Abstract
Objective
To prospectively compare the interobserver variability of combined transrectal ultrasound (TRUS)/computed tomography (CT)- vs. CT only- vs. magnetic resonance imaging (MRI) only-based contouring of the high-risk clinical target volume (CTVHR) in image-guided adaptive brachytherapy (IGABT) for locally advanced cervical cancer (LACC).
Methods
Five patients with LACC (FIGO stages IIb–IVa) treated with radiochemotherapy and IGABT were included. CT, TRUS, and T2-weighted MRI images were performed after brachytherapy applicator insertion. 3D-TRUS image acquisition was performed with a customized ultrasound stepper device and software. Automatic applicator reconstruction using optical tracking was performed in the TRUS dataset and TRUS and CT images were fused with rigid image registration with the applicator as reference structure. The CTVHR (based on the GEC-ESTRO recommendations) was contoured by five investigators on the three modalities (CTVHR_CT, CTVHR_TRUS-CT, and CTVHR_MRI). A consensus reference CTVHR_MRI (MRIref) was defined for each patient. Descriptive statistics and overlap measures were calculated using RTslicer (SlicerRT Community and Percutaneous Surgery Laboratory, Queen’s University, Canada), comparing contours of every observer with one another and with the MRIref.
Results
The interobserver coefficient of variation was 0.18 ± 0.05 for CT, 0.10 ± 0.04 for TRUS-CT, and 0.07 ± 0.03 for MRI. Interobserver concordance in relation to the MRIref expressed by the generalized conformity index was 0.75 ± 0.04 for MRI, 0.51 ± 0.10 for TRUS-CT, and 0.48 ± 0.06 for CT. The mean CTVHR_CT volume of all observers was 71% larger than the MRIref volume, whereas the mean CTVHR_TRUS-CT volume was 15% larger.
Conclusion
Hybrid TRUS-CT as an imaging modality for contouring the CTVHR in IGABT for LACC is feasible and reproducible among multiple observers. TRUS-CT substantially reduces overestimation of the CTVHR volume of CT alone while maintaining similar interobserver variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In image-guided adaptive brachytherapy (IGABT) of locally advanced cervical cancer (LACC), magnetic resonance imaging (MRI) with applicator in place is currently considered as the gold standard for tumor visualization and dose optimization [1,2,3,4,5,6,7,8,9]. MRI is, however, not always available in centers with fewer resources, which is why computed tomography (CT) has been evaluated as alternative, as most radiotherapy departments are equipped with a CT scanner [10]. Contouring on CT enables excellent visualization of the applicator and organs at risk (OAR), but also generally leads to an overestimation of the target volume compared to MRI [10].
Increasing evidence shows that transrectal ultrasound (TRUS) might be a more promising and inexpensive alternative. TRUS is extensively used in prostate brachytherapy, but apart from tumor assessment and confirming tandem placement, it has not been generally adopted in LACC brachytherapy to date [11]. First studies investigating TRUS for target definition in LACC brachytherapy indicated that TRUS appears non-inferior to MRI for assessing the high-risk clinical target volume (CTVHR) dimensions [12, 13]. As TRUS and CT are widely available in radiotherapy departments, combining their assets could possibly provide an equivalent for MRI in contouring target volumes in IGABT for LACC. A clinical workflow for combined TRUS/CT treatment planning in LACC brachytherapy has already been successfully simulated in a patient [14].
If TRUS is further developed as a treatment planning modality, a TRUS/CT-based target delineation protocol should be developed to allow for volumetric TRUS-based contouring in routine clinical practice. As ultrasound is known for its observer dependence and previous studies on TRUS for LACC brachytherapy were performed only with a single observer, further research with multiple observers is mandatory as a key step towards development of TRUS-based brachytherapy. The objective of this analysis was to investigate the feasibility of combined TRUS/CT-based contouring using a dedicated prototype system for LACC brachytherapy and to analyze interobserver variability for CTVHR within a prospective pilot study with multiple observers.
Methods
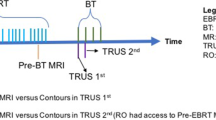
Five patients with squamous cell carcinoma of the uterine cervix treated with curative intent were included in this prospective pilot study (EK no. 1998/2014). Two patients had FIGO stage IIB disease, three had stage IIIC2 (local: IIB, IIIB, IVA, respectively; FIGO version 2018 [15]). All patients underwent a staging MRI at diagnosis. Treatment consisted of external beam radiation therapy (EBRT) with concomitant cisplatin and IGABT. During EBRT, the elective clinical target volume (CTV) received 45 Gy in 1.8-Gy fractions, with a simultaneously integrated boost to pathologic lymph nodes to 55–57.5 Gy in 2.2–2.3-Gy fractions. MRI-based IGABT was performed at the end or after EBRT in two applications of two fractions each, using high-dose-rate brachytherapy with a planning aim of delivering a D90 >85 Gy EQD210 to the CTVHR (EBRT + IGABT). A tandem-ring applicator (“Vienna-type” applicator, Elekta, Sweden) without or with (straight, oblique, or freehand) interstitial titanium needles was used.
Imaging modalities for IGABT
TRUS, CT, and MRI were performed after applicator placement. A Somatom Plus S scanner was used for CT (Siemens, Erlangen, Germany), in 2‑mm slice intervals without contrast medium. T2-weighted MRI was performed with a 0.35 T system (Magnetom C, Siemens) with 5‑mm slice thickness, acquiring axial, para-axial, sagittal, and coronal images.
TRUS system
TRUS was performed with a dedicated prototype system developed for LACC brachytherapy allowing for 3D-TRUS imaging with automatic applicator reconstruction by optical tracking of the applicator (MedCom, Germany; Elekta; ACMIT, Austria). The main principle of the system is that the TRUS probe and the applicator are fixed to each other by a dedicated positioning arm and that tracking tools are positioned at specific reference geometries, which can be identified by the optical tracking system. Overall, the prototype consists of (1) a mobile cart with a PC equipped with (2) an imaging software (GynUS v2.0, MedCom); (3) a 5–10-MHz integrated TRUS transducer (BiopSee, MedCom) with a biplanar probe (BIPC6.5/10/128, BIPL7.5/70/128), mounted to (4) a stepper unit (ECRM, Elekta) with affixed (5) tracking tools (ACMIT) containing factory NDI trackers (single-faced rigid bodies) tracked by (6) a ceiling-mounted Polaris Spectra system (NDI, Canada); and (7) multi-DOF passive positioning arms connecting the applicator to the stepper unit (Baitella, Switzerland), and connecting the complete imaging unit to the operating table’s side rail (MFA, iSYS Medizintechnik, Austria). Further technical details have been published elsewhere [16, 17].
TRUS imaging and tracking procedure
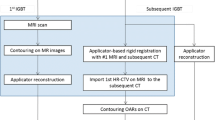
After applicator insertion, the TRUS probe with the stepper unit and loose MFA arms was placed manually in the rectum at the optimal depth and angle according to the position and flexure of the uterus/applicator. The MFA arm was locked and the stepper unit subsequently connected to the applicator with the second positioning arm. Image acquisition was performed during automated rotation of the TRUS probe, generating a 3D volume using the longitudinal array of the probe from the level of the ring applicator to the fundus of uterus, if reachable. Optical tracking of the applicator was based on a set of retroreflective marker spheres mounted on both the TRUS probe and the applicator. The relative position of the two components was tracked with a stereo camera (Polaris, NDI, Canada). The system has already been described by Jelinek et al. [17]. Using spatial information from the tool tracking system, automatic applicator reconstruction using applicator library models was performed ([14]; Fig. 1). The reconstruction process was verified within a plausibility check based on several reference structures (tandem, posterior curvature of the ring, rotation of the ring as indicated by the holes, interstitial needles) and fine-tuned, if necessary. The positioning arms were then unlocked and the imaging unit was removed. The resulting TRUS image volumes were rigidly registered to the post-implant CT using the applicator as a reference coordinate system as previously described [14].
All further image registration and contouring operations were performed in the Oncentra brachytherapy treatment planning system (TPS; Elekta).
Contouring protocol and image analysis
Five investigators were available for analysis. The investigators were all radiation oncologists with experience in contouring brachytherapy target volumes on CT and MRI in LACC. For TRUS-based contouring, only one observer was experienced.
All investigators were given an introductory course on TRUS contouring by the experienced investigator and two test cases were contoured by all investigators. After approval of these cases, the study cases could be contoured. Investigators were provided with the following material: clinical information of each patient, a pelvic MRI at diagnosis, and a 3D clinical drawing of the gynecological examination at diagnosis and at the time of brachytherapy [18].
All investigators contoured each case independently following the workflow defined in Fig. 2. First, the CTVHR was contoured on CT (CTVHR _CT) according to the consensus guidelines for CT-based brachytherapy, defined by the complete mass (cervical + parametrial) at intermediate density (grey/white). The CTVHR height on CT was determined by the GTV height on the MRI at diagnosis [10].
Secondly, the CTVHR was contoured on TRUS (CTVHR_TRUS), defined by a hypoechogenic mass (cervical + parametrial) on grey-scale imaging [13]. After contouring this preliminary target volume on TRUS, the structures were resampled to CT and adapted according to the OAR depicted on CT (CTVHR_TRUS-CT). The CTVHR height on TRUS-CT was determined by the GTV height on the MRI at diagnosis.
Next, the CTVHR was contoured on MRI (CTVHR_MRI) according to the recommendations for MR-based brachytherapy as the whole cervix and any residual disease at time of brachytherapy including “grey zones” [19, 20].
Finally, a reference contour on MRI (MRIref) was generated for each patient, attained by reaching consensus amongst all observers.
Analysis of contouring deviations
For each imaging modality, the maximum width (maximum latero-lateral diameter found in all para-axial slices), the maximum thickness (maximum antero-posterior diameter found in all para-axial slices), the maximum height (maximum cranio-caudal diameter found in all sagittal slices), and the total volume of each CTVHR from every observer was measured for each patient.
CT and MRI images were automatically registered with TRUS-CT images based on the applicator position, serving as a common reference coordinate system defined on the TPS [21]. Finally, all contours were superimposed on a single CT image set per patient, including all contours from all imaging modalities from all observers, allowing for direct comparison to each other (Fig. 3). To assess interobserver variations, all images and contours were exported to the SlicerRT software system, version 4.5.0‑1 (SlicerRT Community and Percutaneous Surgery Laboratory, Queen’s University, Canada) [22]. Contours of every observer were compared with one another and with the MRIref.
Example contours of a patient with FIGO IIB cervical carcinoma at time of brachytherapy with the applicator in place on computed tomography (CT) (a), magnetic resonance imaging (MRI) (b), and transrectal ultrasound (TRUS) (c). Contours from all observers are projected on the CT dataset: MRI contours in pink, TRUS contours in green, CT contours in blue
The coefficient of variation (COV) was defined as the standard deviation divided by the mean value and is larger with increasing variability (more dispersion in the data). A generalized conformity index (CIgen) was defined as the ratio of the sum of all overlapping volumes between pairs of observers and the sum of all overlapping and all additional volumes between the same pairs [23]. A CIgen of 1 indicates a total overlap, while 0 means there is no concordance. Descriptive statistics, mean differences between the groups, and a paired t‑test were calculated. Statistics were performed with Excel 2010 software (Microsoft, Washington). A p-value <0.05 was considered as statistically significant.
Results
The proposed workflow of hybrid TRUS-CT with optical tracking of the applicator was successfully applied. 3D-TRUS imaging, automatic applicator reconstruction, volumetric contouring on TRUS, and image fusion with CT were feasible in all patients. All observers were able to contour the CTVHR on TRUS-CT for all patients, with an acceptable agreement between the observers (CIgen: 0.66 ± 0.08; COV: 0.10 ± 0.04).
The mean maximum width of the CTVHR was 55 mm ± 8 mm, 52 mm ± 6 mm, and 64 mm ± 6 mm, and the mean volume was 46 cm3 ± 9 cm3, 43 cm3 ± 10 cm3, and 67 cm3 ± 9 cm3 on TRUS-CT, MRI, and CT, respectively. The mean CIgen was 0.66 ± 0.08, 0.75 ± 0.04, and 0.66 ± 0.06, and the mean COV was 0.10 ± 0.04, 0.07 ± 0.03, and 0.18 ± 0.05 for TRUS-CT, MRI, and CT, respectively (Table 1).
No significant difference was observed in the mean maximum width, height, thickness, or volume on TRUS-CT compared to MRI. The mean maximum width and volume were significantly larger on CT compared to MRI (Tables 3 and 4).
The mean volume of the CTVHR was 15% larger on TRUS-CT (14%, 9%, 28%, 20%, and 5% for patients 1–5, respectively) and 71% larger than on CT (55%, 50%, 70%, 94%, and 85% for patients 1–5, respectively) than on the MRIref volume (Fig. 4a). The mean maximum width of the CTVHR was 5% larger on TRUS-CT (14%, 13%, 18% larger for patients 1, 2, and 3; 15% and 3% smaller for patients 4 and 5, respectively) and 24% larger on CT (24%, 29%, 29%, 8%, and 31% for patients 1–5, respectively) than on the MRIref contour (Fig. 4b). The CIgen was 0.51 ± 0.1, 0.75 ± 0.04, and 0.48 ± 0.06 for all TRUS-CT, MRI, and CT contours, respectively, in relation to the MRIref contour (Table 2).
Relative over-/underestimation of the mean volume of the high-risk clinical target volume (CTVHR; a) and mean width of the CTVHR (b) on CT (black) and on TRUS-CT (grey) compared to the reference CTVHR on MRI for all patients. Pt patient, CT computed tomography, TRUS-CT transrectal ultrasound combined with computed tomography
The differences in mean maximum width, height, and volume were significantly smaller for TRUS-CT compared to MRIref than for CT compared to MRIref (Tables 3 and 4).
Discussion
Contouring uncertainties due to poor target volume visualization have a substantial impact on tumor coverage and dose to OAR. MRI is currently considered the gold standard for target volume contouring in IGABT in LACC because of the high tissue contrast in pelvic anatomy [10, 20], but is not always accessible due to its cost. Therefore, CT could serve as a plausible alternative, but literature on the comparison of CT- and MRI-based contouring showed a substantial overestimation of CTVHR volume on CT [18, 24, 25]. Contouring of the extent of parametrial invasion remains challenging due to lacking soft tissue contrast on CT, specifically in large tumors with extensive invasion [24]. Recently, TRUS has been considered as an imaging modality for IGABT, since it provides excellent soft tissue contrast and has already proven its advantages in prostate cancer brachytherapy [26]. Schmid et al. suggested implementing TRUS in the pre- and intraoperative setting to assess the CTVHR before applicator insertion, for preplanning purposes, and for TRUS-guided tandem and needle insertion [12]. A comparison of the target volume dimensions between TRUS and MRI showed no statistically significant difference for the CTVHR width, indicating the high potential of TRUS for IGABT in LACC [13]. To integrate TRUS into IGABT and to outweigh subsequent limitations, a workflow for combined TRUS-CT was established by Nesvacil et al. [14], showing that TRUS-CT-based volumetric contouring and treatment planning is feasible and may be clinically comparable to the MRI-based approach.
In this report, volumetric contouring for LACC on 3D-TRUS with multiple observers was investigated for the first time. The previously described workflow for TRUS-CT treatment planning proved feasible within this prospective pilot study and all observers managed to follow a contouring protocol for combined TRUS-CT-based CTVHR contouring. A dedicated prototype comprising standard equipment from different fields including a standard TRUS probe, fixation devices, and an optical tracking system were safely applied. Particularly the TRUS probe positioning and imaging process using a table-mounted flexible stepper unit in combination with fixation devices was a substantial improvement in comparison to the system used in our previous studies. 3D-TRUS imaging with the applicator in place could therefore be applied for all patients in this report. It appears that with such a system, the previously reported substantial proportion (≈20%) of patients not suitable for TRUS imaging with applicator could be significantly reduced. Since the location of the applicator, particularly the ring-component, can’t be defined on TRUS with reasonable accuracy for subsequent treatment planning, optical tracking of the applicator was implemented in the system to allow for automatic applicator reconstruction in the TRUS dataset. This is a crucial step within the proposed workflow to enable further image fusion with CT and treatment planning. Extensive phantom and clinical tests were performed in advance to learn and improve the tracking procedure; however, optical tracking is sensitive to various interferences and shows limitations in daily clinical practice for LACC brachytherapy in its current form. Nevertheless, automatic applicator reconstruction using applicator library models was successfully performed, with some minor manual adjustments, in all patients in this study. Further optimization and investigation of alternative tracking modalities, such as electromagnetic or mechanical tracking, are necessary.
In accordance with our previous study, the mean maximum width of the CTVHR on TRUS-CT was smaller than on CT alone and comparable to MRI, indicating the reproducibility of these previous findings with multiple observers. Furthermore, combining TRUS and CT reduced the mean CTVHR volume by approximately one third, reduced the mean COV from 0.18 to 0.10, and slightly improved the CIgen from 0.48 to 0.51 compared with the MRIref volume. As expected, the variation for the MRI contours was smaller (mean COV = 0.1 and mean CIgen = 0.75 between all observers and with regard to the MRIref volume), whereas the mean CTVHR volume was similar between TRUS-CT (46 cm3) and MRI (43 cm3). These results are in line with the literature on interobserver variations in target delineation of IGABT in LACC: Petric et al. reported a CIgen of 0.78 for MRI-based contouring based on 13 cases and two observers [27], Viswanthan et al. reported a CIgen of 0.48 for CT-based contouring based on 3 cases and 23 observers [24], and Pötter et al. reported a CIgen of 0.54 for CT-based contouring with support of a pre-brachytherapy MRI scan [25]. Performance of TRUS appears to have a similar impact on interobserver variation as a pre-brachytherapy MRI scan, but with the advantage of having the applicator already in place as a reference structure. In addition, Mahantshetty et al. recently investigated the use of TRUS assistance for CT-based target contouring by using measurements of reference points in relation to the cervical canal instead of full volumetric image fusion, and did not observe major deviations compared to MRI-based contouring [28].
The limitations of TRUS are the limited field-of-view and applicator-induced artefacts, implying that parts of the target volume are hardly or not visible on TRUS images alone (Fig. 1). Especially the limited depth of insertion of the transrectal probe in the presence of the applicator hampers depiction of the most cranial parts of the uterus, as was the case for the presented cases. To reach a consensus amongst observers, the initial GTV height as seen on the diagnostic MRI was adopted as the CTVHR height on CT or TRUS-CT. Also, the anterior border of the CTVHR adjacent to the bladder was more difficult to assess, due to the distance from the probe and acoustic shadowing by the applicator, resulting in a wider range in the maximum thickness on TRUS-CT compared to MRI. The presence of the TRUS probe can also cause compression of the uterus, leading to a reduced thickness of the CTVHR as shown in our previous study [13]. In our present study, however, no significant difference between TRUS-CT and MRI was observed. The posterior and lateral parts of the cervix were well defined on TRUS, resulting in a consistently comparable range in the maximum width on TRUS-CT and MRI. In two patients, the maximum width on TRUS-CT was smaller compared to the MRIref contour (15% and 3% in patients 4 and 5, respectively), which should be interpreted with caution as the absolute differences are minor (8 mm ± 2 mm for patient 4 and 1 mm ± 2 mm for patient 5), but needs to be taken seriously, as this could result in a potential topographical miss by TRUS-CT. A random geometric uncertainty of up to 2 mm between contours from different modalities was noticed during conformity analysis. This may be reducible by further technical refinement of the applicator-based TRUS/CT registration methods. The dosimetric impact of the interobserver variations will be presented separately. In the present study, low-field MRI was used as planning modality. Although high-field MRI (1.5–3 T) MRI is more widely available, both are considered suitable for IGABT [20], and a previous report comparing target volume dimensions between TRUS and 1.5 T MRI showed similar results [13].
Another limitation of TRUS could be the operator dependence. Four out of five observers had very limited to no experience with TRUS-based contouring. Therefore, an introductory course into TRUS-based contouring was given and two training cases were contoured. Despite minimal training, excellent results with an acceptable interobserver variation could be achieved. Further improvement with increasing experience can be expected. Of note, while image interpretation and contouring were performed by multiple observers, TRUS image acquisition was performed by only one observer. The operator dependence of TRUS image acquisition is another possible source of variation, which was not addressed in the current study and has to be investigated separately. A similar learning curve as described for TRUS-based prostate brachytherapy can be assumed for TRUS in LACC brachytherapy [29, 30].
The present study covers interobserver contouring using TRUS-CT as one step further in the development of TRUS as a possible treatment modality in IGABT. However, other key questions remain to be answered, in particular regarding optimization of the imaging and tracking procedure, the performance of TRUS by multiple observers, and the actual treatment of patients by any form of TRUS-based brachytherapy within a clinical study.
Conclusion
TRUS-CT-based contouring in IGABT for LACC with automatic applicator reconstruction by optical tracking is feasible after a minimal amount of training, is consistent amongst multiple observers, and leads to reduced volumes compared to CT alone. This prospective interobserver analysis could be the next step in the shift from TRUS being just an aid for tumor assessment and tandem placement verification during the procedure, to an accessible alternative to MRI for CTVHR contouring.
References
Pötter R, Georg P, Dimopoulos JC et al (2011) Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 100:116–123
Charra-Brunaud C, Harter V, Delannes M et al (2012) Impact of 3D image based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother Oncol 103:305–313
Lindegaard JC, Fokdal LU, Nielsen SK et al (2013) MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 52:1510–1519
Sturdza A, Potter R, Fokdal L et al (2016) Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in Retro-EMBRACE, a multicenter cohort study. Radiother Oncol 120:428–433
Rijkmans EC, Nout RA, Rutten IH et al (2014) Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol 135:231–238
Pötter R, Dimopoulos J, Georg P et al (2007) Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 83:148–155
Lindegaard JC, Tanderup K, Nielsen SK et al (2008) Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys 71:756–764
Minkoff D, Gill BS, Kang J et al (2015) Cervical cancer outcome prediction to high-dose rate brachytherapy using quantitative magnetic resonance imaging analysis of tumor response to external beam radiotherapy. Radiother Oncol 115:78–83
Vojtíšek R, Sukovská E, Baxa J et al (2019) Late side effects of 3T MRI-guided 3D high-dose rate brachytherapy of cervical cancer: Institutional experiences. Strahlenther Onkol 195:972–981
Viswanathan AN, Dimopoulos J, Kiritist C et al (2007) Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys 68:491–498
Ordeanu C, Pop DC, Badea R et al (2015) Local experience in cervical cancer imaging: Comparison in tumour assessment between TRUS and MRI. Rep Pract Oncol Radiother 20:223–230
Schmid MP, Pötter R, Brader P et al (2013) Feasibility of transrectal ultrasonography for assessment of cervical cancer. Strahlenther Onkol 189:123–128
Schmid MP, Nesvacil N, Pötter R et al (2016) Transrectal ultrasound for image-guided adaptive brachytherapy in cervix cancer—an alternative to MRI for target definition? Radiother Oncol 120:467–472. https://doi.org/10.1016/j.radonc.2016.01.021
Nesvacil N, Schmid MP, Pötter R et al (2016) Combining transrectal ultrasound and CT for image-guided adaptive brachytherapy of cervical cancer: Proof of concept. Brachytherapy 15:839–844
Bhatla N, Aoki D, Sharma DN et al (2018) Cancer of the cervix uteri. Int J Gynaecol Obstet 143(Suppl 2):22
Jelinek F, Kronreif G, Van de Wardt C (2017) Compact rotational ultrasound probe holder for brachytherapy. Proceedings of the 2017 Design of Medical Devices Conference.
Jelinek F, Kronreif G, Nesvacil N et al (2016) Image-guided adaptive brachytherapy of cervical cancer—workflow for transrectal ultrasound and optical tracking. In: Abstract book, SMIT 2016
Hegazy N, Pötter R, Kirisits C et al (2013) High-risk clinical target volume delineation in CT-guided cervical cancer brachytherapy: impact of information from FIGO stage with or without systematic inclusion of 3D documentation of clinical gynecological examination. Acta Oncol 52:1345–1352
Haie-Meder C, Pötter R, Van Limbergen E et al (2005) Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 74:235–245
Dimopoulos J, Petrow P, Tanderup K et al (2012) Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol 103:113–122
Nesvacil N, Pötter R, Sturdza A et al (2013) Adaptive image guided brachytherapy for cervical cancer: a combined MRI-/CT-planning technique with MRI only at first fraction. Radiother Oncol 107:75–81
Pinter C, Lasso A, Wang A et al (2012) SlicerRT: Radiation therapy research toolkit for 3D Slicer. Med Phys 39:6332–6338
Kouwenhoven E, Giezen M, Struikmans H et al (2009) Measuring the similarity of target volume delineations independent of the number of observers. Phys Med Biol 54:2863–2873
Viswanathan AN, Erickson B, Gaffney DK et al (2014) Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 90:320–328
Pötter R, Federico M, Sturdza A et al (2016) Value of magnetic resonance imaging without or with applicator in place for target definition in cervix cancer brachytherapy. Int J Radiat Oncol Biol Phys 94:588–597
Batchelar D, Gaztañaga M, Schmid M et al (2014) Validation study of ultrasound-based high-dose-rate prostate brachytherapy planning compared with CT-based planning. Brachytherapy 13:75–79
Petric P, Dimopoulos J, Kirisits C et al (2008) Inter- and intraobserver variation in HR-CTV contouring: intercomparison of transverse and paratransverse image orientation in 3D-MRI assisted cervix cancer brachytherapy. Radiother Oncol 89:164–171
Mahantshetty U, Naga CP, Khadanga CR et al (2018) A prospective comparison of computed tomography with transrectal ultrasonography assistance and magnetic resonance imaging-based target-volume definition during image guided adaptive brachytherapy for cervical cancers. Int J Radiat Oncol Biol Phys 102:1448–1456
Beaulieu L, Evans DA, Aubin S et al (2007) Bypassing the learning curve in permanent seed implants using state-of-the-art technology. Int J Radiat Oncol Biol Phys 67(1):71–77
Prada PJ, Cardenal J, García Blanco A et al (2020) Focal high-dose-rate brachytherapy for localized prostate cancer: toxicity and preliminary biochemical results. Strahlenther Onkol. https://doi.org/10.1007/s00066-019-01561-3
Funding
The Department of Radiotherapy at the Medical University of Vienna receives/received financial and/or equipment support for research and educational purposes from Nucletron, an Elekta company. This work was supported by ACMIT, the Austrian Center for Medical Innovation and Technology, which is funded within the scope of the COMET, Competence Centers for Excellent Technologies, program and funded by the federal government (BMVIT and BMWFW) and the governments of Lower Austria and Tyrol, Austria. This project was funded in part via a research contract from Elekta.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Smet, N. Nesvacil, J. Knoth, A. Sturdza, D. Najjari-Jamal, F. Jelinek, G. Kronreif, R. Pötter, J. Widder, C. Kirisits, and M.P. Schmid declare the following: the Department of Radiotherapy at the Medical University of Vienna receives/received financial and/or equipment support for research and educational purposes from Nucletron, an Elekta company. This work was supported by ACMIT, the Austrian Center for Medical Innovation and Technology, which is funded within the scope of the COMET, Competence Centers for Excellent Technologies, program and funded by the federal government (BMVIT and BMWFW) and the governments of Lower Austria and Tyrol, Austria. This project was funded in part via a research contract from Elekta.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethik Kommission Medizinische Universität Wien, no. 1998/2014) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smet, S., Nesvacil, N., Knoth, J. et al. Hybrid TRUS/CT with optical tracking for target delineation in image-guided adaptive brachytherapy for cervical cancer. Strahlenther Onkol 196, 983–992 (2020). https://doi.org/10.1007/s00066-020-01656-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01656-2