Abstract
Purpose
To review existing scientific literature on mobile applications (apps) in the field of radiation oncology and to evaluate characteristics of commercially available apps across different platforms.
Methods
A systematic review of the literature for publications presenting apps in the field of radiation oncology was carried out using the PubMed database, Cochrane library, Google Scholar, and annual meetings of major radiation oncology societies. Additionally, the two major marketplaces for apps, App Store and Play Store, were searched for available radiation oncology apps for patients and health care professionals (HCP).
Results
A total of 38 original publications which met the inclusion criteria were identified. Within those publications, 32 apps were developed for patients and 6 for HCP. The vast majority of patient apps focused on documenting electronic patient-reported outcomes (ePROs). In the two major marketplaces, 26 apps were found, mainly supporting HCP with dose calculations.
Conclusion
Apps used in (and for) scientific research in radiation oncology are rarely available for patients and HCP in common marketplaces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smartphones have revolutionized people’s lives, including the way they seek medical information [1]. In 2020, 78% of the worldwide population was in possession of a smartphone and the forecast predicts a further increase in the future [2]. There is a wide variety of health care applications (apps) for smartphones available. The top two categories are wellness management/fitness and disease management apps, whereas other categories include self-diagnosis, medication reminders, and electronic patient portal apps [1]. The World Health Organization (WHO) classifies such tools under the label mHealth or eHealth, and defines them as “medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices” [3].
Cancer is a leading cause of disability and mortality worldwide [4]. Accordingly, a variety of cancer-focused apps exist and the number of research articles that study the use of apps in the field of general/clinical oncology is increasing [5]. The majority of health care professionals (HCP) are in favor of the use of oncological apps by patients according to Kessel et al. [6]. Moreover, the acceptance of mobile apps for the surveillance and follow-up of cancer patients undergoing radiotherapy is high [7, 8]. Indeed, there are several advantages like monitoring patient-reported outcomes (ePRO) and education of patients to increase compliance and optimize the HCP interaction. In addition, these applications can have positive psychologic effects by empowering patients, e.g., to track treatment, monitor side effects, or to schedule follow-up appointments [9].
Despite the large selection of apps in the field of oncology in general and the resonance in the scientific literature, little is known about their particular impact and use in the field of radiation oncology. Therefore, this review aims to summarize data on the scientific reception of apps in radiation oncology in the literature and to gain information on currently available apps for patients and HCP online.
Methods
A systematic review of the literature in the PubMed database and Cochrane Library was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10] in August 2022 using the terms (“radiotherapy” OR “radiation oncology” OR “radio-oncology”) AND (“smartphone app” OR “mobile application” OR “app”).
To improve the retrieval rate of studies, the reference sections of eligible articles were additionally screened. Moreover, Google Scholar was searched using the identical search terms. To further enhance search output, a manual search was conducted within the table of contents of annual meetings of the European Society for Radiotherapy and Oncology (ESTRO), the German Society of Radiation Oncology (DEGRO), and the American Society of Radiation Oncology (ASTRO), including oral and (e-) poster contributions (past 10 years). Furthermore, ClinicalTrials.gov was searched using the same search terms to detect ongoing trials (search field: “all studies” and “other terms”).
Inclusion and exclusion criteria were defined before the search. Inclusion criteria were as follows:
-
1.
Original articles in English or German language.
-
2.
Presentation of a mobile app for HCP working in the field of radiation oncology or patients being treated with radiotherapy (RT; in cases of mixed cohorts, the majority of patients had to be involved in RT; in cases of a concomitant use of web-based tools and smartphone apps, e.g., for ePRO collection, the majority had to be smartphone apps).
Exclusion criteria were:
-
1.
Review articles.
-
2.
Surveys.
-
3.
Apps aimed at general/clinical oncology, without a focus on RT.
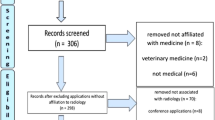
The respective PRISMA flowchart is given in Fig. 1.
Concomitantly, a search was conducted in the two major app stores to find commercially available smartphone apps. These two app stores were the Play Store (Android, Google; https://play.google.com/store) and App Store (iOS, Apple; https://www.apple.com/de/app-store). Search terms were “radiotherapy,” “radiation oncology,” and “radiation therapy.” The search was carried out in August 2022 independently by two authors (S.J. and L.K.) using an iPhone 13 and a Samsung Galaxy S10. The respective PRISMA flowchart is given in Fig. 2.
Results
Review of scientific literature
In total, 38 publications meeting the inclusion criteria were found (Table 1, 2 and 3). Different publications by the same study group concerning the same or mostly the same study cohort (e.g., study protocol, publication of preliminary and final results) were merged. Publications were categorized mainly in two different categories: apps developed for HCP (6 of 38, 16%) and apps developed for patients receiving RT or radiochemotherapy (RCT; 32 of 38, 84%). The latter publications were subdivided according to the patients’ underlying primary tumors: a mixed cohort of cancer patients receiving RT or RCT was the basis for 15 studies, followed by apps for head and neck cancer patients (n = 10), lung cancer patients (n = 3), breast cancer patients (n = 2), esophageal cancer patients (n = 1), and prostate cancer patients (n = 1). Two studies focused on palliative patients [11, 12], while the majority set a focus on definitive or adjuvant RT/RCT in a curative setting. Most of the studies were designed as single-arm studies testing the feasibility of a certain app or were only descriptive in nature (n = 26). Seven studies had a two-arm design (mostly randomized) [13,14,15,16,17,18,19].
Most patient-centered apps (n = 22) focused on ePROs during or/and after RT. This included recording of all types of RT-related side effects and symptoms like pain, general performance, and quality of life. Sometimes validated questionnaires like the EORTC (European Organisation for Research and Treatment of Cancer) QLQ C30 were used [13, 14, 20,21,22]; in other studies, this was not stated in detail. Side effects were usually evaluated concerning the irradiated area, e.g., swallowing difficulties, dryness of mouth, skin reaction, and mucositis in head and neck cancer patients. The time period of ePRO data collection was either during RT (n = 12), during follow-up (n = 8), or in both periods (n = 10).
Although not clearly stated in every publication, in seven ePRO-centered studies, the possibility of direct contact with a specialized HCP was provided beyond the general contact form.
Three apps supplied an activity tracker [12, 23, 24]. Two reminder apps for head and neck cancer patients were found, one with the goal of completing skin care four times a day [13] and one to support adherence to swallowing therapy during RT [25]. Three apps supplied information on therapy (films, references) [16, 26], one study especially for pediatric patients receiving proton therapy [27]. One study gave assistance in scheduling appointments [28]. Three apps supplied multipurpose features including collection of ePRO as well as an appointment calendar, reminder, and/or knowledge databases [17, 29, 30].
As described in Tables 1 and 2 in detail, most patient-centered studies were single-arm studies or descriptive in nature. Overall, those studies showed high compliance [20, 31, 32], high acceptance [22, 23, 29, 33,34,35,36,37], effectiveness [28], satisfaction [31], usability [29, 38, 39], simplicity [38], and feasibility [12, 25, 32, 35,36,37, 39]. The randomized two-arm studies analyzing the impact of an app on the standard of care showed a significant reduction in adverse effects in head and neck cancer patients [13, 15, 17] and prostate cancer patients undergoing RT [19]. Five studies did not provide any results because they have so far only published study protocols [16, 18, 21, 24, 26].
The six apps designed for HCP focused on the following topics: RT-related calculations for physicists (n = 3) [40,41,42], semiautomatic segmentation tool for gliomas (n = 1) [43], resources for self-directed learning for trainees (n = 1) [44], and dose calculations (n = 1) [45]. All apps for HCP were descriptive in nature and showed good feasibility, in particular “easy to use” [44], “time efficient, accurate, and simple” [42], “user satisfaction” [45], and “efficient” [43].
Search in app marketplaces
Twenty apps were found in iOS App Store (Apple) and a total of 13 in Google’s Play Store. Seven apps could be found simultaneously in both stores. In a total of 26 apps, four were designed for patients (workflow management, n = 1; guidance of breath-hold management, n = 1; information on RT, n = 2). One app was specifically dedicated to prostate cancer patients in need of RT. In contrast, 22 apps were designed for HCP. Those can be divided into BED (biological effective dose)/EQD2 (2-Gy equivalent dose) calculators (n = 10), reference tools/e-journals (n = 5), learning tools for students/young professionals (n = 4), physics calculations (n = 1), and patient/workflow management (n = 2). Most of the apps were free of charge; only two apps for HCP ranged between 0.99 € and 2.99 €. Five apps disclosed in-app purchases. In summary, all identified apps had a maximum of one review (in App Store) and had been downloaded > 100–5000 times (Play Store). Only one app had been downloaded > 10,000 times.
Of all scientific publications included in the first part of this review, seven featured apps (one app was included in two independent studies) were also available in the two major app stores (18%) [11, 16, 20, 27, 37, 45,46,47]. Three of them were designed specifically for the field of radiation oncology (one dose calculation app [45] and two information apps [16, 27]), while four apps did not have a direct reference to radiation oncology but were tested in a patient collective receiving RT (two digital health management apps [20, 37, 46], one symptom tracker [11], and one body weight management app [47]). Figure 3 shows two representative examples of apps included in this review found in Apple’s App store.
a Example of a representative app for digital care management for health care professionals and patients: Care Hub Mobile App (Care Hub Digital Limited, v3.3.4, via App Store). Developed in the UK. App download and use free of charge. Password-restricted access. Features: monitoring of medication and task changes. Automation of assessments. Use not restricted to radiotherapy. Evaluation results: no user reviews available so far. b Example of a representative app for digital therapy and health care assistance: MyOnCare App (ONCARE GmbH, v1.6.2, via App Store). Developed in Germany. App download and use free of charge. Password-restricted access (via QR code of participating medical center). Features: exchange of medical data, (e.g., medication plans, support, activities, etc.) between health care professionals and patients. Use not restricted to radiotherapy. Evaluation results: 5 out of 5 (12 user reviews)
Discussion
Along with the increase in smartphone usage in all age groups, there is a growing interest in mobile health apps. Both HCP and patients are in favor of the use of oncological apps to support treatment adherence, to monitor side effects, and to improve communication [6, 7]. A body of research has been published focused on apps in the general/clinical oncological setting [5]. RT is an innovative, future-oriented discipline [48], representing a major pillar in cancer treatment and requiring special considerations. To our knowledge, this is the first review article to give a comprehensive overview of existing scientific literature on radiation oncology apps and the availability of commercial RT apps in major digital marketplaces.
In comparison to clinical oncology [5], our search revealed a limited number of research papers focusing on apps in radiation oncology. Of those, most studies consisted of a one-arm testing of a certain app or were descriptive in nature. Still, the vast majority of single-arm studies showed feasibility, high acceptance, and patient satisfaction. In the prospective two-arm studies with published results, apps showed improved exercise compliance in follow-up, with reduced adverse reactions for head and neck cancer [13, 15, 17] and increased compliance/communication in patients with prostate cancer [19] compared to the regular standard of care without the use of an additional app. However, the positive impact was largely limited to improving symptom control/reducing side effects. These results are in line with a study of Osborn et al. reviewing mHealth apps of patients with cancer (no special focus on RT). Their overview article included 17 studies with smartphone apps or internet portals collecting data on symptoms or patient activity. In summary, they showed statistically significant differences in ePROs when symptom monitoring using an app was compared to usual care [49]. The authors concluded that apps might improve aspects of symptom control in patients with cancer, but there was only little evidence for impact on other outcomes (e.g., mortality, cancer-related morbidity, long-term outcomes) [49]. Another systemic review of studies on symptom management interventions in people with advanced cancer also showed web- and mobile-based interventions to be efficient in decreasing the overall physical symptom burden [50].
ePROs, as seen in the majority of articles analyzed in our review, are still collected in a generally uncoordinated fashion. In recent years, the European Organisation for Research and Treatment of Cancer (EORTC) has tried to provide guidance in terms of ePRO measurement [51]. However, the problem of unstandardized measurements has already been stated by Giordano et al. [52]. In comparison to laboratory data, there is no common terminology or a common standard for accessing and analyzing ePROs [52]. This is in line with our results showing a wide variety of ePRO measurements in different studies. Mostly, evaluation focused on possible symptoms according to the RT field, but no standard was defined. This also applies to the times of PRO measurements: some study groups focused on the time period during RT (ranging from multiple daily to once-a-week collections), while others gathered data exclusively after RT (during follow-up), or both.
In addition to their importance in clinical day-to-day life, the collection of ePROs or other data (e.g., blood pressure, heart rate, etc.) via apps might also be useful and convenient in randomized controlled trials (so called smartRCTs), especially in the field of radiation oncology [53]. While legal limitations concerning data protection might be a hurdle, apps could potentially reduce costs, study duration, and subjectivity bias [53]. However, to our knowledge, there are currently no existing smartRCTs in the radiation oncology research field.
Collection of app-based ePROs could also hold some disadvantages: Hauth et al. stated that strategies to handle the large amount of data are among the major challenges to be addressed in the future [34]. Moreover, in an early paper published in 2011, Cox et al. pointed out some concerns particularly for palliative patients, where personal contact and the intuition of experienced HCP play an important role. Some clinicians found the age of patients often too advanced, with concomitant rapid deterioration of their condition, to be able to genuinely participate in a study using e‑technology [11]. Still, we found several examples of encouraging results in elderly patient cohorts. Buergy et al. found app-based follow-up feasible in a patient group ≥ 60 years [20]. In the randomized study of Sundberg et al., median age was 69 years [19]. Mean ages of around 60 years were described by others [13, 31, 39]. In line with the demographic transformation and an increase in personal usage of smartphones in the age group ≥ 65 years [54], we believe that the elderly population benefits from technical innovations and should not be excluded.
However, one still has to take the concerns of Cox et al. about personal contact und its impact into account [11]. Nevertheless, the use of new technologies does not automatically imply the neglect of personal contact. On the contrary, apps can be an additional tool to enhance patient empowerment and to personalize the patient–HCP relationship. Future app developers should therefore aspire to these goals.
In 2019, Cunha et al. published a review of the literature on mobile apps for remote support of RT patients similar to our review and found only four articles in the English language [55]. We revealed significantly more publications (n = 38). Apart from the fact that our search was more comprehensive, this shows that the existing literature is increasing, reflecting interest in this topic. More than half of the articles found in our search had been published within the past 3 years.
In the second part of our review, we searched digital marketplaces for available radiation oncology apps. A similar attempt was carried out by the study group of Charbonneau et al. [9]. They included 123 apps for general oncology: 50% of apps focused on general information for cancer, followed by specific apps for breast cancer (15%) and skin cancer (7%). Interactive features, including the ability to monitor symptoms, side effects, and treatment, were found in 20% [9]. These observations were similar to the RT-specific results presented here. However, we identified more apps for head and neck cancer patients, which is probably due to the fact that these patients often receive RT/RCT. Lu et al. found 41 apps, with the majority (73%) being general health/pain symptom trackers, when searching app stores for oncological apps [56], which also confirms our findings. Another review article focused on the more specific topic of apps in radiation oncology and was therefore more comparable to our search [57]. In total, Calero et al. identified 31 apps. However, the study group also included tumor staging apps which are not specific to radiation oncology. After subtracting these apps, 13 apps which would have met our inclusion criteria remained. The fact that we identified 39 apps in our search further underscores the emerging interest in this topic in the past few years.
Interestingly, we found a great discrepancy between our search results in scientific literature and search results for existing apps in the two major app marketplaces. While the literature search mainly returned apps for patients (ePROs, information, reminder, and workflow management), the two major app stores contained only four patient-centered apps (three for information/teaching and one workflow management). Only for one app found in app stores was a corresponding scientific publication found. On the other hand, 18% of scientific research articles dealt with an app that was available commercially. Most of those apps were not specifically designed for radiation oncology, but rather general health care apps tested in a radiation oncology setting. This was also seen in the review of Ana, where several apps tended not to be available after completion of the studies [5]. One reason for this discrepancy could be legal difficulties in setting up patient apps outside a clinical trial, with expensive accreditation procedures, e.g., CE marking or accreditation to the European Device Regulations (EU MDR), and reservations on data safety as well as an obligation to update information [58, 59]. Amortization of development, validation, and accreditation costs of RT apps may be facilitated by reimbursement frameworks with prescriptions for RT apps by the healthcare provider [59]. However, the potential target group for radiation oncology apps might be too small to compensate accreditation and maintenance costs. Nonetheless, the demand for digital education in radiooncology has increased since the beginning of the COVID-19 pandemic, especially among medical students [60,61,62]. In conclusion, while more and more apps are being developed and tested, currently existing apps for HCP and patients found in app stores lack scientific background, whereas clinically validated apps do not become available through app stores or as prescribable medical products.
There are certain limitations to our study. First, in both parts of the review, i.e., the literature search and the app marketplace, search terms for general/clinical oncology were excluded. While beyond the scope of this review, we acknowledge that oncology apps could significantly contribute to advances in radiotherapy, especially for patients undergoing chemotherapy in addition to radiotherapy. Moreover, certain synergistic intersections between oncology and radiotherapy regarding the collection of ePROs (e.g., EORTC QLQ C30 quality of life forms) are conceivable.
Secondly, apps found in our research were downloaded for evaluation but not tested in full detail or even rated.
Conclusion
The current scientific literature provides some evidence for helpful apps in the field of radiation oncology, with a clear emphasis on apps for patients. The vast majority cover ePROs during or after RT. Most studies are single armed, showing the “feasibility” of tested apps without further randomized testing against standard of care. In contrast, app marketplaces mainly offered apps for dose calculations for HCP in the field of radiation oncology. Therefore, efforts directing the transfer of existing scientific research into the development of commercially available apps in the field of RT should be undertaken.
In pursuing this goal, creation of quality-assured apps for RT patients which combine information sources, ePROs during and after therapy, and the possibility of contact with an HCP would be desirable. Ideally, such apps could transfer highly standardized ePRO data to a hospital interface for easy analysis. Moreover, app development in the field of radiation oncology should involve a certification process of radiation oncology societies, with subsequent testing in trials. After successful studies, apps should be transferred for continuous support of patients and HCP outside the trials [5]. Furthermore, ePROs could also be used for app-accompanied clinical trials (smartRCTs) [53].
References
Kao CK, Liebovitz DM (2017) Consumer mobile health apps: Current state, barriers, and future directions. PM R 9(5S):S106–S115. https://doi.org/10.1016/j.pmrj.2017.02.018
statistica.com (2022) Number of smartphone subscriptions worldwide from 2016 to 2021, with forecasts from 2022 to 2027. www.statistica.com. Accessed August 2022
mHealth (2011) New Horizons for Health Through Mobile Technologies: Second Global Survey on eHealth. World Health Organization (WHO), Geneva
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Ana FA, Loreto MS, José LM, Pablo SM, María Pilar MJ, Myriam SA (2020) Mobile applications in oncology: A systematic review of health sciencedatabases. Int J Med Inform 133:104001. https://doi.org/10.1016/j.ijmedinf.2019.104001
Kessel KA, Vogel MME, Schmidt-Graf F, Combs SE (2016) Mobile apps in oncology: A survey on health care professionals’ attitude toward telemedicine, mhealth, and oncological apps. J Med Internet Res 18(11):e312. https://doi.org/10.2196/jmir.6399
Kessel KA, Vogel MM, Kessel C, Bier H, Biedermann T, Friess H, Herschbach P, von Eisenhart-Rothe R, Meyer B, Kiechle M, Keller U, Peschel C, Schmid RM, Combs SE (2017) Mobile health in oncology: A patient survey about app-assisted cancer care. JMIR Mhealth Uhealth 5(6):e81–14. https://doi.org/10.2196/mhealth.7689
El Shafie RA, Weber D, Bougatf N, Sprave T, Oetzel D, Huber PE, Debus J, Nicolay NH (2018) Supportive care in radiotherapy based on a mobile app: Prospective multicenter survey. JMIR Mhealth Uhealth 6(8):e10916–30. https://doi.org/10.2196/10916
Charbonneau DH, Hightower S, Katz A, Zhang K, Abrams J, Senft N, Beebe-Dimmer JL, Heath E, Eaton T, Thompson HS (2020) Smartphone apps for cancer: A content analysis of the digital health marketplace. Digit Health 6:2055207620905413. https://doi.org/10.1177/2055207620905413
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Cox A, Illsley M, Knibb W, Lucas C, O’Driscoll M, Potter C, Flowerday A, Faithfull S (2011) The acceptability of e‑technology to monitor and assess patient symptoms following palliative radiotherapy for lung cancer. Palliat Med 25(7):675–681. https://doi.org/10.1177/0269216311399489
Pavic M, Klaas V, Theile G, Kraft J, Tröster G, Guckenberger M (2020) Feasibility and usability aspects of continuous remote monitoring of health status in palliative cancer patients using wearables. Oncology 98(6):386–395. https://doi.org/10.1159/000501433
Rades D, Narvaez CA, Doemer C, Janssen S, Olbrich D, Tvilsted S, Conde-Moreno AJ, Cacicedo J (2020) Radiotherapy-related skin toxicity (RAREST-02): A randomized trial testing the effect of a mobile application reminding head-and-neck cancer patients to perform skin care (reminder app) on radiation dermatitis. Trials 21(1):424. https://doi.org/10.1186/s13063-020-04307-0
Rades D, Werner EM, Glatzel E, Eggert EC, Olbrich D, Tvilsted S, Bohnet S (2020) Pneumonitis after radiotherapy for lung cancer (PARALUC): an interventional study to create a symptom-based scoring system for identification of patients developing radiation pneumonitis. BMC Cancer 20(1):785. https://doi.org/10.1186/s12885-020-07291-5
Di R, Li G (2018) Use of a smartphone medical app improves complications and quality of life in patients with nasopharyngeal carcinoma who underwent radiotherapy and chemotherapy. Med Sci Monit 24:6151–6156. https://doi.org/10.12659/MSM.908146
Fristedt S, Smith F, Grynne A, Browall M (2021) Digi-Do: a digital information tool to support patients with breast cancer before, during, and after start of radiotherapy treatment: an RCT study protocol. BMC Med Inform Decis Mak 21(1):76. https://doi.org/10.1186/s12911-021-01448-3
Liao T, Qiu L, Zhu J, Li J, Zhang Y, Yang L (2022) A mHealth-based nursing model for assessing the health outcomes of the discharged patients with nasopharyngeal carcinoma: a pilot RCT. BMC Nurs 21(1):210. https://doi.org/10.1186/s12912-022-00993-0
Sprave T, Zöller D, Stoian R, Rühle A, Kalckreuth T, Haehl E, Fahrner H, Binder H, Grosu AL, Heinemann F, Nicolay NH (2020) App-controlled treatment monitoring and support for head and neck cancer patients (APCOT): Protocol for a prospective randomized controlled trial. JMIR Res Protoc 9(12):e21693. https://doi.org/10.2196/21693
Sundberg K, Lindström V, Petersson LM, Langius-Eklöf A (2021) Supporting health literacy using an interactive app for symptom management during radiotherapy for prostate cancer. Patient Educ Couns 104(2):381–386. https://doi.org/10.1016/j.pec.2020.08.003
Buergy D, Siefert V, Neumaier C, Ganslandt T, Sperk E, Blessing M, Hesser J, Welzel G, Wenz F, Giordano FA (2020) Prospective trial on telemonitoring of geriatric cancer patients using handheld devices. Strahlenther Onkol 196(3):205–212. https://doi.org/10.1007/s00066-019-01548-0
El Shafie RA, Bougatf N, Sprave T, Weber D, Oetzel D, Machmer T, Huber PE, Debus J, Nicolay NH (2018) Oncologic therapy support via means of a dedicated mobile app (OPTIMISE-1): Protocol for a prospective pilot trial. JMIR Res Protoc 7(3):e70. https://doi.org/10.2196/resprot.8915
Kessel KA, Vogel ME, Alles A, Dobiasch S, Fischer H, Combs SE (2018) Mobile app delivery of the EORTC QLQ-C30 questionnaire to assess health-related quality of life in oncological patients: usability study. JMIR Mhealth Uhealth 6(2):e45. https://doi.org/10.2196/mhealth.9486
Boeke S, Hauth F, Fischer SG, Lautenbacher H, Bizu V, Zips D, Gani C (2022) Acceptance of physical activity monitoring in cancer patients during radiotherapy, the GIROfit phase 2 pilot trial. Tech Innov Patient Support Radiat Oncol 5(22):16–21. https://doi.org/10.1016/j.tipsro.2022.03.004
Wong J (2022) Smartphone pain app for assessing oral mucositis pain in patients (Department of Medicine, Devision of Hematology/Oncology, Medical College of Wisconsin, ClinicalTrials.gov Identifier: NCT02727062)
Starmer HM, Abrams R, Webster K, Kizner J, Beadle B, Holsinger FC, Quon H, Richmon J (2018) Feasibility of a mobile application to enhance swallowing therapy for patients undergoing radiation-based treatment for head and neck cancer. Dysphagia 33(2):227–233. https://doi.org/10.1007/s00455-017-9850-y
Ladbury C (2022) A mobile application, oncpatient, to assist patients undergoing radiation therapy (City of Hope Medical Center, Duarte, California. ClinicalTrials.gov Identifier: NCT05173961)
Stephenson NW, Todd KE, Indelicato DJ, Arce SH (2018p) Designing and developing a mobile application to prepare paediatric cancer patients for proton therapy. Des Health. https://doi.org/10.1080/24735132.2018.1448618
Kauppinen J, Kokkonen M, Kaunisto M, Seppälä J (2019) Mobile application for daily patient scheduling during radiotherapy treatment course. Radiother Oncol 133(Suppl 1):1–1220
Birkhoff SD, Cantrell MA, Moriarty H, Lustig R (2018) The usability and acceptability of a patient-centered mobile health tracking app among a sample of adult radiation oncology patients. Ans Adv Nurs Sci 41(3):243–259. https://doi.org/10.1097/ANS.0000000000000202
Marques da Cruz F, Faria ET, Ghobad PC, Mano Alves LY, Dos Reis PE (2021) A mobile app (AMOR mama) for women with breast cancer undergoing radiation therapy: functionality and usability study. J Med Internet Res 23(10):e24865. https://doi.org/10.2196/24865
Falchook AD, Tracton G, Stravers L, Fleming ME, Snavely AC, Noe JF, Hayes DN, Grilley-Olson JE, Weiss JM, Reeve BB, Basch EM, Chera BS (2016) Use of mobile device technology to continuously collect patient-reported symptoms during radiation therapy for head and neck cancer: A prospective feasibility study. Adv Radiat Oncol 1(2):115–121. https://doi.org/10.1016/j.adro.2016.02.001
Møller KP, Pappot H, Bernchou U, Schytte T, Mortensen ZV, Brúnni MFA, Dieperink KB (2021) Feasibility, usability and acceptance of weekly electronic patient-reported outcomes among patients receiving pelvic CT- or online MR-guided radiotherapy—A prospective pilot study. Tech Innov Patient Support Radiat Oncol 15(21):8–15. https://doi.org/10.1016/j.tipsro.2021.12.001
Gani C, Hauth F, Bizu V, Boecke S, Tenev A, Lautenbacher H, Zips D (2019) Innovative Ansätze zur Optimierung der ambulanten Patientenversorgung mit Biosensoren und Patienten-Apps in der Radioonkologie. Strahlenther Onkol 195(Suppl 1):16
Hauth F, Bizu V, App R, Lautenbacher H, Tenev A, Bitzer M, Malek NP, Zips D, Gani C (2019) Electronic patient-reported outcome measures in radiation oncology: Initial experience after workflow implementation. JMIR Mhealth Uhealth 7(7):e12345. https://doi.org/10.2196/12345
Maguire R, Ream E, Richardson A, Connaghan J, Johnston B, Kotronoulas G, Pedersen V, McPhelim J, Pattison N, Smith A, Webster L, Taylor A, Kearney N (2015) Development of a novel remote patient monitoring system: the advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nurs 38(2):E37–E47. https://doi.org/10.1097/NCC.0000000000000150
Zini EM, Lanzola G, Quaglini S, Bossi P, Licitra L, Resteghini C (2019) A pilot study of a smartphone-based monitoring intervention on head and neck cancer patients undergoing concurrent chemo-radiotherapy. Int J Med Inform 129:404–412. https://doi.org/10.1016/j.ijmedinf.2019.06.004
Teckie S, Solomon J, Kadapa K, Sanchez K, Orner D, Kraus D, Kamdar DP, Pereira L, Frank D, Diefenbach M (2021) A mobile patient-facing app for tracking patient-reported outcomes in head and neck cancer survivors: single-arm feasibility study. JMIR Form Res 5(3):e24667. https://doi.org/10.2196/24667
Friedman J, Ber RM, Ridner S, Dietrich M, Johnson K (2016) PrimeMD, a real-time symptom management smartphone application for head and neck cancer patients undergoing radiation therapy. Int J Radiat Oncol Biol Phys 96(Suppl):E524
Underwood J, McCloskey S, Raldow A, Kishan A, Zalkin C, Navarro D, Scott Holt L, Webb A, Lynch KA, Atkinson TM (2022) Developing a mobile patient-reported outcomes version of the common terminology criteria for adverse events administration system to capture postradiation toxicity in oncology: usability and feasibility study. JMIR Form Res 6(4):e27775. https://doi.org/10.2196/27775
Ataei G, Cham S, Niksirat F, Shabestani Monfared A, Ebrahimnejad Gorji K (2020) Developing a mobile phone application for common radiotherapy calculations. J Biomed Phys Eng 10(2):235–240. https://doi.org/10.31661/jbpe.v0i0.1216
Jermoumi M, Yucel A, Hao Y, Cifter G, Sajo E, Ngwa W (2015) A software app for radiotherapy with in-situ dose-painting using high Z nanoparticles. IFMBE Proc 51:618–621. https://doi.org/10.1007/978-3-319-19387-8_151
Schiefer H, Ingulfsen N, Kluckert J, Peters S, Plasswilm L (2015) Measurements of isocenter path characteristics of the gantry rotation axis with a smartphone application. Med Phys 42(3):1184–1192. https://doi.org/10.1118/1.4906248
Wu YP, Lin YS, Wu WG, Yang C, Gu JQ, Bai Y, Wang MY (2017) Semiautomatic segmentation of glioma on mobile devices. J Healthc Eng 2017:8054939. https://doi.org/10.1155/2017/8054939
Gerard J, Wan B, Di Lalla V, Skamene S, Alfieri J (2022) An interactive smartphone application for trainees in radiation oncology: The Rad Onc Handbook. Int J Radiat Oncol Biol Phys 114(1):e17. https://doi.org/10.1016/j.ijrobp.2022.06.035
Tsang DS, Townsend C, Cao X, Szumacher E (2015) RBApp: Creation and patterns of use of an educational mobile application for radiobiology calculations in radiation therapy. J Med Imaging Radiat Sci 46(2):215–222. https://doi.org/10.1016/j.jmir.2015.03.001
Wöller B, Bruns M, Jäger K, Gabler V, Pachmann S, Riepl M, Panzer M (2022) Erste Erfahrungen mit App basierter Nachsorge einer Strahlentherapie-Praxis im ländlichen Raum. Strahlenther Onkol 198(Suppl 1):S73
Yang K, Oh D, Noh JM, Yoon HG, Sun JM, Kim HK, Zo JI, Shim YM, Ko H, Lee J, Kim Y (2021) Feasibility of an interactive health coaching mobile app to prevent malnutrition and muscle loss in esophageal cancer patients receiving neoadjuvant concurrent chemoradiotherapy: Prospective pilot study. J Med Internet Res 23(8):e28695. https://doi.org/10.2196/28695
Krug D, Hecht M, Ebert N, Mäurer M, Fleischmann DF, Fokas E, Straube C, Nicolay NH, Hörner-Rieber J, Schmitt D, von Neubeck C, Zamboglou C, Sperk E, Kaul D, Hess J, Corradini S, Seidel C, Gani C, Baues C, Frey B, Blanck O, Gauer T, Niyazi M (2021) Innovative radiation oncology together—Precise, personalized, human: vision 2030 for radiotherapy & radiation oncology in Germany. Strahlenther Onkol 197(12):1043–1048. https://doi.org/10.1007/s00066-021-01843-9
Osborn J, Ajakaiye A, Cooksley T, Subbe CP (2020) Do mHealth applications improve clinical outcomes of patients with cancer? A critical appraisal of the peer-reviewed literature. Support Care Cancer 28(3):1469–1479. https://doi.org/10.1007/s00520-019-04945-4
Saeidzadeh S, Kamalumpundi V, Chi NC, Nair R, Gilbertson-White S (2021) Web and mobile-based symptom management interventions for physical symptoms of people with advanced cancer: A systematic review and meta-analysis. Palliat Med 35(6):1020–1038. https://doi.org/10.1177/02692163211006317
www.eortc.org/app/uploads/sites/2/2018/02/EORTC_QLQ_Clinical_Practice_User_Manual-1.0.pdf. Accessed: August 2022
Giordano FA, Welzel G, Siefert V, Jahnke L, Ganslandt T, Wenz F, Grosu AL, Heinemann F, Nicolay NH (2020) Digital follow-up and the perspective of patient-centered care in oncology: what’s the PROblem? Oncology 98(6):379–385. https://doi.org/10.1159/000495294
Vogel ME, Combs SE, Kessel KA (2017) mHealth and application technology supporting clinical trials: Today’s limitations and future perspective of smartRCTs. Front Oncol 13(7):37. https://doi.org/10.3389/fonc.2017.00037
(2022) Share of adults in the United States who owned a smartphone from 2015 to 2021, by age group. www.statistica.com. Accessed: August 2022
Cunha CE, Fernandes R, Santos CX, Boccaletti KW, Pellizzon ACA, Barbosa JHO (1992) Viability of mobile applications for remote support of radiotherapy patients. Rev Assoc Med Bras 65(10):1321–1326. https://doi.org/10.1590/1806-9282.65.10.1321
Lu D, Girgis M, David JM, Chung EM, Atkins KM, Kamrava M (2021) Evaluation of mobile health applications to track patient-reported outcomes for oncology patients: a systematic review. Radiat Oncol 6(1):100576. https://doi.org/10.1016/j.adro.2020.09.016
Calero JJ, Oton LF, Oton CA (2017) Apps for radiation oncology. A comprehensive review. Transl Oncol 10(1):108–114. https://doi.org/10.1016/j.tranon.2016.08.008
https://health.ec.europa.eu/medical-devices-sector/directives_en. Accessed: August 2022
Gordon WJ, Landman A, Zhang H, Bates DW (2020) Beyond validation: getting health apps into clinical practice. npj Digit Med 3:14. https://doi.org/10.1038/s41746-019-0212-z (eCollection 2020)
Oertel M, Pepper NB, Schmitz M, Becker JC, Eich HT (2022) Digital transfer in radiation oncology education for medical students-single-center data and systemic review of the literature. Strahlenther Onkol 198(9):765–772. https://doi.org/10.1007/s00066-022-01939-w
Vorwerk H, Engenhart-Cabillic R (2022) Students’ learning behavior in digital education for radiation oncology. Strahlenther Onkol 198(1):12–24. https://doi.org/10.1007/s00066-021-01858-2
Dapper H, Wijnen-Meijer M, Rathfelder S, Mosene K, Kirchbauer I, Bernhardt D, Berberat PO, Combs SE (2021) Radiation oncology as part of medical education-current status and possible digital future prospects. Strahlenther Onkol 197(6):528–536. https://doi.org/10.1007/s00066-020-01712-x
Hecht M, Goldbrunner L, Kaul L, Winkler S, Ploner N, Lettmaier S, Haderlein M, Kallies A, Pirschel W, Fietkau R, Distel L (2022) Pilotprojekt zur App-basierten Patientenanbindung in der ambulanten Tumortherapie. Strahlenther Onkol 198(Suppl 1):S46
Theile G, Klaas V, Tröster G, Guckenberger M (2017) mHealth technologies for palliative care patients at the interface of in-patient to outpatient care: protocol of feasibility study aiming to early predict deterioration of patient’s health status. JMIR Res Protoc 6(8):e142. https://doi.org/10.2196/resprot.7676
Werner E, Glatzel E, Janssen S, Ohlbrich D, Tvilsted S, Bohnet S, Rades D (2021) Ein symptombasiertes Punktesystem zur Identifikation einer Pneumonitis nach Strahlentherapie beim Lungenkarzinom: Erste Ergebnisse einer prospektiven multizentrischen Studie (PARALUC). Strahlenther Onkol 197(Suppl 1):S141
Rades D, Zwaan I, Cacicedo J, Bruchhage KL, Hakim SG, Olbrich D, Schild SE, Tvilsted S, Janssen S (2022) Impact of a mobile application (reminder app) on acute toxicity during radiotherapy of head-and-neck cancer—results of a randomized phase III trial (RAREST-02). BMC Cancer 22:989. https://doi.org/10.1186/s12885-022-10088-3
Sundberg K, Wengström Y, Blomberg K, Hälleberg-Nyman M, Frank C, Langius-Eklöf A (2017) Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support Care Cancer 25(7):2195–2204. https://doi.org/10.1007/s00520-017-3625-8
Sundberg K, Eklöf AL, Blomberg K, Isaksson AK, Wengström Y (2015) Feasibility of an interactive ICT-platform for early assessment and management of patient-reported symptoms during radiotherapy for prostate cancer. Eur J Oncol Nurs 19(5):523–528. https://doi.org/10.1016/j.ejon.2015.02.013
Langius-Eklöf A, Christiansen M, Lindström V, Blomberg K, Hälleberg Nyman M, Wengström Y, Sundberg K (2017) Adherence to report and patient perception of an interactive app for managing symptoms during radiotherapy for prostate cancer: Descriptive study of logged and interview data. JMIR Cancer 3(2):e18. https://doi.org/10.2196/cancer.7599
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Janssen, R.A. El Shafie, A.M. Ruder, D. Buergy, D. Scafa, F.A. Giordano, N.H. Nicolay, M.M.E. Vogel, S.E. Combs, F.B. Fahlbusch, D. Rades, and L. Käsmann declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janssen, S., El Shafie, R.A., Ruder, A.M. et al. Mobile applications in radiation oncology—current choices and future potentials. Strahlenther Onkol 199, 337–349 (2023). https://doi.org/10.1007/s00066-023-02048-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02048-y