Abstract
Purpose
Patients with head and neck cancer (HNC) are at risk of malnutrition, especially during radiochemotherapy. We aimed to study the impact of a ketogenic diet (KD) versus an unspecified standard diet (SD) on body composition and survival in HNC patients undergoing radio(chemo)therapy.
Methods
As part of a controlled clinical trial, non-metastasized HNC patients were enrolled into either a KD (N = 11) or an SD (N = 21) group between May 2015 and May 2021. Body composition was measured weekly by bioimpedance analysis and analyzed using linear mixed effects models. Overall and progression-free survival was assessed during regular follow-up.
Results
A total of 7 KD and 21 SD patients completed the study and were eligible for comparative analysis. Chemotherapy was significantly associated with declines in all body composition parameters, while the KD had opposing, yet nonsignificant effects. In patients receiving chemotherapy, average weekly reductions of body mass (BM) and skeletal muscle mass (SMM) were 0.9 kg and 0.31 kg in the KD group versus 1.2 kg and 0.57 kg in the SD group, respectively. Patients in the KD group receiving no chemotherapy achieved an average increase of 0.04 kg BM and 0.12 kg SMM per week. After a median follow-up of 42 months (range 6.7–78 months) there were no significant differences in progression-free or overall survival between the groups.
Conclusion
The KD may partially counteract the detrimental effects of radiochemotherapy on body composition in HNC patients. This should encourage further research into KDs in frail cancer patient populations and motivate their implementation as complementary therapy for selected patients.
Similar content being viewed by others
Introduction
Head and neck cancer (HNC) describes cancers originating from the lip, oral and nasal cavity, paranasal sinuses, pharynx, larynx, and trachea. The vast majority (≈ 90%) of HNCs are squamous cell carcinoma (HNSCC) that originate from the mucosal lining (epithelium) of these regions.
Patients with HNC frequently present with feeding difficulties and malnutrition, which are often further aggravated by tobacco and alcohol abuse and a general unhealthy lifestyle. Studies have shown that nutritional status is already inadequate at the time of diagnosis in up to 60% of all HNC patients [1, 2] and usually gets worse during anti-tumor therapy, which can promote xerostomia, mucositis, and anorexia. The negative effects of cancer- and therapy-induced body mass (BM) reduction are mainly due to the loss of skeletal muscle mass (SMM), which leads to declines in strength, quality of life, and tolerability of cancer treatment. A simple and easy-to-obtain indicator of muscle mass and strength exists in the form of the phase angle (PA) measured by tetrapolar bioelectrical impedance analysis (BIA) [3]. The PA is determined by tissue cellularity, hydration, and membrane potential, and therefore is useful to assess malnutrition at the cellular level. Consistently, low PAs strongly predict poor survival in cancer patients [4]. BIA measurements have shown that already in early stages of disease, i.e., with normal body mass index (BMI) and minimal previous weight loss, HNC patients exhibit a significantly lower PA than age-matched healthy controls, which is not explainable by an altered hydration status [5]. This sign of subclinical malnutrition therefore seems to occur early in the progression of disease and may be connected to the early systemic insulin resistance that has been described in a variety of cancer patients [6,7,8].
To counteract subclinical and clinically manifest malnutrition, it has been recommended that even HNC patients who appear healthy should be counseled and advised to eat a high-calorie and high-protein diet [9]; the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend protein intakes up to 1.5 g/kg per day if possible [10]. Nutritional support through counseling and oral supplements has also become an important modality during radiochemotherapy for HNC, as it may support the maintenance of BM and quality of life, increase protein-calorie intake, and improve anti-cancer treatment tolerance [11]. In practice, however, the variety of available supplementary nutrition formulas and general, inconsistent dietary advice for cancer patients [12] pose a challenge for deciding on the optimal diet for preventing body and muscle mass reduction, improving quality of life, reducing inflammation, and withstanding therapy-induced side effects. Many physicians seem unaware of the fact that besides the amount of caloric intake, the composition of the diet may have profound influences on these dietary goals. For example, an investigation of enteral and parenteral feeding practices in a Chinese university teaching hospital [13] revealed that only 2.1% of cancer patients received Supportan® (Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany), a disease-specific high-fat nutrition formula that has been shown to improve nutritional and functional parameters in HNC patients compared to a standard formula [14], while most standard formulas contain large amounts of simple sugars which elevate blood glucose concentrations and in this way may possibly even “feed” the tumor [15]. HNSCC are generally highly glycolytic tumors [16, 17], which in theory also contributes to their radioresistance [18]. It has therefore been suggested that low-carbohydrate diets could target the altered metabolism of such glycolytic tumors [19,20,21,22]. Among low-carbohydrate diet variants, the ketogenic diet (KD) has gained particular interest as a complementary cancer therapy. In oncological settings, KDs can be defined as high-fat (usually ≥ 65% of energy intake), low-carbohydrate (≤ 50 g and day) diets ideally providing an adequate protein supply (≈ 1.5 g/kg per day) and leading to an elevation of circulating ketone bodies into the mM range (≥ 0.5 mmol/L). Preclinical research has shown that KDs may not only impair tumor cell metabolism and growth, but also fight cachexia and therapy-induced side effects [23,24,25]. However, data showing whether HNC patients can comply to KDs are sparse. In a recent uncontrolled phase I study, two thirds (8/12) of HNC patients did not manage to tolerate a KD fed via percutaneous endoscopic gastrostomy (PEG) tubes with an aim of 5 weeks, which would question the clinical implementation of KDs as complementary therapies for HNC patients. After gaining initial experiences with KDs in our clinic [26], we initiated a controlled clinical trial, the KETOCOMP study, in order to investigate the feasibility and impact on BM and body composition in cancer patients, including HNC patients [27]. Here we describe the primary outcomes of the KETOCOMP study for the HNC patient cohort, providing important insights into the clinical implementation and the benefits of KDs for such patients.
Materials and methods
Study design
KETOCOMP was a non-randomized controlled clinical phase I trial performed at the Department of Radiotherapy and Radiation Oncology at a peripheral hospital in Bavaria, Germany. The protocol was registered on August 6, 2015, under ClinicalTrials.gov identifier NCT02516501 after study approval was granted by the ethics committee of the Bavarian Medical Association (Landesaerztekammer Bayern). The KETOCOMP study comprised three different cohorts of breast, rectal, and HNC patients [27]. An interim report of all cohorts [28] as well as final results for the breast [29, 30] and rectal cancer [31, 32] cohorts have already been published. Here we present the final primary outcomes for the HNC cohort consisting of patients which presented at our clinic for curative radio(chemo)therapy.
The initial study protocol was designed to investigate two intervention groups: intervention group 1 was supposed to receive a ketogenic breakfast consisting of increasing doses up to 225 ml of a ketogenic drinkFootnote 1 containing 20 g medium-chain triglycerides (MCTs) per 100 ml (betaquik®, vitaflo, Bad Homburg, Germany) plus essential amino acids in form of the Master Amino Acid Pattern supplement (MAP, re-branded as MyAMINO; dr. reinwald health-care gmbh + co kg, Altdorf, Germany) while being free to follow their usual diet for the remainder of the day. Intervention group 2 was advised to follow a KD supplemented with MAP. Similar to the rectal cancer patient cohort [31], HNC patients in intervention group 1 displayed poor tolerability of the maximum target dose of 225 ml betaquik® (most frequently due to diarrhea and stomach pain), so that this group was closed after recruiting four patients of whom two were unable to finish the study per protocol. The protocol was subsequently amended to shift focus to intervention group 2, which will hereafter be referred to as the KD group, and the target numbers for the KD and control group consuming an unspecified standard diet (SD) were increased to 10 and 20 patients, respectively. In what follows, only results for the KD and SD groups are analyzed and discussed.
Participants
Inclusion criteria were age between 18 and 75 years, diagnosis of a non-metastatic HNC, Karnofsky index ≥ 70, and body mass index (BMI) between 18 and 34 kg/m2. Exclusion criteria included metallic implants (because of possible interference with body composition measurements), pregnancy, cognitive impairment, inability to speak or understand German, and metabolic disorders that would pose a contraindication for a KD [27].
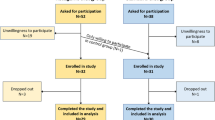
Fig. 1 shows a flowchart of the recruitment process. In general, patients fulfilling the inclusion criteria were asked at first consultation by their treating oncologist if they would be willing to participate in a particular group. Thereby, we first aimed to fill the SD group with consecutive patients, followed by the KD breakfast group (intervention group 1), and then the KD group, each with the prespecified number of patients. The reason for this consecutive recruitment pattern was to minimize patients’ self-selection of a particular diet (through interpatient discussions, e.g., in the waiting room). However, the protocol allowed patients showing a large interest in a KD during the initial consultation to enter the KD group irrespective of recruitment phase, and vice versa, i.e., patients were allowed to act as controls if they wanted to join the study but maintain their usual diet [27]. In this way, the research team wanted to ensure compliance with a chosen diet type. A total of three patients decided to take that option and switch to the KD group during recruitment to the SD group, among them one woman who had metallic implants but was included due to her wish and used for analyzing BM changes. Two other patients decided to undertake a full KD instead of entering the ketogenic breakfast group. Finally, four patients who were foreseen for the KD group switched to the SD group. In total, 41 eligible patients had been asked to participate (Fig. 1).
Measurements
In general, patients presented fasted and with an empty bladder on the same morning they received their radiotherapy planning computed tomography for initial (baseline) measurements, approximately 1 week prior to the start of radiotherapy. Baseline measurements consisted of:
-
weight and bioimpedance analysis (BIA) on a calibrated seca 515/514 medical Body Composition Analyzer (mBCA; seca Deutschland, Hamburg, Germany),
-
the validated EORTC QLC-C30 questionnaire version 3.0 together with the H&N35 module,
-
blood draw with subsequent analysis in the hospital laboratory.
BIA and weighing were repeated weekly during radiotherapy. In addition, laboratory blood analysis and completion of the quality of life questionnaire were repeated once during and in the final week of radiotherapy.
The primary outcome measures for this study were as follows:
-
dropout rate as a measure of feasibility,
-
changes from baseline to the final week of radiotherapy in body composition parameters: BM, fat-free mass (FFM), fat mass (FM = BM − FFM), SMM, extracellular water (ECW), total body water (TBW), and intracellular water (ICW = TBW − ECW),
-
Changes in bioimpedance PA at 50 kHz (PA).
The secondary outcomes analyzed here concern overall and progression-free survival of the KD and SD groups. Other secondary outcomes concerning quality of life and blood parameter changes will be the topic of a future paper.
Dietary intervention
The KD was started after baseline measurements, about 2–3 days prior to the first RT fraction. It was supposed to last until the final week of RT. The last measurement day in the final week of RT was considered the stop date of the diet.
The intervention comprised a self-administered, whole-food KD (aiming for ≤ 50 g of carbohydrates per day), with emphasis on high-quality protein (meat, eggs, fish) and micronutrient-dense foods (vegetables, bone broth, organ meats). The meals were not controlled and no calories were counted. Participants were instructed by a dietitian on how to implement the KD and were provided with written guidelines (Supplementary File 1), recipes, and sample menus. During weekly appointments for BIA measurement, patients had the possibility to get further dietary advice and to address difficulties. In case of swallowing difficulties or utilization of a PEG tube, ketogenic formulas were provided to the patients. Furthermore, all patients in the KD group received a medium-chain triglyceride (MCT) oil (Dr. Schaer AG/SpA, Burgstall, Italy), which they were asked to use according to their own tolerance. Additionally, all patients in the KD group received 10 g MAP after each RT fraction. MAP contains all essential amino acids (L-isoleucine, L‑leucine, L‑lysine, L‑valine, L‑methionine, L‑phenylalanine, L‑threonine, L‑tryptophane) and has been found to have a net nitrogen utilization of 99% when consumed as sole protein source [33]. No additional micronutrient supplements were advised, apart from vitamin D in case of low serum 25-hydroxyvitamin D levels, which we aimed to correct in both KD and SD groups.
Statistical analysis
Data were analyzed using the statistical software R (R Foundation for Statistical Computing, Vienna, Austria) version 4.1.2. Differences between groups were compared using the Wilcoxon rank sum and Fisher’s exact test for continuous and categorical variables, respectively. We did not adjust for multiple comparisons [34] but used a more stringent threshold of p < 0.005 for claiming statistical significance in order to minimize non-replicable, false-positive findings [35].
Baseline-to-final changes were analyzed using linear mixed effects models, thereby using all weekly repeated measurements and assuming a linear gradual change. The slope and intercept of the variable t (time since start of RT) were modeled as random effects varying by the individual patient. Let yij, \(i=1,\ldots ,n_{j}\), denote the ith measurement on patient \(j=1,\ldots ,N\) at time ti during the study. We constructed basic models predicting an individual measurement yij based on time, group (0 = SD; 1 = KD), a time × group interaction, the corresponding baseline measure y1j, and systemic therapy (abbreviated as “chemo” with 0 = no; 1 = yes) and a time × systemic therapy interaction as confounding variables:
The average time trends of patients in the SD and KD group not receiving chemotherapy are therefore given by β1 and \(\beta _{1}+\beta _{3}\), respectively, or by \(\beta _{1}+\beta _{5}\) and \(\beta _{1}+\beta _{3}+\beta _{5}\) for those patients who had received chemotherapy. By adding additional covariates to the basic model (Eq. 1), we tried to further improve the model fit which was judged by the bias-corrected Akaike information criterion (AICc) [36]. The set of considered covariates consisted of age, gender, baseline Karnofsky index, PTV size, and PEG use. It was decided that a new covariate would be included into the model only if it resulted in a reduction of the AICc by at least 2.
Models were fit using the lmer function in the R package lmerTest [37] with restricted maximum likelihood, and p-values of fixed effects calculated by means of a t test.
Results
Dropouts
Recruitment began in May 2015 and ended in May 2021 due to slow recruitment and the impact of the COVID-19 crisis. Of 11 patients enrolled into the KD group, four patients (36%) dropped out and seven patients completed the study. Patients dropped out 1, 5, 7, and 12 days after starting radiotherapy and median time on the diet was 8 days. One of the patients that dropped out had switched voluntarily to the KD group and then quit the study due to strong bronchitis and craving for carbohydrate-rich foods (potatoes). The rest of the patients that dropped out had been recruited during the KD block recruitment phase and quit due to psychological problems (N = 1) and non-compliance with the KD (N = 2). Of the 21 patients that were enrolled into the SD group, all patients terminated the study successfully and were included into the analysis. The difference in dropout percentages between the KD and SD groups was almost statistically significant (p = 0.0092).
The median study duration (time from start of RT until final measurement) was 39 days (35–46 days) in the KD and 40 days (34–55 days) in the SD group (p = 0.422). The median time on the diet for the patients in the KD group was 47 days (42–49 days). These patients had started their KD on average 7 days (range 0–14 days) prior to the first irradiation.
Patient characteristics
The baseline characteristics of patients included in the analysis are displayed in Table 1. Overall, the block recruitment had resulted in roughly balanced groups with respect to most baseline variables (all p-values for differences > 0.05). One patient each from the KD and SD groups had elevated β‑hydroxybutyrate concentrations (≥ 0.5 mmol/l) already at baseline. The patient from the SD group had not eaten for 3 days prior to baseline measurement, while the patient from the KD group had probably already started to reduce carbohydrates after agreeing to participate in the study.
As a measure of cellular malnutrition, we compared baseline PAs at 50 kHz with the rectal [31] and breast cancer [29] cohorts. Women with HNC (N = 7) had significantly lower PAs (median 4.10°) than women with breast cancer (N = 59, 4.86°; p = 0.0033), but not compared to women with rectal cancer (N = 14, 4.56°; p = 0.218). Men with HNC (N = 21) had lower PAs than men with rectal cancer (N = 27), although this was not statistically significant (4.50° versus 5.14°, p = 0.0672).
Ketogenic diets
While on the KD, median capillary β‑hydroxybutyrate concentrations in the KD group were 0.7 mmol/L (0.2–3.2 mmol/L). Thereby, all 7 patients in the KD group achieved at least one capillary or laboratory β‑hydroxybutyrate measurement ≥ 0.5 mmol/L, showing that all patients tried to comply with the diet.
Absolute changes in body composition
Table 2 shows the absolute changes that occurred within both groups between the baseline and final measurement. Significant absolute reductions in all body composition parameters occurred only in the SD but not the KD group. However, all changes in relative body composition parameters were statistically nonsignificant. Noteworthily, SMM was reduced less in the KD group in absolute terms and did not change relative to BM, while it decreased by 0.5% in the SD group.
Fig. 2 displays the absolute body composition changes as boxplots and shows the p-values corresponding to a comparison of changes between both groups. While all body composition parameters were reduced to a lesser degree in the KD group, the difference to the SD group was not significant for any of the parameters. We note that the initial BM loss in the KD group (change from the first to the second measurement) measured 0.24 ± 1.4 kg BM (mean ± SD), which was almost equal to the reduction of TBW measuring 0.23 ± 1.4 L; this indicates that initial weight loss was mostly due to initial water losses. Fig. 2 also shows that the changes in FFM and SMM were paralleled by those in TBW and ICW, respectively. Indeed, the correlation between absolute changes in FFM and TBW (Spearman’s ρ = 0.984, p = 2.2 × 10−16) as well as between changes in SMM and ICW (ρ = 0.994, p = 2.2 × 10−16) were highly significant. On average, 0.38 ± 0.55 kg FFM and 0.43 ± 0.34 kg SMM loss could not be accounted for by losses of TBW and ICW, respectively. This indicates that the reductions that occurred in FFM and SMM were not only due to extracellular and glycogen-bound water losses, but also reflect a reduction in structural tissue mass.
Gradual changes in body composition
The results of the linear mixed effects models (Eq. 1) are displayed in Table 3 in form of the best-fit regression coefficients. Additional covariates to the ones shown in Table 3 were not considered because their inclusion into the basic models increased the AICc values and hence did not improve model fits. Overall model fits were judged as good, since the R2 values indicated that the included covariates were able to explain most of the variance. Patients in the KD having received chemotherapy experienced an average gradual reduction of 0.9 kg BM, 0.44 kg FM, and 0.31 kg SMM per week, while those who did not receive chemotherapy achieved an average gradual increase by 0.04 kg BM and 0.12 kg SMM per week. In the SD group, average gradual reductions in BM, FM, and SMM for patients who had received chemotherapy were somewhat larger, namely 1.2 kg, 0.57 kg, and 0.48 kg per week, and even those patients who did not receive chemotherapy experienced a gradual reduction of BM and FM by 0.30 ± 0.16 (p = 0.071) and 0.24 ± 0.10 (p = 0.029) per week (Table 3). Noteworthily, compared to the SD, the KD was associated with a gradual SMM increase of 0.17 ± 0.08 kg per week with a p-value of 0.06. However, really significant changes in body composition were only associated with chemotherapy, which tended to reduce especially lean mass and ICW.
Overall and progression-free survival
Survival outcomes of radio(chemo)therapy were evaluated between the SD and KD group (Fig. 3). In the KD group, median follow-up for OS and PFS was 35.2 months (12.4–63.7 months) and 35.2 months (4.3–63.7 months), respectively. In the SD group, median follow-up was 45.8 months (6.7–78.0 months) and 36.9 months (6.7–70.4 months), respectively. One patient in the KD group developed locoregional progression 4.3 months after starting radiochemotherapy and died 8.1 months thereafter; another patient experienced locoregional relapse at 48.5 months follow-up and was reirradiated (he is currently alive). In the SD group, a total of five patients experienced tumor progression and four patients had died after a median follow-up of 17 months (12.1–20.2 months). Differences in PFS or OS were not significant between both groups (Fig. 3), and this result did not change qualitatively when performing an intention-to-treat analysis (Fig. 4).
Discussion
In this analysis, we show that an individualized KD supplemented with essential amino acids consumed during curative radio(chemo)therapy of HNC patients was able to slow down the negative consequences of therapy on body composition to some extent. The major factor inducing BM, FM, and SMM reductions was the administration of chemotherapy, while the KD was nonsignificantly associated with opposing trends, in particular a gradual SMM increase of 0.17 ± 0.08 kg per week (p = 0.060). A difference between the KD and SD groups with respect to either OS or PFS could not be demonstrated, nor was the study powered to show this. However, the results regarding body composition should encourage further research of KDs in frail cancer patient populations and motivate their implementation as complementary therapies for select patients.
Compliance and dropouts
“Is it realistic to restrict carbohydrates for HNC patients?” is the question which was addressed in a review article written by one of us (RJK), and it was concluded that carbohydrate restriction in the form of KDs or intermittent fasting “seems feasible and therefore realistic, but requires additional time and effort as it has to be tailored towards the individual patient” [38]. In an uncontrolled phase I study, Ma et al. found that two thirds of HNSCC patients did not manage to consume a KD during 5 weeks of radiochemotherapy [25]. Thereby, all patients had received a PEG tube prior to the study, which was used to provide a ketogenic formula that accounted for the main calories of the KD. Reasons for discontinuation included fatigue, nausea, and vomiting, two cases of grade 4 hyperuricemia, and one case of acute grade 3 pancreatitis; the attribution of these side effects to the KD was unclear in all cases [25]. Thus, a KD—even if applied via a PEG tube—may not be feasible in the general HNC population. The results of our study also go into this direction, although compliance was higher (64%) in our patients of whom 2/7 had a PEG tube. It appears that the possibility to voluntarily switch to the KD group may have contributed to the higher compliance rate in our study. Our experience was that patients who consented to join the KD group because they were asked to appeared motivated at the beginning, but soon became noncompliant and consumed nonketogenic foods such as potato squash in one case or high-sugar formula foods prescribed by other physicians during a brief stationary stay in another case. Frequently, patients were also not able to care for themselves and depended on family members to prepare foods. We admit that we underestimated these facts in the beginning. We think that compliance problems in HNC patients are not KD-specific, because they also occur with other individualized dietary prescriptions as has been shown for example in a Turkish study in which only 18/48 (37.5%) of patients undergoing radio(chemo)therapy were able to achieve at least 75% of the prescribed energy and protein intake [39]. We think that with broader support from patients’ social environment and oncological colleagues, a significantly higher fraction of HNC patients would be able to maintain a KD.
Survival outcomes
Ma et al. also showed that the combination of a ketogenic formula with ionizing radiation significantly prolonged the survival time of HNC xenograft-bearing mice compared to irradiation alone [25]. However, the survival benefit vanished when a new version of the formula with another fatty acid composition was given. This indicates that the heterogeneity of individually consumed KDs may affect whether they act synergistically with ionizing radiation or not. Nevertheless, there are also more general effects that would lead one to expect synergism between KDs and therapies acting through oxidative DNA damage [22, 40]. In particular lactate and, to a lesser extent, pyruvate have been shown to act as antioxidants and protect tumor cells against ionizing radiation [18, 41, 42]. Lactate concentrations in HNSCCs are higher than in normal tissue, correlate positively with tumor stage, and predict worse OS [43]. It is therefore interesting that Schroeder et al. measured a decline in lactate concentration in HNSCC tissue after only a few days of a KD [44]. Despite these mechanistic insights predicting synergistic effects between a KD and radiotherapy, we did not observe a clear benefit of the KD group with respect to either PFS or OS. We point out that both high pretreatment carbohydrate intake [45] and pretreatment nutritional support with presumably high-carbohydrate supplements [46] are associated with poor prognosis in HNC patients, which may have influenced survival outcomes but was unfortunately not measured. Furthermore, the sample size was too small to detect potentially small survival benefits in the KD group.
Body composition changes
Compared to breast and rectal cancer patients, our HNC patient cohort already had low PA values at the start of radiotherapy, indicative of subclinical or clinically manifest malnutrition. As expected, the administration of platinum-based chemotherapy further worsened the condition of patients, being the most significant predictor of body composition decline. Patients having received adjuvant chemotherapy experienced a significant average reduction of 0.94 ± 0.18 BM, 0.59 ± 0.13 FFM, and 0.43 ± 0.07 SMM per week (all p-values < 0.005). The KD appeared to counter these trends, being associated with an average increase of BM, FFM, and SMM by 0.34 ± 0.20 kg, 0.22 ± 0.15 kg, and 0.17 ± 0.08 kg, respectively. Although these effects associated with the KD were nominally not significant, they are consistent with clinical and mechanistic evidence for the hypothesis that KDs are able to counteract muscle catabolism in frail patient populations [23, 24, 47, 48].
The impact of nutritional counselling alone on nutritional status of HNC patients during radiochemotherapy is hard to predict, as recent studies found either no [49] or a significant difference to the no-counselling group [50]. A better strategy appears to be nutritional counselling plus the delivery of calorie-dense, high-protein, and omega‑3 fatty acid-enriched supplements, which was shown to lead to significantly less BM loss than nutritional counselling alone [11]. Our intervention mostly relied on nutritional counselling, but patients were also provided with 10 g essential amino acids per day in the form of the MAP supplement. While MAP makes no significant contribution to energy intake, it may have helped to maintain SMM. It is noteworthy that we did not find any association between MAP intake and better preservation of FFM or SMM in the KETOCOMP breast cancer cohort, which we explained by the fact that these women had no difficulties in consuming ample quantities of high-quality protein in their KD. It is possible that the addition of MAP had a larger effect in the HNC patients who had more difficulties with food intake. In addition, the rise of ketone bodies during the KD may have counteracted SMM degradation, in line with the muscle-sparing effect of ketosis that is essential for surviving longer periods of starvation [51]. Rat studies have shown that ketosis inhibits oxidation of the branched-chain amino acids in muscle [52] and decreases the release of the gluconeogenic amino acid alanine [53]. Finally, our KD prescription emphasized the intake of high-quality animal protein from meat, organs, and eggs. Protein of animal origin has a higher anabolic potential in general compared to plant proteins, because it has a higher digestibility and no limiting dietary indispensable (essential) amino acids [54]. Together, these factors may have been effective in reducing the loss of SMM compared to the SD group, although it was not statistically significant, possibly due to the small sample size.
Limitations
The major limitation of this study is the small sample size of patients in the KD group, which was partly due to the rare presentation of HNC patients in our clinic and partly to the fact that some patients declined to participate in the KD group, switched to the SD group, or dropped out of the study. While patient numbers were large enough to detect a clear signal of chemotherapy as a highly significant predictor of body composition changes, it is possible that the opposing influence of the KD would have been more significant with a larger patient number.
Another limitation could be that patients were allowed to self-select to enter the KD group, and that the KD food composition was not standardized but rather highly individual. While the KD and SD groups were comparable with respect to many confounding factors, there might have been some residual confounders influencing outcomes that remained unaccounted for. Individualization of diet prescriptions is usually considered essential in order to maximize compliance in cancer patients, and therefore our study reflects the real-world clinical situation, which increases the degree to which our results apply to any “real world” HNC population (external validity).
Finally, the validity of BIA for assessing body composition may be criticized. First, however, we were mainly interested in average (group-wise) time trends of body composition, and a study in non-metastasized colorectal cancer patients has shown that on a group level, FFM estimated by our BIA device (the seca mBCA 515) was not different from FFM estimated by the gold standard dual-energy X‑ray absorptiometry (DXA) [55]. Second, by looking at gradual changes, any systematic biases in individual BIA measurements would not affect the time trends. Third, measurements were strictly standardized by telling patients to appear fasted and with an empty bladder, in order to minimize individual measurement errors.
Notes
Initially, the maximum volume of the ketogenic drink was 250 ml (one container) [27], but was reduced to 225 ml after a re-sizing of the containers took place by the manufacturing company.
References
Donaldson SS, Lenon RA (1979) Alterations of nutritional status: impact of chemotherapy and radiation therapy. Cancer 43:2036–2052
Bassett MR, Dobie RA (1983) Patterns of nutritional deficiency in head and neck cancer. Otolaryngol Head Neck Surg 91:119–125
Selberg O, Selberg D (2002) Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol 86:509–516. https://doi.org/10.1007/s00421-001-0570-4
Arab A, Karimi E, Vingrys K, Shirani F (2021) Is phase angle a valuable prognostic tool in cancer patients’ survival? A systematic review and meta-analysis of available literature. Clin Nutr 40:3182–3190. https://doi.org/10.1016/j.clnu.2021.01.027
De Luis DA, Aller R, Izaola O, Terroba M, Cabezas G, Cuellar L (2006) Tissue electric properties in head and neck cancer patients. Ann Nutr Metab 50:7–10. https://doi.org/10.1159/000089484
McCall JL, Tuckey JA, Parry BR (1992) Serum tumour necrosis factor alpha and insulin resistance in gastrointestinal cancer. Br J Surg 79:1361–1363
Noguchi Y, Yoshikawa T, Marat D, Doi C, Makino T, Fukuzawa K et al (1998) Insulin resistance in cancer patients is associated with enhanced tumor necrosis factor‑α expression in skeletal muscle. Biochem Biophys Res Commun 253:887–892
Yoshikawa T, Noguchi Y, Doi C, Makino T, Nomura K (2001) Insulin resistance in patients with cancer: relationships with tumor site, tumor stage, body-weight loss, acute-phase response, and energy expenditure. Nutrition 17:590–593
Alshadwi A, Nadershah M, Carlson ER, Young LS, Burke PA, Daley BJ (2013) Nutritional considerations for head and neck cancer patients: a review of the literature. J Oral Maxillofac Surg 71:1853–1860. https://doi.org/10.1016/j.joms.2013.04.028
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F et al (2017) ESPEN guidelines on nutrition in cancer patients. Clin Nutr 36:11–48. https://doi.org/10.1016/j.clnu.2016.07.015
Cereda E, Cappello S, Colombo S, Klersy C, Imarisio I, Turri A et al (2017) Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol 126:81–88. https://doi.org/10.1016/j.radonc.2017.10.015
Champ CE, Mishra MV, Showalter TN, Ohri N, Dicker AP, Simone NL (2013) Dietary recommendations during and after cancer treatment: consistently inconsistent? Nutr Cancer 65:430–439. https://doi.org/10.1080/01635581.2013.757629
Zhu X‑P, Zhu L‑L, Zhou Q (2013) Prescribing practice and evaluation of appropriateness of enteral nutrition in a university teaching hospital. Ther Clin Risk Manag 9:37–43
Fietkau R, Lewitzki V, Kuhnt T, Hölscher T, Hess C‑F, Berger B et al (2013) A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized, controlled, multicenter trial. Cancer 119:3343–3353. https://doi.org/10.1002/cncr.28197
Bozzetti F, Stanga Z (2020) Does nutrition for cancer patients feed the tumour? A clinical perspective. Crit Rev Oncol Hematol 153:103061. https://doi.org/10.1016/j.critrevonc.2020.103061
Richtsmeier WJ, Dauchy R, Sauer LA (1987) In vivo nutrient uptake by head and neck cancers. Cancer Res 47:5230–5233
Sandulache VC, Ow TJ, Pickering CR, Frederick MJ, Zhou G, Fokt I et al (2011) Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer 117:2926–2938. https://doi.org/10.1002/cncr.25868
Sattler UGA, Meyer SS, Quennet V, Hoerner C, Knoerzer H, Fabian C et al (2010) Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol 94:102–109. https://doi.org/10.1016/j.radonc.2009.11.007
Seyfried TN, Shelton LM (2010) Cancer as a metabolic disease. Nutr Metab (Lond) 7:7. https://doi.org/10.1186/1743-7075-7-7
Holm E, Kämmerer U (2011) Lipids and carbohydrates in nutritional concepts for tumor patients. Aktuel Ernahrungsmed 36:286–298
Klement RJ, Kämmerer U (2011) Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond) 8:75. https://doi.org/10.1186/1743-7075-8-75
Klement RJ (2019) The influence of ketogenic therapy on the 5 R’s of radiobiology. Int J Radiat Biol 95:394–407. https://doi.org/10.1080/09553002.2017.1380330
Tisdale MJ, Brennan RA, Fearon KC (1987) Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer 56:39–43. https://doi.org/10.1038/bjc.1987.149
Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P et al (2014) Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab 2:18. https://doi.org/10.1186/2049-3002-2-18
Ma DC, Anderson CM, Rodman SN, Buranasudja V, McCormick ML, Davis A et al (2021) Ketogenic diet with concurrent chemoradiation in head and neck squamous cell carcinoma: preclinical and phase 1 trial results. Radiat Res. https://doi.org/10.1667/RADE-20-00150.1
Klement RJ, Sweeney RA (2016) Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res Notes 9:143. https://doi.org/10.1016/j.clnesp.2015.11.001
Klement RJ, Sweeney RA (2016) Impact of a ketogenic diet intervention during radiotherapy on body composition: II. Protocol of a randomised phase I study (KETOCOMP). Clin Nutr ESPEN 12:e1–6. https://doi.org/10.1016/j.clnesp.2015.11.001
Klement RJ, Schäfer G, Sweeney RA (2020) A ketogenic diet exerts beneficial effects on body composition of cancer patients during radiotherapy: an interim analysis of the KETOCOMP study. J Tradit Complement Med 10:180–187. https://doi.org/10.1016/j.jtcme.2019.03.007
Klement RJ, Champ CE, Kämmerer U, Koebrunner PS, Krage K, Schäfer G et al (2020) Impact of a ketogenic diet intervention during radiotherapy on body composition: III—final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res 22:94. https://doi.org/10.1186/s13058-020-01331-5
Klement RJ, Weigel MM, Sweeney RA (2021) A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr 40:4267–4274. https://doi.org/10.1016/j.clnu.2021.01.023
Klement RJ, Koebrunner PS, Meyer D, Kanzler S, Sweeney RA (2021) Impact of a ketogenic diet intervention during radiotherapy on body composition: IV. Final results of the KETOCOMP study for rectal cancer patients. Clin Nutr 40:4674–4684. https://doi.org/10.1016/j.clnu.2021.05.015
Klement RJ, Meyer D, Kanzler S, Sweeney RA (2021) Ketogenic diets consumed during radio-chemotherapy have beneficial effects on quality of life and metabolic health in patients with rectal cancer. Eur J Nutr 40:4674–4684. https://doi.org/10.1007/s00394-021-02615-y
Lucà-Moretti M (1998) Discovery of the master amino acid pattern. Ann R Natl Acad Med Spain CXV:397–416
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316:1236–1238
Benjamin DJ, Berger JO, Johannesson M, Nosek BA, Wagenmakers EJ, Berk R et al (2018) Redefine statistical significance. Nat Hum Behav 2:6–10. https://doi.org/10.1038/s41562-017-0189-z
Anderson DR (2008) Model based inferences in the life sciences: a primer on evidence, 1st edn. Springer Science+Business Media, LLC, New York
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmertest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Klement RJ (2014) Restricting carbohydrates to fight head and neck cancer—is this realistic? Cancer Biol Med 11:145–161. https://doi.org/10.7497/j.issn.2095-3941.2014.03.001
Hopanci Bicakli D, Ozkaya Akagunduz O, Meseri Dalak R, Esassolak M, Uslu R, Uyar M (2017) The effects of compliance with nutritional counselling on body composition parameters in head and neck cancer patients under radiotherapy. J Nutr Metab 2017:8631945. https://doi.org/10.1155/2017/8631945
Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA et al (2014) Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol 2:963–970. https://doi.org/10.1016/j.redox.2014.08.002
Quennet V, Yaromina A, Zips D, Rosner A, Walenta S, Baumann M et al (2006) Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol 81:130–135. https://doi.org/10.1016/j.radonc.2006.08.012
Sattler UGA, Mueller-Klieser W (2009) The anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol 85:963–971. https://doi.org/10.3109/09553000903258889
Voelxen NF, Blatt S, Knopf P, Henkel M, Appelhans C, Righesso LAR et al (2018) Comparative metabolic analysis in head and neck cancer and the normal gingiva. Clin Oral Investig 22:1033–1043. https://doi.org/10.1007/s00784-017-2185-0
Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B (2013) Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer 65:843–849. https://doi.org/10.1080/01635581.2013.804579
Arthur AE, Goss AM, Demark-Wahnefried W, Mondul AM, Fontaine KR, Chen YT et al (2018) Higher carbohydrate intake is associated with increased risk of all-cause and disease-specific mortality in head and neck cancer patients: results from a prospective cohort study. Int J Cancer 143:1105–1113. https://doi.org/10.1002/ijc.31413
Rabinovitch R, Grant B, Berkey BA, Raben D, Ang KK, Fu KK et al (2006) Impact of nutrition support on treatment outcome in patients with locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: a secondary analysis of RTOG trial 90-03. Head Neck 28:287–296. https://doi.org/10.1002/hed
Fearon KC, Borland W, Preston T, Tisdale MJ, Shenkin A, Calman KC (1988) Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr 47:42–48
Ok JH, Lee H, Chung H‑Y, Lee SH, Choi EJ, Kang CM et al (2018) The potential use of a ketogenic diet in pancreatobiliary cancer patients after pancreatectomy. Anticancer Res 38:6519–6527. https://doi.org/10.21873/anticanres.13017
Löser A, Ramke K, Grohmann M, Krause L, Roser P, Greinert F et al (2021) The impact of nutritional counseling on thyroid disorders in head and neck cancer patients after (chemo)radiotherapy: results from a prospective interventional trial. Strahlenther Onkol. https://doi.org/10.1007/s00066-021-01865-3
Ho YW, Yeh KY, Hsueh SW, Hung CY, Lu CH, Tsang NM et al (2021) Impact of early nutrition counseling in head and neck cancer patients with normal nutritional status. Support Care Cancer 29:2777–2785. https://doi.org/10.1007/s00520-020-05804-3
Sherwin RS, Hendler RG, Felig P (1975) Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest 55:1382–1390. https://doi.org/10.1172/JCI108057
Buse MG, Biggers JF, Friderici KH, Buse JF (1972) Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem 247:8085–8096
Palaiologos G, Felig P (1976) Effects of ketone bodies on amino acid metabolism in isolated rat diaphragm. Biochem J 154:709–716
Tome D (2012) Criteria and markers for protein quality assessment—a review. Br J Nutr 108:S222–9. https://doi.org/10.1017/S0007114512002565
Ræder H, Kværner AS, Henriksen C, Florholmen G, Henriksen HB, Bøhn SK et al (2018) Validity of bioelectrical impedance analysis in estimation of fat-free mass in colorectal cancer patients. Clin Nutr 37:292–300. https://doi.org/10.1016/j.clnu.2016.12.028
Acknowledgements
We are thankful to all patients who participated in this study. We also thank the staff of our Department of Radiation Oncology and the colleagues on the ward who helped with the conduct of this study as well as the companies vitaflo, dr. reinwald healthcare gmbh + co kg, and Dr. Schär (Kanso) for providing us product samples for this study.
Funding
No external funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: RJK, RAS; formal analysis: RJK; investigation: RJK, RAS; methodology: RJK, RAS; project administration: RAS; resources: RAS; supervision: RAS; visualization: RJK; writing—original draft preparation: RJK; writing—review and editing: RAS
Corresponding author
Ethics declarations
Conflict of interest
R.J. Klement has received an honorarium from the company vitaflo for giving a talk about the objectives and preliminary results of the KETOCOMP study. R.J. Klement and R.A. Sweeney follow a KD occasionally. No other potential conflicts of interest, in particular no financial conflicts of interest, exist. The companies providing product samples for this study had no influence on the design, data collection, or analysis, and none of the authors has any business relationship with them.
Ethical standards
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The protocol was registered on August 6, 2015, under ClinicalTrials.gov identifier NCT02516501 after study approval was granted by the ethics committee of the Bavarian Medical Association (Landesaerztekammer Bayern). Informed consent was obtained from all individual participants included in the study.
Additional information
Data share statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending reasonable application.
Rights and permissions
About this article
Cite this article
Klement, R.J., Sweeney, R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: V. Final results of the KETOCOMP study for head and neck cancer patients. Strahlenther Onkol 198, 981–993 (2022). https://doi.org/10.1007/s00066-022-01941-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-022-01941-2