-5467); Niels D. Martin (ORCID 0000-0002-2157-0825); Ian J. McCurry (ORCID 0000-0002-9701-338X); Emily A. Vail (ORCID 0000-0002-1849-5780); Bradford C. Bormann (ORCID 0000-0001-7746-0249). Maine Medical Center: Daniel C. Cullinane (ORCID 0000-0002-0414-1949); Jaswin S. Sawhney (ORCID 0000-0003-3693-0229); Jonathan Dreifus; Forest R. Sheppard (ORCID 0000-0001-5461-2297). Riverside University Health System Medical Center: Raul Coimbra (ORCID 0000-0002-3759-6851); Paul Albini (ORCID 0000-0002-1115-2419); Sara Edwards.
Funding
Open access funding provided by Örebro University. No funding was received for the execution of the current study.
Author information
Authors and Affiliations
Consortia
Contributions
All collaborators will be listed on PubMed as authors; see end of manuscript for list of Manuscript Writing Group, SnapAppy Steering Committee and Study Collaborators, and their affiliations.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bass, G.A., Kaplan, L.J., Forssten, M.P. et al. Techniques for mesoappendix transection and appendix resection: insights from the ESTES SnapAppy study. Eur J Trauma Emerg Surg 49, 17–32 (2023). https://doi.org/10.1007/s00068-022-02191-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-022-02191-8