Abstract
Purpose
The purpose of the present prospective study was to evaluate the significance of geriatric conditions measured by a comprehensive geriatric assessment (GA) for the prediction of the risk of high-grade acute radiation-induced toxicity.
Methods
A total of 314 prostate cancer patients (age ≥ 65 years) undergoing definitive radiotherapy at a tertiary academic center were included. Prior to treatment, patients underwent a GA. High-grade toxicity was defined as acute toxicity grade ≥ 2 according to standard RTOG/EORTC criteria. To analyze the predictive value of the GA, univariable and multivariable logistic regression models were applied.
Results
A total of 40 patients (12.7%) developed acute toxicity grade ≥ 2; high grade genitourinary was found in 37 patients (11.8%) and rectal toxicity in 8 patients (2.5%), respectively. Multivariable analysis revealed a significant association of comorbidities with overall toxicity grade ≥ 2 (odds ratio [OR] 2.633, 95% confidence interval [CI] 1.260–5.502; p = 0.010) as well as with high-grade genitourinary and rectal toxicity (OR 2.169, 95%CI1.017–4.625; p = 0.045 and OR 7.220, 95%CI 1.227–42.473; p = 0.029, respectively). Furthermore, the Activities of Daily Living score (OR 0.054, 95%CI 0.004–0.651; p = 0.022), social status (OR 0.159, 95%CI 0.028–0.891; p = 0.036), and polypharmacy (OR 4.618, 95%CI 1.045–20.405; p = 0.044) were identified as independent predictors of rectal toxicity grade ≥ 2.
Conclusion
Geriatric conditions seem to be predictive of the development of high-grade radiation-induced toxicity in prostate cancer patients treated with definitive radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is predominantly a disease of older adults with a median age at diagnosis of 68 years [1]. With the exponential aging of the population and increasing life expectancy, especially in developed countries, the burden due to prostate cancer is expected to increase substantially in the future.
External beam radiotherapy represents a highly efficacious treatment modality for prostate cancer and offers a particular advantage in patients who are unsuitable for surgery because of comorbidity or evidence of extraprostatic extension of the cancer. However, management of PCa is often challenging because, even without treatment, disease progression can occur slowly in many patients but it has also been demonstrated that death as a result of PCa increases with age, despite increasing death rates from competing causes [2].
Life-expectancy is a major determinant of the potential to benefit from curative therapy. In prostate cancer, predicted life expectancy has directly been incorporated into treatment guidelines [3]. However, life expectancy varies substantially between individuals within a given age group [4]. Thus, advanced age alone should not preclude effective treatment for prostate cancer but it is necessary to assess the risks and benefits of treatment in elderly patients to avoid interventions that might decrease health-related quality of life without prolonging survival.
The geriatric assessment (GA) is defined as a multidimensional diagnostic process, focusing on determining an older person’s medical, psychosocial, and functional capabilities to objectively appraise the health status of older people in order to develop a coordinated and integrated plan for treatment and long-term follow-up [5]. GA-guided treatment plans have been shown to improve overall survival (OS), quality of life, physical function, and decrease the risk of hospitalization [6, 7]. The available data also support the value of GA as an effective tool to predict the patient’s tolerance of cytotoxic interventions [8]. Most previous studies focused on the prediction of chemotherapy toxicity and showed that the factors most consistently associated with toxicity were functional status and comorbidity [9,10,11,12,13,14]. Other identified risk factors were cognitive deficiencies, lack of social support, poor mood status, falls, and nutritional status [9, 15]. Other publications also showed a correlation between impairments measured with GA tools and risk of premature termination of cancer treatment [13, 16,17,18,19].
Geriatric conditions are also likely to influence analogous facets of radiation treatment, specifically the patient’s ability to complete the intended radiation treatment duration and the ability to tolerate radiotherapy-related side-effects. Thus, implementation of GA might be an effective tool for the identification of older adults who are at high risk of radiotherapy complications. However, to date, little is known about the ability of a GA to predict toxicity in elderly cancer patients undergoing curative radiotherapy [20,21,22,23].
The aim of the present study was to identify geriatric conditions measured by GA that are predictive of the development of high-grade radiation-induced toxicities in a cohort of prostate cancer patients treated with definitive radiotherapy.
Materials and methods
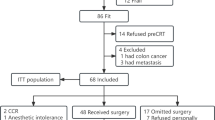
This single-center prospective cohort study was performed including 314 prostate cancer patients treated at the tertiary academic center.
Patients were eligible for inclusion if aged 65 years or above, candidates to definitive radiation treatment and provided written informed consent. At inclusion in the cohort, all study participants completed a routine clinical questionnaire including family history, medication, previous surgery, comorbidities, and smoking habits.
Before initiation of the radiotherapy, a comprehensive GA was completed. The measures included in the GA were chosen for their reliability, validity, brevity, and prognostic ability to determine risk for morbidity or mortality in older patients. The GA tools are summarized in Table 1 and comprised a health care provider and a patient portion. The health care provider portion consisted of two items: Timed Up and Go Test (TUG) and the Mini-Mental State Examination (MMSE). The patient portion consisted of self-reported measures of functional status, nutrition, social support/function, mood, comorbidity and medications. A member of the radiation oncologist team assisted those who needed help with completing the questionnaires; the assessments were performed under the supervision of a geriatric oncologist.
Image-guided radiotherapy with high energy photons was generally performed using volumetric intensity-modulated arc therapy (VMAT) techniques to encompass the prostate and seminal vesicles. The total dose, prescribed to the International Commission on Radiation Units and Measurement point, ranged from 74–78 Gy delivered in 2 Gy per fraction dependent on risk situation. Hypofractionated radiotherapy with a total dose of 60 Gy (3 Gy per single fraction) was delivered in 13 patients. Treatment was performed daily, 5 days/week.
In patients with low risk disease, the clinical target volume (CTV) encompassed the prostate. In intermediate and high-risk disease, the CTV included the prostate and 75% of the seminal vesicles (SV), in case of SV involvement, the entire seminal vesicles were included. The planning target volume (PTV) was defined as the CTV with a margin of 7 mm in all dimensions, except for the posterior aspect (prostate–rectal interface), where the margin was 5 mm. Pelvic lymph node irradiation was performed in 7 patients with clinical lymph node involvement.
The rectum was segmented from above the anal verge to the turn into the sigmoid colon, including the rectal contents. The dose–volume constraints were defined according to the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) recommendations [24, 25]. In patients treated with conventionally fractionated radiotherapy, the following dose constraints had to be fulfilled: V50 < 50%, V60 < 35%, V65 < 25%, V70 < 20%, and V75 < 10%. The urinary bladder was outlined as entire organ. The bladder dose constraints to be fulfilled were as follows: V50 < 50%, V60 < 40%, V70 < 25%.
The dose constraints for the rectum and bladder in patients treated with hypofractionated radiotherapy were derived from the CHHIP trial [26].
Acute genitourinary and gastrointestinal toxicity was regularly assessed during the radiation therapy course and graded according to standard European Organisation for Research and Treatment of Cancer (EORTC)/Radiation Therapy Oncology Group (RTOG) criteria.
The study complied with the Declaration of Helsinki and was performed according to the national law. The protocol has been approved by the local Ethical Committee (approval number: EK 31-437 ex 18/19). Written informed consent was obtained from all participants.
The primary endpoint was the development of high-grade acute genitourinary or rectal toxicity that was defined as acute toxicity grade ≥ 2 according to standard RTOG/EORTC criteria. Comparison of groups was done using student’s t‑test, rank sum test, χ2 test, and analysis of variance (ANOVA). The relationship between GA results and age was studied by nonparametric tests. The association of GA variables with acute toxicity was assessed using univariable logistic regression analysis. The variables that reached a p-value of less than 0.1 were further examined in a multivariable logistic regression model that adjusted GA variables to relevant clinical or treatment related factors. P-values < 0.05 were considered statistically significant. Statistical analyses were carried out using SPSS 28.0 for Windows (IBM, Armonk, NY, USA).
Results
Baseline patient characteristics
The study cohort consisted of 314 prostate cancer patients aged ≥ 65 years. Median age at diagnosis was 74 years (mean 73.5 ± 5.59 years). A total of 28 patients (8.9%) presented with low-risk prostate cancer, 139 patients (44.3%) with intermediate-risk cancer, and 147 patients (46.8%) with high-risk prostate cancer. Neoadjuvant ADT was administered in 294 patients (93.6%). Patient and treatment characteristics are summarized in Table 2.
Results of comprehensive geriatric assessments
Comprehensive GA was performed in 282 patients; polypharmacy and comorbidities were recorded in 289 and 295 patients, respectively. The mean score on the Activities of Daily Living (ADL) scale was 99.04 ± 7.742; a score ≤ 90 indicating at least moderate dependency was detected in 6 patients (1.9%). The Instrumental Activities of Daily Living (IADL) scale had a mean score of 7.75 ± 0.874, and 18 patients (7.5%) had a score < 7, indicating decreased function. The mean time required for the Timed Up and Go test (TUG) was 10.54 ± 2.8 and 58 patients (18.5%) had reduced mobility as defined by a TUG > 10. The Mini Nutritional Assessment (MNA) scale had a mean value of 25.57 ± 1.825 and only 5 patients (1.6%) were at risk of malnutrition with an MNA score < 24.
The mean value on the Social Status and Support scale (SOS) was 23.05 ± 1.881 with a score ≤ 22 indicating a slightly higher need for social support detected in 82 patients (26.1%). Cognitive disorders defined as a score < 26 on the Mini-Mental State Examination (MMSE) were detected in 14 patients, a Geriatric Depression Scale (GDS) 15 score > 3 indicating a higher level of depression and anxiety was found in 57 patients (18.2%).
Polypharmacy defined as the intake of at least 6 medications was observed in 18.2% of patients, furthermore, the Charlson Comorbidity Index (CCI) revealed comorbidity in 31.5% of patients. The results of the geriatric assessment are displayed in Table 3.
Correlation between geriatric assessment results and age
We detected a significant association of the IADL score (p = 0.006) and the TUG result (p = 0.006) with age indicating a decreased function and mobility with increasing age. We also found a significant correlation between the GDS score and age (p = 0.026) suggesting a higher level of depression and anxiety among patients aged > 80 years. For the remaining geriatric assessment results, significant associations with age were not detected.
Analysis of radiation-induced acute toxicity
Acute genitourinary and/or rectal toxicity grade ≥ 2 was detected in 40 patients (12.7%), acute genitourinary toxicity grade ≥ 2 was found in 37 patients (11.8%) and acute rectal toxicity grade ≥ 2 in 8 patients (2.5%), respectively. The association of baseline patient and treatment characteristics with acute radiation-induced toxicity is shown in Table 5.
Association between geriatric parameters and radiation-induced acute toxicity
Univariable analysis showed a significant association between the presence of comorbidities and the development of acute rectal and/or genitourinary toxicity grade ≥ 2 (OR 2.269, 95%CI 1.130–4.557; p = 0.021). Regarding the relationship between GA variables and the development of acute genitourinary toxicity grade ≥ 2, a trend for increased toxicity in case of the presence of comorbidities was detected (OR 1.842, 95%CI 0.894–3.794; p = 0.098). Furthermore, univariable analysis revealed a significant relationship between the results on the SOS and the MMSE with rectal toxicity grade ≥ 2 (OR 0.156, 95%CI 0.030–0.823; p = 0.029 and OR 0.114, 95%CI 0.020–0.647; p = 0.014). A significant association between the intake of > 6 medications and the risk of rectal toxicity grade ≥ 2 was also detected (OR 4.415, 95%CI 1.070–18.222; p = 0.040). Furthermore, we observed a marginally significant association of reduced functionality represented by an ADL score ≤ 90 and the presence of comorbidities with acute rectal toxicity grade ≥ 2 (OR 0.111, 95%CI 0.011–1.102; p = 0.061 and OR 5.000, 95%CI 0.952–26.255; p = 0.057, respectively).
In multivariable analysis, GA results associated with toxicity grade ≥ 2 in univariable analysis (p < 0.1) were adjusted to variables deemed to be of clinical importance (age, smoking status, risk group, radiation fractionation, radiation dose, and pelvic node irradiation).
In multivariable analysis, the pretreatment CCI remained a significant predictor of acute genitourinary and/or rectal toxicity grade ≥ 2 (OR 2.633, 95%CI 1.260–5.502; p = 0.010), in addition, the CCI was significantly associated with acute genitourinary toxicity grade ≥ 2 (OR 2.169, 95%CI 1.017–4.625; p = 0.045) as well as with rectal toxicity grade ≥ 2 (OR 7.220, 95%CI 1.227–42.473; p = 0.029).
Furthermore, multivariable analysis revealed a significant association of the ADL score (OR 0.054, 95%CI 0.004–0.651; p = 0.022) with the risk of rectal toxicity grade ≥ 2. In addition, the score on the SOS scale (OR 0.159, 95%CI 0.028–0.891; p = 0.036) and polypharmacy (OR 4.618, 95%CI 1.045–20.405; p = 0.044) remained significant predictors of acute rectal toxicity grade ≥ 2 in multivariable analysis, whereas for the pretreatment MMSE, a marginally significant association with acute rectal toxicity grade ≥ 2 was detected (OR 2.144, 95%CI 0.019–1.083; p = 0.060). The results of uni- and multivariable analyses of the associations between geriatric parameters and radiation-induced acute toxicity are displayed in Table 6.
Discussion
Since chronological age alone is not an adequate indicator of the diverse aging process, a comprehensive assessment of older individuals’ medical, psychological, and functional abilities has become increasingly recognized as a means of distinguishing between those elderly patients who are good candidates for standard cancer treatment and those who are too vulnerable to tolerate aggressive therapies.
In this prospective observational study, we evaluated the usefulness of a comprehensive GA in predicting radiation-induced acute side effects in a group of prostate cancer patients over the age of 65 who received definitive radiotherapy. Our analysis revealed significant correlations between the incidence of acute radiation-induced toxicity and factors such as comorbidities, functional capacity, social standing, and polypharmacy.
In addition, we explored the occurrence of geriatric disorders across various age categories (< 70, 70–80, and > 80 years) and identified significant distinctions in measures of functional capacity (IADL and TUG) and mental state (GDS) across these age groups. These results imply that functionality tends to decrease and the likelihood of depression tends to increase with age. However, no significant differences between age groups were found in the other domains of geriatric assessment. These findings underscore the fact that age alone is inadequate for characterizing a patient’s medical, psychosocial, and functional abilities [27, 28].
However, it is well-established that geriatric patients are particularly vulnerable to toxicity due to age-related decline and heightened sensitivity to toxic exposures and it is essential to identify predictive factors for treatment response and toxicity.
Previous studies have primarily focused on the predictive role of a comprehensive GA for chemotherapy toxicity and have revealed that functional capacity and comorbidities were the factors most frequently linked with toxicity [11, 12, 29, 30]. For instance, in a study by Hurria et al. that analyzed risk factors for the toxicity of chemotherapy in geriatric cancer patients aged 65–91 years, a need for assistance in activities of daily living such as mobility, housework, and medication intake and also reduced mobility in walking test correlated with treatment-related side effects [31]. In addition, a systematic review and meta-analysis of 13 studies by Edwards et al. revealed that patients with comorbidities had a higher risk of experiencing severe chemotherapy-induced toxicity than those without comorbidities [32]. Furthermore, cognitive impairment, social dependency, and depression have been found to represent predictors of chemotherapy-related side effects [12, 33,34,35]. Previous studies have also developed predictive models for chemotherapy toxicity in older adults with cancer that incorporate geriatric assessment variables. For example, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) and the Cancer Aging Research Group (CARG) toxicity score have both been shown to represent reliable predictors of chemotherapy toxicity and overall survival in older cancer patients [8, 12, 26, 36].
To date, evidence is scarce on what type of GA tools or predictors of health status can be employed to anticipate the risks of radical prostatectomy in older males. Previous studies that have investigated the relationship between frailty and complication rates among patients undergoing radical prostatectomy have revealed an increased risk of overall and major complications in frail patients [37, 38]. Patients with elevated frailty scores are prone to experience higher rates of wound disruptions, bleeding transfusions, and 30-day mortality [34]. Nevertheless, further research is necessary to ascertain the specific clinical tools that can directly predict the surgical tolerability of elderly men.
In the present study, the pretreatment CCI was significantly associated with the risk of high-grade rectal and genitourinary side effects. Moreover, our findings revealed that ADL, social status, and polypharmacy were significantly linked to rectal toxicity, and we also observed a trend suggesting a higher risk of rectal toxicity among patients with cognitive impairment.
The results of our study can be attributed to the assumption that the presence of comorbidities indicates inflammatory processes or microvascular alterations that negatively influence the recuperative capacity of healthy tissues and result in heightened toxicity. Additionally, the observed trend towards a heightened risk of rectal side effects among patients with cognitive impairments supports to the hypothesis of a link with vascular changes, which are also prevalent in individuals with dementia. ADL and social status serve as measures of individual independence in performing daily tasks. For patients with difficulties in performing basic activities and those with unfulfilled social requirements, it might be challenging to adhere to recommended diets and other supportive measures. This group of patients may also subjectively experience a greater degree of discomfort from gastrointestinal symptoms compared to those who do not face similar limitations in their daily lives. Polypharmacy is a widely acknowledged risk factor for heightened toxicity among older patients receiving cancer treatment [39]. The consumption of numerous medications may heighten the susceptibility of healthy tissues to radiation exposure; moreover, certain drugs are known to cause gastrointestinal reactions, which, when coupled with an additional irritating factor, such as ionizing radiation, can lead to the manifestation of drug-related side effects that were not clinically apparent prior to the radiotherapy.
To date, data on the role of a CGA for the prediction of radiation-induced toxicity are sparse. Goineau et al. aimed to identify predictors of impaired quality of life (QoL) in men aged 75 years or older who underwent curative radiotherapy with or without androgen deprivation therapy (ADT) for localized prostate cancer. Comprehensive GA and QoL questionnaires were administered to 208 elderly prostate cancer patients. However, none of the parameters studied, including tumor characteristics, treatment, or oncogeriatric parameters, were predictive of a decrease in QoL following radiotherapy [40]. DeVries et al. investigated whether GA items or frailty screening instruments could predict the likelihood of acute toxicity in 160 head and neck cancer patients undergoing radiation therapy and also did not find any significant association between frailty or geriatric parameters and the risk of acute toxicity [22]. In contrast, Ulger et al. detected a significant correlation between lower gait speed and emesis when evaluating the predictive value of a comprehensive GA for radiation therapy toxicity and tolerability in 30 geriatric cancer patients with a mean age of 70 years [41].
In the present prospective study, we performed a comprehensive and systematic analysis of the predictive role of geriatric parameters. To the best of our knowledge, our study cohort is the largest to date that has examined the correlation between the GA and the risk of radiation-induced toxicity. It is also characterized by a high degree of homogeneity in terms of patient, tumor, and treatment characteristics. In addition, we focused on the predictive value of the CGA for grade ≥ 2 side effects, which are regarded as clinically significant adverse events.
The integration of a comprehensive GA in treatment planning and follow-up has been associated with improved overall survival, quality of life, and physical function [42,43,44]. In prostate cancer radiotherapy, the pretherapeutic GA may assist in determining the appropriate fractionation schedule or target volume. For instance, in patients who are particularly vulnerable, the GA might indicate the omission of elective lymph node irradiation or avoiding fractionation schedules that are known to result in higher toxicity rates.
A potential drawback of our study is a potential selection bias, whereby certain vulnerable patients who were deemed eligible for radiation therapy may have declined to participate due to their overall diminished performance status. Moreover, unaccounted factors such as prostate volume and drug treatment such as alpha-blocker administration may have influenced the occurrence of side effects. Furthermore, in patients undergoing hypofractionated radiotherapy, acute side effects are frequently observed following treatment completion, so that possibly acute side effects were not fully captured in this subgroup of patients. In addition, detailed analyses of dose–volume parameters were beyond the scope of the present study. However, due to our strict dose constraints, we were able to achieve a high level of homogeneity regarding the dose–volume parameters in our study population. It has also to be taken into account that a dose–volume histogram of the bladder or rectum obtained from a single planning computed tomography is unlikely to represent the true dose distribution delivered to the bladder or rectum during the treatment course.
Nevertheless, validation of our data in additional prospective large-scale studies is imperative before firm conclusions about the effectiveness of geriatric assessment in predicting radiation-induced toxicity in prostate cancer can be drawn.
In conclusion, the significant correlation we observed between comorbidities, functionality, social status, and polypharmacy and the risk of developing acute radiation-induced side effects supports the hypothesis that the GA may serve as a useful tool in predicting radiotherapy toxicity in elderly cancer patients. If confirmed by additional studies, the GA could contribute to the identification of patients at high risk of adverse events, leading to individualized treatment plans that can minimize toxicity in elderly cancer patients.
References
Chin HW, Kim J, Rasp G, Hristov B (2015) Prostate Cancer in Seniors. Fed Pract 32(Suppl 4):41–44
Scosyrev E, Messing EM, Mohile S, Golijanin D, Wu G (2012) Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer 118:3062–3070. https://doi.org/10.1002/cncr.26392
Droz JP, Aapro M, Balducci L et al (2014) Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol 15:e404–e414
Walter LC, Covinsky KE (2001) Cancer screening in elderly patients. JAMA 285:2750. https://doi.org/10.1001/jama.285.21.2750
Extermann M, Aapro M, Bernabei R et al (2005) Use of comprehensive geriatric assessment in older cancer patients. Crit Rev Oncol Hematol 55:241–252. https://doi.org/10.1016/j.critrevonc.2005.06.003
Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D (2011) Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006211.pub2
Cohen HJ, Feussner JR, Weinberger M et al (2002) A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med 346:905–912. https://doi.org/10.1056/NEJMsa010285
Cavdar E, Iriagac Y, Karaboyun K, Avci O, Seber ES (2022) Prospective comparison of the value of CARG, G8, and VES-13 toxicity tools in predicting chemotherapy-related toxicity in older Turkish patients with cancer. J Geriatr Oncol 13:821–827. https://doi.org/10.1016/j.jgo.2022.03.004
Aparicio T, Gargot D, Teillet L et al (2017) Geriatric factors analyses from FFCD 2001-02 phase III study of first-line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 74:98–108. https://doi.org/10.1016/j.ejca .2016.09.029
Pilnik NG, Ibero MM, Carri D, Mareca O (2010) Influence of some clinical parameters in the tolerance to treatment in elderly patients with advanced lung cancer. J Clin Oncol 28:e19600–e19600. https://doi.org/10.1200/jco.2010.28.15_suppl.e19600
Shin DY, Lee JO, Kim YJ et al (2012) Toxicities and functional consequences of systemic chemotherapy in elderly Korean patients with cancer: a prospective cohort study using comprehensive geriatric assessment. J Geriatr Oncol 3:359–367. https://doi.org/10.1016/j.jgo.2012.06.002
Extermann M, Boler I, Reich RR et al (2012) Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age patients (CRASH) score. Cancer 118:3377–3386. https://doi.org/10.1002/cncr.26646
Marinello R, Marenco D, Roglia D et al (2009) Predictors of treatment failures during chemotherapy: a prospective study on 110 older cancer patients. Arch Gerontol Geriatr 48:222–226. https://doi.org/10.1016/j.archger.2008.01.011
Feliu J, Jiménez-Munárriz B, Basterretxea L et al (2020) Predicting chemotherapy toxicity in older patients with cancer: a multicenter prospective study. Oncologist 25:e1516–e1524. https://doi.org/10.1634/theoncologist.2019-0701
Biesma B, Wymenga ANM, Vincent A et al (2011) Quality of life, geriatric assessment and survival in elderly patients with non-small-cell lung cancer treated with carboplatin–gemcitabine or carboplatin–paclitaxel: NVALT‑3 a phase III study. Ann Oncol 22:1520–1527. https://doi.org/10.1093/annonc/mdq637
Wymenga M, Biesma B, Vincent A, Dalesio O, Groen H (2009) Platinum-based combination chemotherapy in the treatment of older non-small cell lung cancer (NSCLC) patients (pts): Is there a role for Complete Geriatric Assessment (CGA)? Final results from the prospective multicenter NVALT-3 study. J Clin Oncol 27:e20547–e20547. https://doi.org/10.1200/jco.2009.27.15_suppl.e20547
Wymenga M, Biesma B, Vincent A et al (2007) Can baseline complete geriatric assessment (CGA) predict toxicity in elderly non-small cell lung cancer (NSCLC) patients (pts) receiving combination chemotherapy? Results from the first 100 pts in the prospective multicenter NVALT-3 study. J Clin Oncol 25(18_suppl):7537–7537. https://doi.org/10.1200/jco.2007.25.18_suppl.7537
Bang HJ, Shim HJ, Kim GR et al (2022) Geriatric functional assessment for decisionmaking on adjuvant chemotherapy in older colon cancer patients. Korean J Intern Med 37:660–672. https://doi.org/10.3904/kjim.2021.324
Spyropoulou D, Pallis AG, Leotsinidis M, Kardamakis D (2014) Completion of radiotherapy is associated with the vulnerable elders survey-13 score in elderly patients with cancer. J Geriatr Oncol 5:20–25. https://doi.org/10.1016/j.jgo.2013.08.002
Osborne GEC, Appleyard SA, Gilbert DC et al (2017) Comprehensive geriatric assessment in men aged 70 years or older with localised prostate cancer undergoing radical radiotherapy. Clin Oncol 29:609–616. https://doi.org/10.1016/j.clon.2017.05.003
VanderWalde NA, Deal AM, Comitz E et al (2016) Comprehensive geriatric assessment as a predictor of tolerance, quality of life, and toxicity in older patients receiving radiation. Int J Radiat Oncol Biol Phys 94:885. https://doi.org/10.1016/j.ijrobp.2015.12.070
de Vries J, Poelman A, Sidorenkov G et al (2022) The association of frailty and outcomes of geriatric assessment with acute radiation-induced toxicity in patients with head and neck cancer. Oral Oncol. https://doi.org/10.1016/j.oraloncology.2022.105933
Szumacher E, Sattar S, Neve M et al (2018) Use of comprehensive geriatric assessment and geriatric screening for older adults in the radiation oncology setting: a systematic review. Clin Oncol 30:578–588. https://doi.org/10.1016/j.clon.2018.04.008
Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU (2010) Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys 76(3 Suppl):S116–S122. https://doi.org/10.1016/j.ijrobp.2009.02.090
Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO (2010) Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 76(3 Suppl):S123–S129. https://doi.org/10.1016/j.ijrobp.2009.03.078
Dearnaley D, Syndikus I, Mossop H et al (2016) Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5‑year outcomes of the randomised, non-inferiority, phase 3 CHhiP trial. Lancet Oncol 17(8):1047–1060. https://doi.org/10.1016/S1470-2045(16)30102-4
Mayer A, Wenzel W, Wollschläger D, Bostel T, Krüger M, Matthias C, Schmidberger H (2022) Adjuvant chemoradiotherapy in elderly patients with head and neck cancer: a monoinstitutional, two-to-one pair-matching analysis. Strahlenther Onkol 198:159–170. https://doi.org/10.1007/s00066-021-01890-2
Sprave T, Verma V, Fabian A, Rühle A, Baltas D, Grosu AL, Nicolay NH (2022) Cost effectiveness and health-related quality of life of chemoradiotherapy versus radiation therapy alone in elderly head and neck cancer patients. Strahlenther Onkol 198:1008–1015. https://doi.org/10.1007/s00066-022-01975-6
Zhang J, Liao X, Feng J, Yin T, Liang Y (2019) Prospective comparison of the value of crash and carg toxicity scores in predicting chemotherapy toxicity in geriatric oncology. Oncol Lett 18:4947–4955. https://doi.org/10.3892/ol.2019.10840
Feliu Batlle J, Basterretxea L, Torregrosa MD et al (2019) Predictive factors of grade 3–5 toxicity in older patients with cancer treated with chemotherapy: a prospective multicenter study. J Clin Oncol 37:11509–11509. https://doi.org/10.1200/JCO.2019.37.15_suppl.11509
Hurria A, Togawa K, Mohile SG et al (2011) Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 29:3457–3465. https://doi.org/10.1200/JCO.2011.34.7625
Edwards MJ, Campbell ID, Lawrenson RA, Kuper-Hommel MJ (2017) Influence of comorbidity on chemotherapy use for early breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 165:17–39. https://doi.org/10.1007/s10549-017-4295-4
Puts MTE, Hardt J, Monette J, Girre V, Springall E, Alibhai SMH (2012) Use of geriatric assessment for older adults in the oncology setting: a systematic review. JNCI J Natl Cancer Inst 104:1134–1164. https://doi.org/10.1093/jnci/djs285
Puts MTE, Santos B, Hardt J et al (2014) An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 25:307–315. https://doi.org/10.1093/annonc/mdt386
Freyer G, Geay JF, Touzet S et al (2005) Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol 16:1795–1800. https://doi.org/10.1093/annonc/mdi368
Mittal A, Rangaraju RR, Agarwal A, Chandragouda D, Batra S, Qureshi S (2021) Estimating the risk of chemotherapy toxicity in Indian geriatric patient population and utility of chemotherapy risk assessment scale for high age patients (CRASH) score. South Asian J Cancer 10:161–166. https://doi.org/10.1055/s-0041-1729447
Levy I, Finkelstein M, Bilal KH, Palese M (2017) Modified frailty index associated with Clavien-Dindo IV complications in robot-assisted radical prostatectomies: a retrospective study. Urol Oncol 35:425–431. https://doi.org/10.1016/j.urolonc.2017.01.005
Rosiello G, Palumbo C, Knipper S et al (2020) Preoperative frailty predicts adverse short-term postoperative outcomes in patients treated with radical prostatectomy. Prostate Cancer Prostatic Dis 23:573–580. https://doi.org/10.1038/s41391-020-0225-3
Mohamed MR, Mohile SG, Juba KM et al (2023) Association of polypharmacy and potential drug-drug interactions with adverse treatment outcomes in older adults with advanced cancer. Cancer 129:1096–1104. https://doi.org/10.1002/cncr.34642
Goineau A, Campion L, d’Aillières B et al (2018) Comprehensive geriatric assessment and quality of life after localized prostate cancer radiotherapy in elderly patients. PLoS ONE 13:e194173. https://doi.org/10.1371/journal.pone.0194173
Ulger S, Kizilarslanoglu MC, Kilic MK et al (2015) Estimating radiation therapy toxicity and tolerability with comprehensive assessment parameters in geriatric cancer patients. Asian Pac J Cancer Prev 16:1965–1969. https://doi.org/10.7314/APJCP.2015.16.5.1965
Paillaud E, Brugel L, Bertolus C et al (2022) Effectiveness of geriatric assessment-driven interventions on survival and functional and nutritional status in older patients with head and neck cancer: a randomized controlled trial (EGeSOR). Cancers (Basel) 14:3290. https://doi.org/10.3390/cancers14133290
Chen Z, Ding Z, Chen C et al (2021) Effectiveness of comprehensive geriatric assessment intervention on quality of life, caregiver burden and length of hospital stay: a systematic review and meta-analysis of randomised controlled trials. BMC Geriatr 21:377. https://doi.org/10.1186/s12877-021-02319-2
Briggs R, McDonough A, Ellis G, Bennett K, O’Neill D, Robinson D (2022) Comprehensive geriatric assessment for community-dwelling, high-risk, frail, older people. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012705.pub2
Katz S (1983) Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31:721–727. https://doi.org/10.1111/j.1532-5415.1983.tb03391.x
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x
Guigoz Y, Vellas B, Garry PJ (1994) Mini nutritional assessment: a practical assessment tool for grading the nutritional state of elderly patients. Facts Res Gerontol 4(Suppl 2):15–59
Nikolaus T, Specht-Leible N, Bach M, Oster P, Schlierf G (1994) Soziale Aspekte bei Diagnostik und Therapie hochbetagter Patienten. Erste Erfahrungen mit einem neu entwickelten Fragebogen im Rahmen des geriatrischen Assessment. Z Gerontol 27:240–245
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Yesavage JA, Sheikh JI (1986) 9/Geriatric Depression Scale (GDS). Clin Gerontol 5(1–2):165–173
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Funding
Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Paal, B. Stranz, E.-M. Thurner, U. Langsenlehner, W. Renner, T.B. Brunner and T. Langsenlehner declare that they have no competing interests.
Additional information
Data Availability Statement for this Work
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paal, K., Stranz, B., Thurner, EM. et al. Comprehensive geriatric assessment predicts radiation-induced acute toxicity in prostate cancer patients. Strahlenther Onkol 200, 208–218 (2024). https://doi.org/10.1007/s00066-023-02132-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02132-3